The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel
Abstract
:1. Introduction
2. Neutron-Absorption Elements and Shielding Mechanism
3. Neutron-Shielding Materials
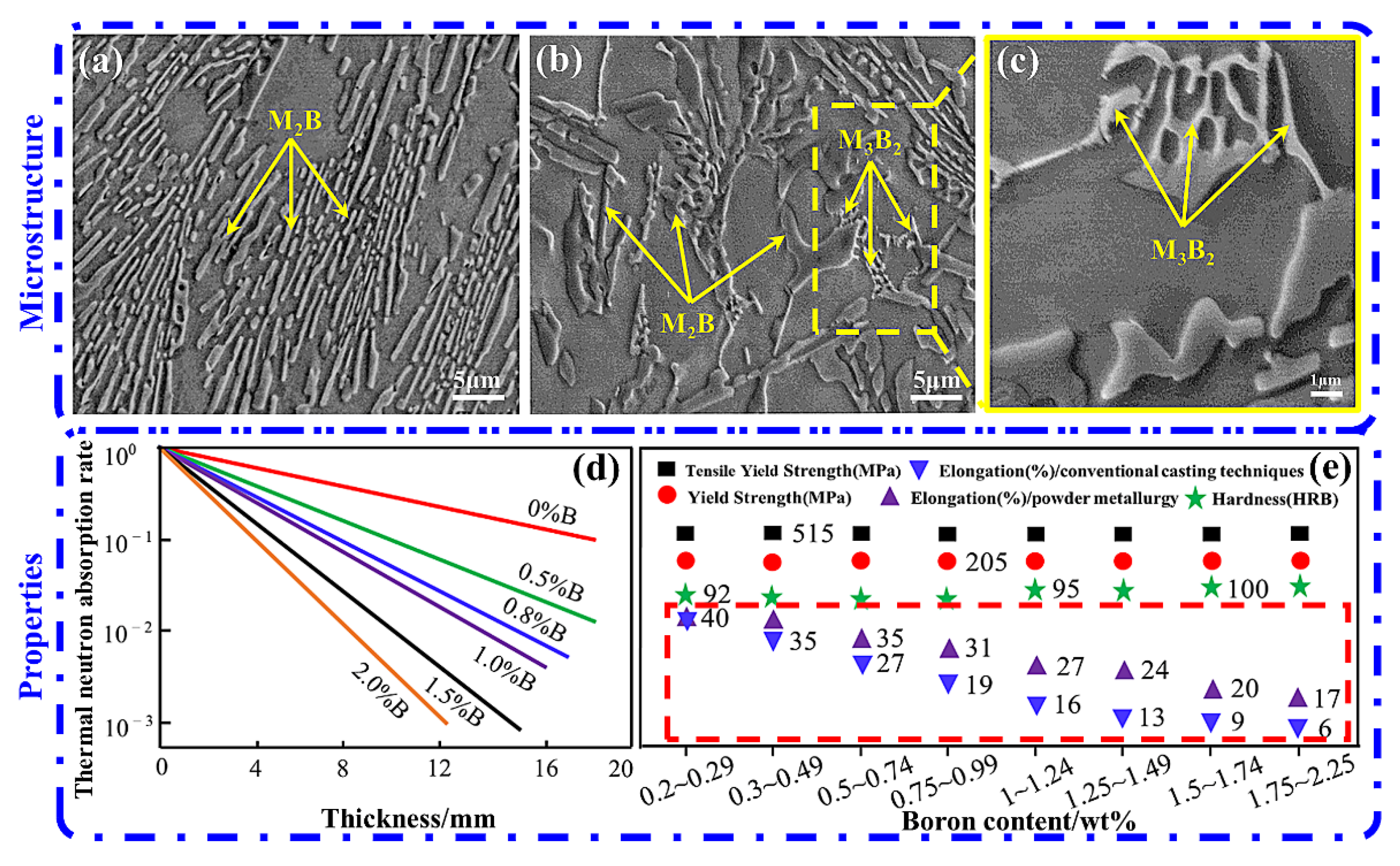
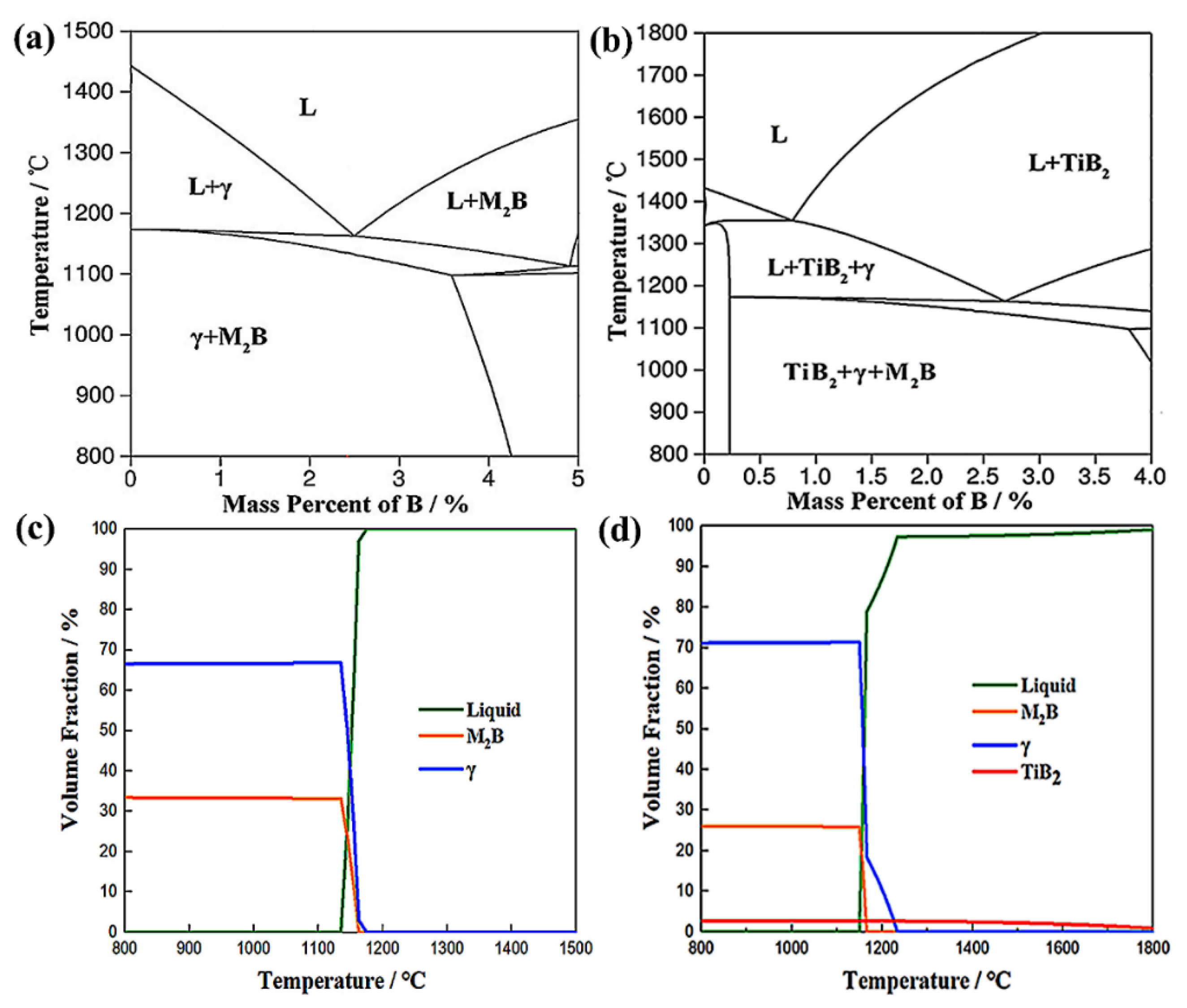
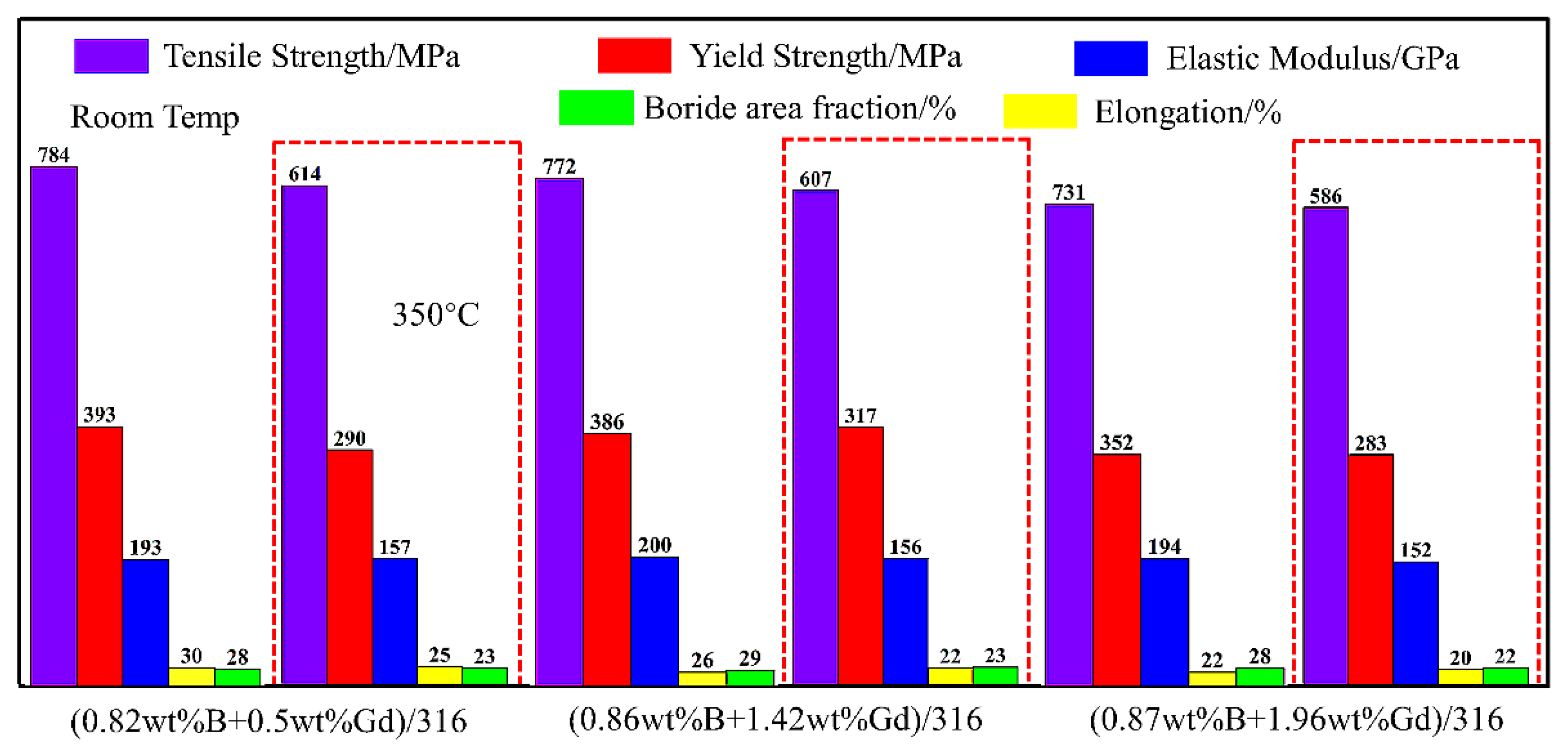
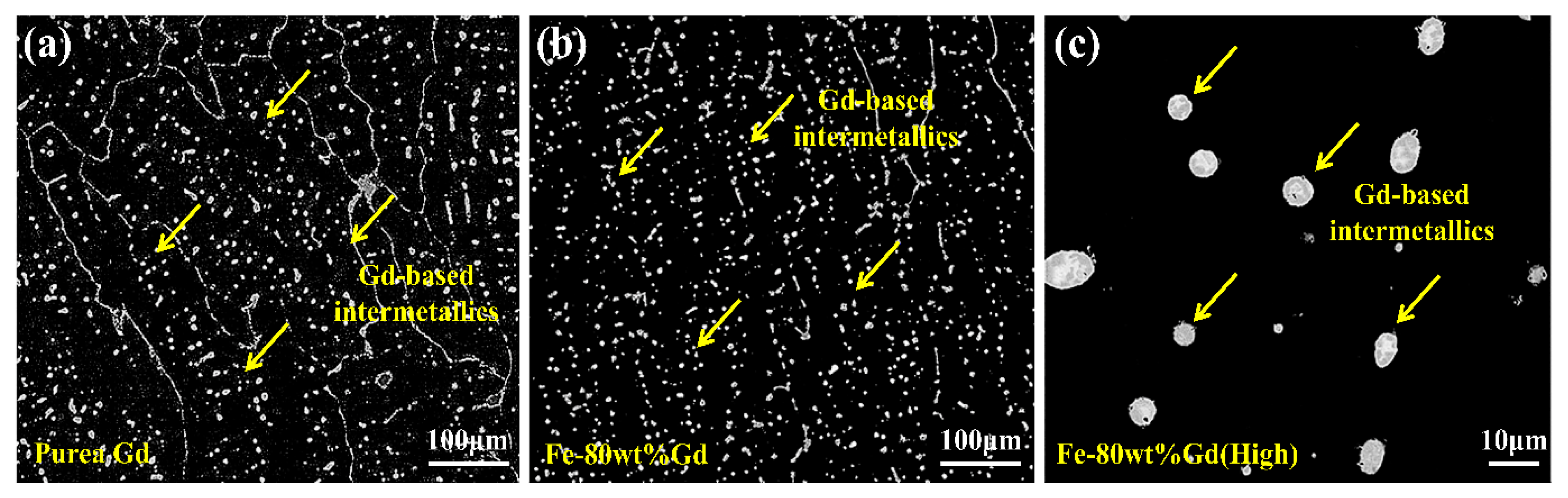
3.1. Borated Stainless Steels
3.2. B/Al Alloy
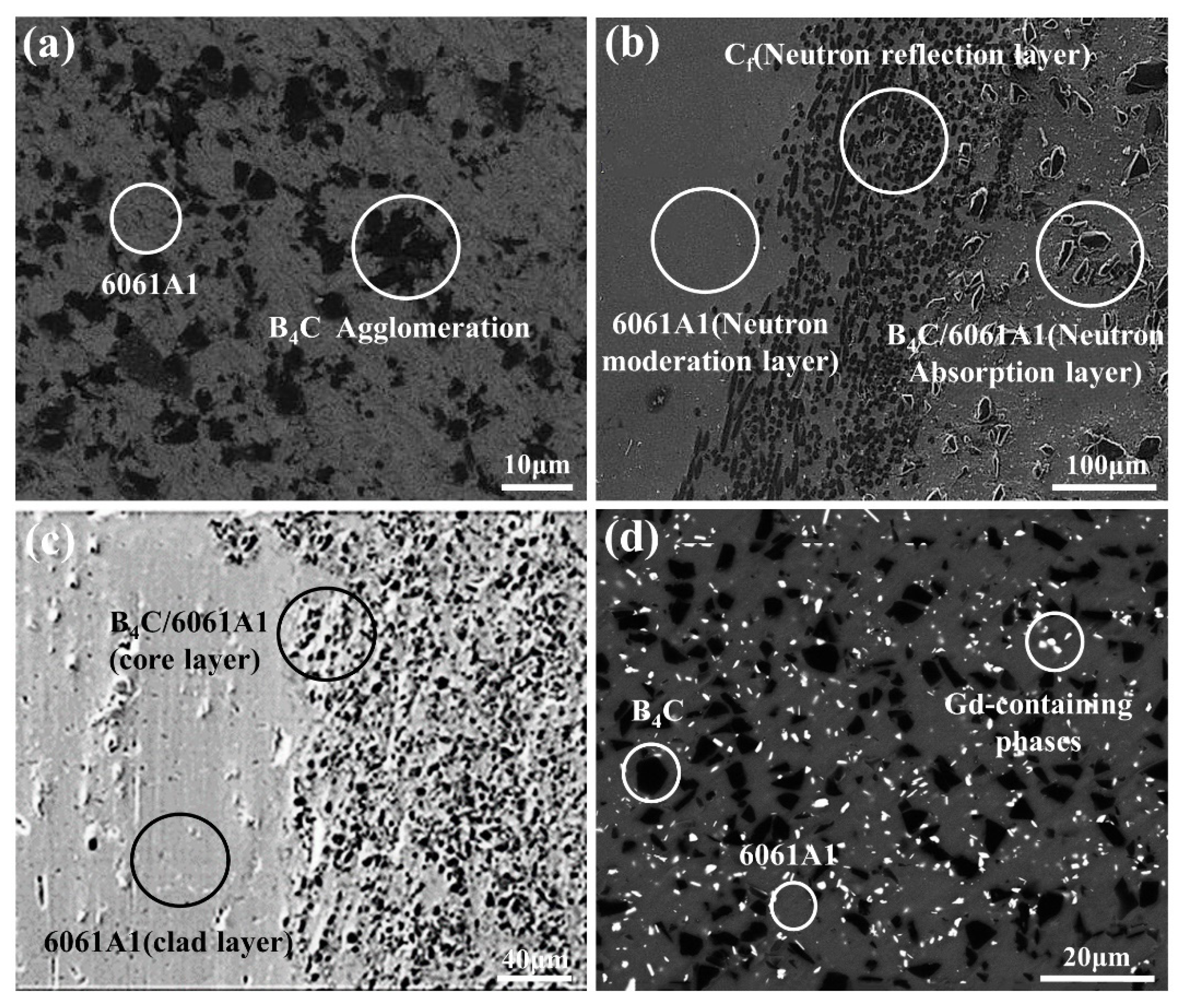
3.3. B4C/Al Composite
3.3.1. Stirring Casting
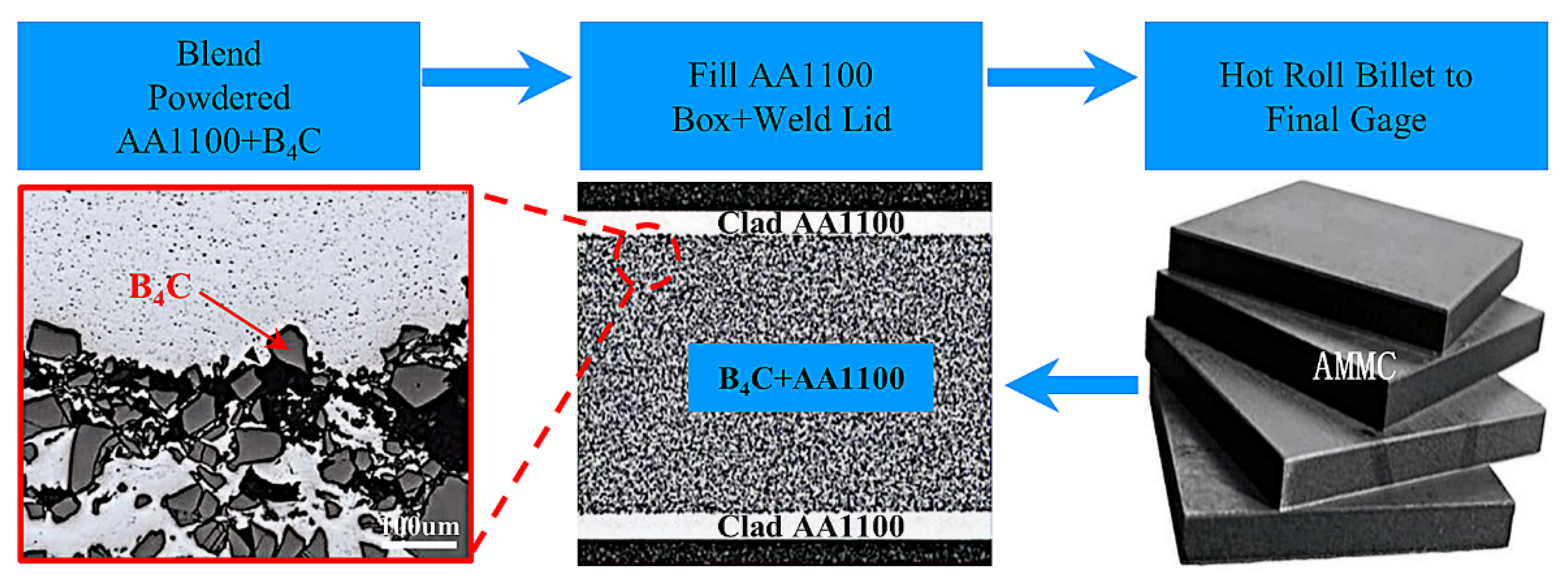
3.3.2. Powder Metallurgy
3.3.3. Infiltration Process
3.3.4. New Materials
3.4. Polymer-Based Materials
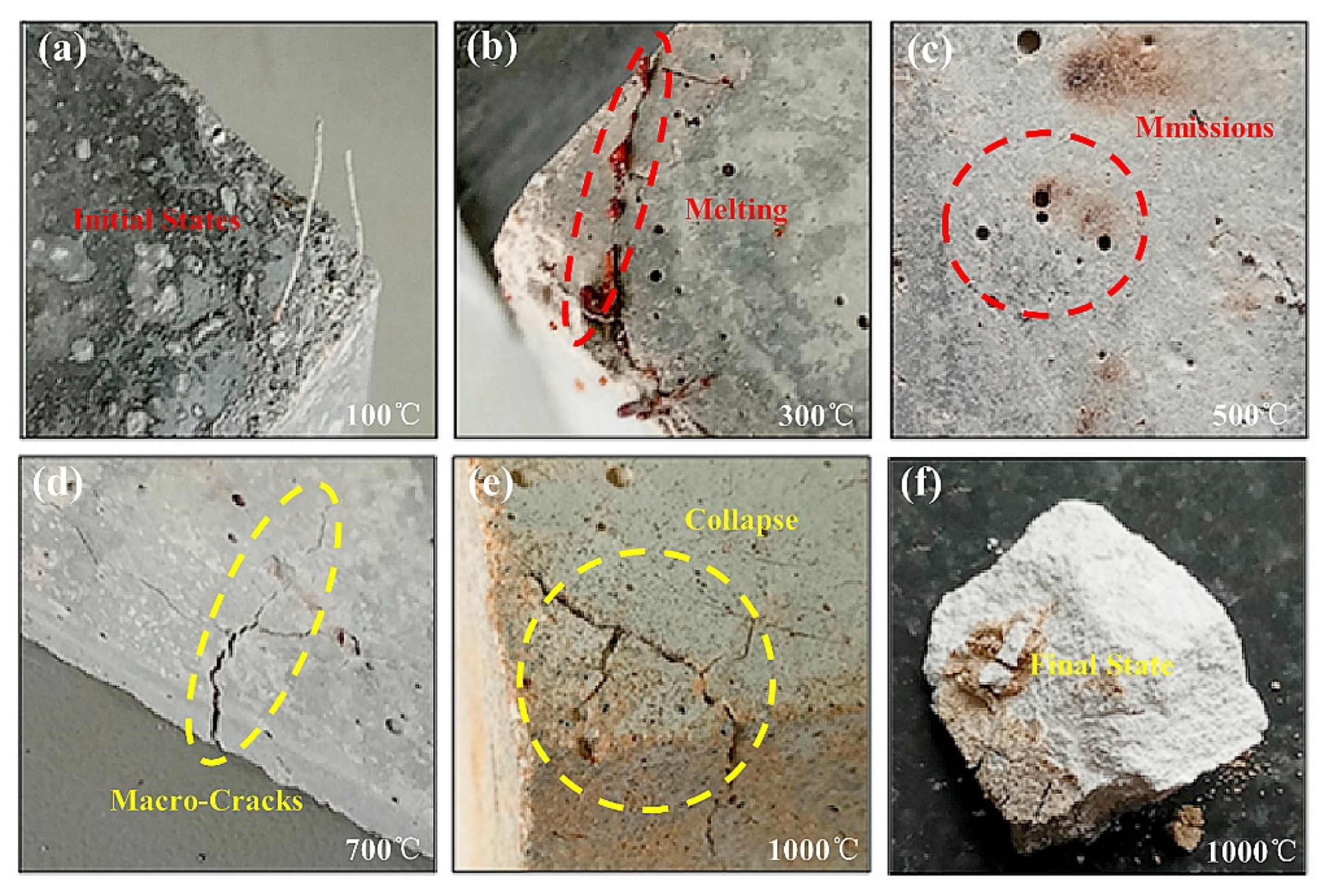
3.5. Shielding Concrete
4. Conclusions
- Rare-earth monomers and their oxides have large neutron-absorption cross section values, which can effectively avoid the formation of He bubbles in the radiation capture reaction, prolong the service life of neutron-absorbing materials, and increase the storage capacity of spent fuel. There is a great potential for the development of new-structure, functionally integrated, neutron-absorbing materials using rare-earth monomers or their oxides as fillers, and two or more fillers acting together in combination to obtain new properties.
- The radiation shielding performance is strong. It not only has the functions of slowing down and absorbing neutrons, but also has high shielding performance for the primary gamma rays released by spent fuel and the secondary gamma rays released by the interaction between target nucleus and neutron in shielding materials. It can shield neutrons and gamma rays at the same time. In the process of use, the three-dimensional size of the shielding material is stable and has good compatibility with other related material components. It is easy to replace and repair when it is necessary to replace or repair.
- Excellent mechanical properties, sufficient structural performance, and strength for extremely harsh environments. The utility model has the advantages of high thermal stability, corrosion resistance, light weight, simple production process, low cost, easy processing, environmentally friendly, and long service cycle. The release rate of fissile gas generated by spent fuel is high, it has strong resistance to radiation damage, and it has a certain self-healing function for irradiation damage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadi, A.; Hassanzadeh, M.; Gharib, M. Shielding calculation and criticality safety analysis of spent fuel transportation cask in research reactors. Appl. Radiat. Isot. 2016, 108, 129–132. [Google Scholar] [CrossRef] [PubMed]
- El-Samrah, M.G.; Tawfic, A.F.; Chidiac, S.E. Spent nuclear fuel interim dry storage; Design requirements, most common methods, and evolution: A review. Ann. Nucl. Energy 2021, 160, 108408–108421. [Google Scholar] [CrossRef]
- Sun, W.; Hu, G.; Yu, X.; Shi, J.; Xu, H.; Wu, R.; He, C.; Yi, Q.; Hu, H. Study on a High-Boron-Content Stainless Steel Composite for Nuclear Radiation. Materials 2021, 14, 7004. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Chung, S.; Hong, J. Performance evaluation of METAMIC neutron absorber in spent fuel storage rack. Nucl. Eng. Technol. 2018, 50, 788–793. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Tang, C.; He, Z.; Yuan, P.; Sun, Y.; Lau, W.; Zhang, K.; Mei, J.; Huang, Y. High temperature resistant polyimide/boron carbide composites for neutron radiation shielding. Compos. Part B Eng. 2019, 159, 355–361. [Google Scholar] [CrossRef]
- Kim, T.M.; Dho, H.S.; Cho, C.H.; Ko, J.H. Preliminary Shielding Analysis of the Concrete Cask for Spent Nuclear Fuel Under Dry Storage Conditions. J. Nucl. Fuel Cycle Waste Technol. 2017, 15, 391–402. [Google Scholar] [CrossRef]
- Piotrowski, T. Shielding concrete with neutron attenuating and absorbing components. In Micro and Nanostructured Composite Materials for Neutron Shielding Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 117–218. [Google Scholar]
- Tomoyuki, T.; Kaito, H.; Enrico, G.; Ajmi, A.; Iwa, O.; Takashi, S.; Pretam, K.D.; Mandeep, S.R.; Rohit, D.; Yusuke, K. Gamma-ray spectra from thermal neutron capture on gadolinium-155 and natural gadolinium. Prog. Theor. Exp. Phys. 2020, 2020, 43–45. [Google Scholar]
- Mellow, B.D.; Thomas, D.J.; Joyce, M.J.; Kolkowski, P.; Roberts, N.J.; Monk, S.D. The replacement of cadmium as a thermal neutron filter. Nucl. Inst. Methods Phys. Res. A 2007, 577, 690–695. [Google Scholar]
- Pandiyarajan, R.; Prabakaran, M.P.; Rajkumar, T.; Vetrivel Kumar, K.; Manikandan, R. Metallurgical and mechanical properties of SiC/ B4C reinforced with aluminum composites synthesized by mechanical alloying. Mater. Today Proc. 2021, 37, 1794–1798. [Google Scholar] [CrossRef]
- Güngör, A.; Akbay, I.K.; Özdemir, T. EPDM Rubber with hexagonal Boron Nitride: A Thermal Neutron Shielding Composite. Radiat. Phys. Chem. 2019, 165, 108391–108421. [Google Scholar] [CrossRef]
- Özdemir, T.; Akbay, I.K.; Uzun, H.; Reyhancan, I.A. Neutron shielding of EPDM rubber with boric acid: Mechanical, thermal properties and neutron absorption tests. Prog. Nucl. Energy 2016, 89, 102–109. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Wang, W.; Tan, X.; Xie, L.; Guo, F. The design, microstructure and mechanical properties of a novel Gd2O3/6061Al neutron shielding composite. Vacuum 2019, 162, 92–100. [Google Scholar] [CrossRef]
- Castley, D.; Goodwin, C.; Liu, J. Computational and experimental comparison of boron carbide, gadolinium oxide, samarium oxide, and graphene platelets as additives for a neutron shield. Radiat. Phys. Chem. 2019, 165, 108435–108443. [Google Scholar] [CrossRef]
- Cong, S.; Ran, G.; Li, Y.; Chen, Y. Ball-milling properties and sintering behavior of Al-based Gd2O3–W shielding materials used in spent-fuel storage. Powder Technol. 2020, 369, 127–136. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Wang, W.; Tan, X.; Xie, L.; Chen, X. Design, shielding mechanism and tensile property of a novel (B4C+6061Al)/Cf/6061Al laminar neutron-shielding composite. Vacuum 2020, 177, 109383–109390. [Google Scholar] [CrossRef]
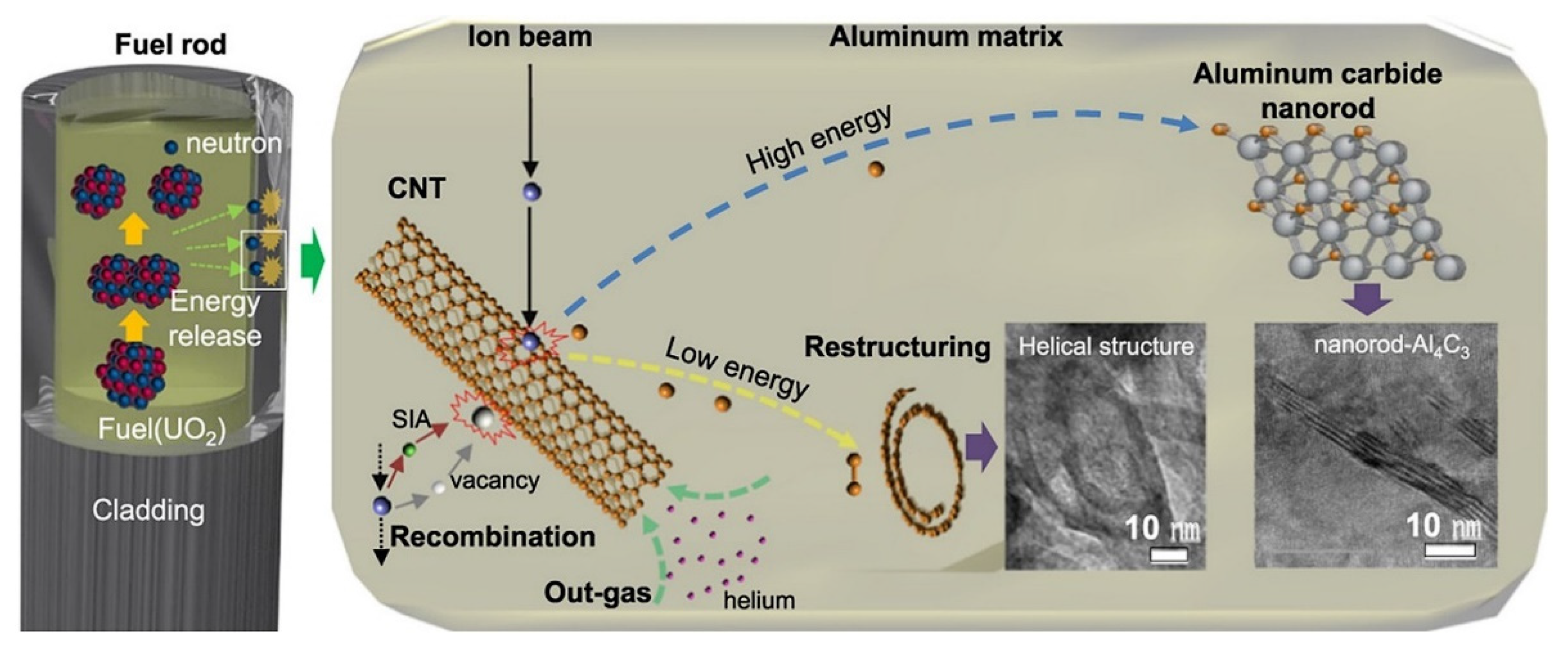
- So, K.P.; Chen, D.; Kushima, A.; Li, M.; Kim, S.; Yang, Y.; Wang, Z.; Park, J.G.; Lee, Y.H.; Gonzalez, R.I.; et al. Dispersion of carbon nanotubes in aluminum improves radiation resistance. Nano Energy 2016, 22, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Roslan, M.K.A.; Ismail, M.; Kueh, A.B.H.; Zin, M.R.M. High-density concrete: Exploring Ferro boron effects in neutron and gamma radiation shielding. Constr. Build. Mater. 2019, 215, 718–725. [Google Scholar] [CrossRef]
- Fu, X.; Ji, Z.; Lin, W.; Yu, Y.; Wu, T. The Advancement of Neutron Shielding Materials for the Storage of Spent Nuclear Fuel. Sci. Technol. Nucl. Install. 2021, 2021, 5541047. [Google Scholar] [CrossRef]
- Pei, Y.; Qu, X.; Ge, Q.; Wang, T. Evolution of Microstructure and Elements Distribution of Powder Metallurgy Borated Stainless Steel during Hot Isostatic Pressing. Metals 2022, 12, 19. [Google Scholar] [CrossRef]
- Yılmaz, D.; Aktaş, B.; Çalık, A.; Aytar, O.B. Boronizing effect on the radiation shielding properties of Hardox 450 and Hardox HiTuf steels. Radiat. Phys. Chem. 2019, 161, 55–59. [Google Scholar] [CrossRef]
- Gan, B.; Liu, S.; He, Z.; Chen, F.; Niu, H.; Cheng, J.; Tan, B.; Yu, B. Research Progress of Metal-Based Shielding Materials for Neutron and Gamma Rays. Acta Metall. Sin. 2021, 34, 1609–1617. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T. A comparative study of high boron alloys with 2.0 wt% B based on 304 and 316 stainless steels. Mater. Lett. 2021, 285, 129035–129045. [Google Scholar] [CrossRef]
- EPRI. Standard Specification for Borated Stainless Steel Plate, Sheet, and Strip for Nuclear Application; Revision 1: 2022 Update; EPRI: Palo Alto, CA, USA, 2022; p. 3002018496. [Google Scholar]
- Wang, Z.; Li, Y.; Wang, G.; Liu, H. Effects of boron content on the microstructure and mechanical properties of twin-roll strip casting borated steel sheets. Mater. Sci. Eng. A 2020, 793, 139847–139860. [Google Scholar] [CrossRef]
- Li, Y.W.; Liu, G.J.; Wang, Z.; Zhang, B.; Zhang, X.M.; Liu, H. Microstructure and Tensile Elongation Enhancement of 1.6 wt% B stainless Steel Plates Fabricated by Composite Rolling. Steel Res. Int. 2019, 90, 1800491–1800499. [Google Scholar] [CrossRef]
- Ren, X.; Tang, S.; Fu, H.; Xing, J. Effect of Titanium Modification on Microstructure and Impact Toughness of High-Boron Multi-Component Alloy. Metals 2021, 11, 193. [Google Scholar] [CrossRef]
- Xu, G.; Wang, K.; Dong, X.; Yang, L.; Jiang, H.; Wang, Q.; Ding, W. Effects of Titanium Addition on the Microstructural and Mechanical Property Evolution of FeCrB Alloys. Metall. Mater. Trans. A 2020, 51, 4610–4622. [Google Scholar] [CrossRef]
- Grebennikov, R.V.; Chirkin, A.V.; Pereverzeva, R.K.; Vukolova, V.N.; Demidov, P.I. Influence of vanadium on the phase composition and structure of high-boron steel. Sov. At. Energy 1966, 20, 174–175. [Google Scholar] [CrossRef]
- Lin, H.; Ying, L.; Li, J.; Li, B. Effects of hot rolling and titanium content on the microstructure and mechanical properties of high boron Fe–B alloys. Mater. Des. 2012, 36, 88–93. [Google Scholar]
- Gol Dshtein, Y.E.; Mizin, V.G. Some peculiarities of the structure of high boron steels. Met. Sci. Heat Treat. 1988, 30, 479–484. [Google Scholar] [CrossRef]
- Grebennikov, R.V.; Chirkin, A.V.; Vukolova, V.N.; Shavrova, T.N.; Balanik, I.S. Influence of titanium on the phase composition and deformability of high boron steels. Sov. At. Energy 1967, 22, 481–486. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, Y.W.; Zhang, W.N.; Wang, G.D.; Liu, H.T. Microstructural evolution and mechanical properties of titanium-alloying high borated steel sheets fabricated by twin-roll strip casting. Mater. Sci. Eng. A 2021, 811, 141067–141076. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wan, S.; Zhang, T. Design, fabrication and comprehensive properties of the novel thermal neutron shielding Gd/316L composites. Fusion Eng. Des. 2021, 171, 112566–112573. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Del Corso, G.J.; Klankowski, K.A.; Lherbier, L.W.; Novotnak, D.J. Review of the Development and Testing of a New Family of Boron and Gadolinium-bearing Dual Thermal Neutron Absorbing Alloys-13026. In Proceedings of the WM2013: Waste Management Conference: International Collaboration and Continuous Improvement, Phoenix, AZ, USA, 24–28 February 2013. [Google Scholar]
- Robino, C.V.; Michael, J.R.; DuPont, J.N.; Williams, D.B.; Mizia, R.E.; Shaber, E. Development of Gd-enriched alloys for spent nuclear fuel applications—Part 1: Preliminary characterization of small scale Gd-enriched stainless steels. J. Mater. Eng. Perform. 2003, 12, 206–214. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, J.; Moon, B.; Kim, D.; Oh, S.; Kim, Y.; Jung, H. Preliminary study on FeGd alloys as binary alloys and master alloys for potential spent nuclear fuel (SNF) application. Mater. Des. 2020, 194, 108906–108916. [Google Scholar] [CrossRef]
- Loria, E.A.; Isaacs, H.S. Type 304 stainless steel with 0.5% boron for storage of spent nuclear fuel. JOM 1980, 32, 10–17. [Google Scholar] [CrossRef]
- Changlong, S.; Min, J.; Daogui, F.; Xiaozhen, L.; Meiling, W.; Gang, L.; Huawei, H. Effect of hot rolling process on microstructure and properties of boron-containing stainless steel. Hot Work. Technol. 2014, 43, 52–54. [Google Scholar]
- Kim, M.J.; Kim, W.; Lee, D.; Lemaire, M.; Lee, H.; Sohn, D.; Kwon, H. Development of integral type spent fuel pool storage rack with gadolinium and europium-containing structure materials. Ann. Nucl. Energy 2019, 130, 107–117. [Google Scholar] [CrossRef]
- Abenojar, J.; Martinez, M.A.; Velasco, F. Effect of the boron content in the aluminium/boron composite. J. Alloy. Compd. 2006, 422, 67–72. [Google Scholar] [CrossRef]
- Lindquist, K. Handbook of Neutron Absorber Materials for Spent Nuclear Fuel Transportation and Storage Applications; Technical Report; Electric Power Research Institute: Palo Alto, CA, USA, 2022. [Google Scholar]
- Huang, M.; Fan, G.H.; Geng, L.; Cao, G.J.; Du, Y.; Wu, H.; Zhang, T.T.; Kang, H.J.; Wang, T.M.; Du, G.H.; et al. Revealing extraordinary tensile plasticity in layered Ti-Al metal composite. Sci. Rep. 2016, 6, 38461–38470. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Yong-hun, G.; Feng, X.; Hong-bo, D.; Pei-hu, G.; Jian-ping, L. Effect of laser remelting treatment on microstructure and tensile property of piston aluminum alloy. Trans. Mater. Heat Treat. 2015, 36, 49–55. [Google Scholar]
- 3M™ Advanced Metal Matrix Composite. Available online: https://multimedia.3m.com/mws/media/1324320O/3m-advanced-metal-matrix-composite.pdf (accessed on 20 April 2022).
- Boralcan. Available online: https://www.nrc.gov/docs/ML1308/ML13080A005.pdf (accessed on 22 April 2022).
- Ramanathan, A.; Krishnan, P.K.; Muraliraja, R. A review on the production of metal matrix composites through stir casting—Furnace design, properties, challenges, and research opportunities. J. Manuf. Process. 2019, 42, 213–245. [Google Scholar] [CrossRef]
- Novich, K.A.; Pedersen, S.V.; Borrelli, R.A.; Christensen, R.; Jaques, B.J. Synthesis of boron carbide reinforced aluminum castings through mechanical stir casting. J. Compos. Mater. 2021, 55, 2165–2177. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Bhandari, R.; Aherwar, A.; Pinca-Bretotean, C. A study of fabrication methods of aluminum based composites focused on stir casting process. Mater. Today Proc. 2020, 27, 1608–1612. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Xu, G.; Ying, F.; Lu, Y.; Wang, T.; Guo, Q. Microstructure and Performance of a Three-Layered Al/7075–B4C/Al Composite Prepared by Semi Continuous Casting and Hot Rolling. Metals 2018, 8, 600. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Lee, D.; Kim, J.; Park, B.; Jo, S.-K.; Kim, Y.; Lee, S.-B. Mechanical and Thermal Neutron Absorbing Properties of B4C/Aluminum Alloy Composites Fabricated by Stir Casting and Hot Rolling Process. Metals 2021, 11, 413–424. [Google Scholar]
- Lee, D.; Kim, J.; Lee, S.; Kim, Y.; Lee, S.; Cho, S. Experimental and thermodynamic study on interfacial reaction of B4C–Al6061 composites fabricated by stir casting process. J. Alloy. Compd. 2021, 859, 157813–157822. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q. Effect of Magnetic-Mechanical Coupled Stirring on the Distribution of B4C Particles in Al-B4C Composites. J. Mater. Eng. Perform. 2021, 31, 907–917. [Google Scholar] [CrossRef]
- Gudipudi, S.; Nagamuthu, S.; Subbian, K.S.; Chilakalapalli, S.P.R. Enhanced mechanical properties of AA6061-B4C composites developed by a novel ultra-sonic assisted stir casting. Eng. Sci. Technol. Int. J. 2020, 23, 1233–1243. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, W.X.; Nie, H.H.; Zhou, J.; Li, Y.L.; Liu, R.F.; Zhang, Y.Y.; Zhang, P. Microstructure evolution and mechanical properties of B4C/6061Al neutron absorber composite sheets fabricated by powder metallurgy. J. Alloy. Compd. 2018, 730, 342–351. [Google Scholar] [CrossRef]
- Hongsheng, C.; Wenxian, W.; Huihui, N.; Yuli, L.; Ruifeng, L.; Run’Ai, L.; Tao, Y. Research Progress and Development of Neutron-Absorbing Materials for Nuclear Shielding. Rare Met. Mater. Eng. 2020, 49, 4358–4364. [Google Scholar]
- Li, Y.; Wang, W.; Zhou, J.; Chen, H.; Zhang, P. 10B areal density: A novel approach for design and fabrication of B4C/6061Al neutron absorbing materials. J. Nucl. Mater. 2017, 487, 238–246. [Google Scholar] [CrossRef]
- Park, J.; Hong, S.; Lee, M.; Rhee, C.; Rhee, W. Enhancement in the microstructure and neutron shielding efficiency of sandwich type of 6061Al–B4C composite material via hot isostatic pressing. Nucl. Eng. Des. 2015, 282, 1–7. [Google Scholar] [CrossRef]
- Jiang, L.T.; Xu, Z.G.; Fei, Y.K.; Zhang, Q.; Qiao, J.; Wu, G.H. The design of novel neutron shielding (Gd+B4C)/6061Al composites and its properties after hot rolling. Compos. Part B Eng. 2019, 168, 183–194. [Google Scholar] [CrossRef]
- Tamayo, P.; Thomas, C.; Rico, J.; Cimentada, A.; Polanco, J.A. Review on neutron-absorbing fillers. In Micro and Nanostructured Composite Materials for Neutron Shielding Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 25–52. [Google Scholar]
- Viala, J.C.; Bouix, J.; Gonzalez, G.; Esnouf, C. Chemical reactivity of aluminium with boron carbide. J. Mater. Sci. 1997, 32, 4559–4573. [Google Scholar] [CrossRef]
- Peng, K.; Heli, M.A.; Chen, R.; Wenyuan, W.U. Study on Phase and Microstructure of B4C/Al Composite. Hot Work. Technol. 2010, 39, 96–98. [Google Scholar]
- Dongshan, W.; Xiangxin, X. Preparation of A1B12powder by powder metallugy method. Powder Metall. Technol. 2008, 26, 298–303. [Google Scholar]
- Zhang, L.; Shi, G.; Xu, K.; Hao, W.; Li, Q.; Junyan, W.; Wang, Z. Phase transformation and mechanical properties of B4C/Al composites. J. Mater. Res. Technol. 2020, 9, 2116–2126. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, Y.; Wu, Y.; Li, D. Neutron Shielding Performance of 3D-Printed Boron Carbide PEEK Composites. Materials 2020, 13, 2314. [Google Scholar] [CrossRef]
- Cramer, C.L.; Elliott, A.M.; Kiggans, J.O.; Haberl, B.; Anderson, D.C. Processing of complex-shaped collimators made via binder jet additive manufacturing of B4C and pressureless melt infiltration of Al. Mater. Des. 2019, 180, 107956. [Google Scholar] [CrossRef]
- Yang, L.; Shen, P.; Guo, R.; Jiang, Q. The role of TiO2 incorporation in the preparation of B4C/Al laminated composites with high strength and toughness. Ceram. Int. 2018, 44, 15219–15227. [Google Scholar] [CrossRef]
- Hei, D.; Chen, R.; Liu, F.; Lao, D.; Jia, W. A novel design of neutron shielding composite materials with three-dimensionally interwoven structure and excellent properties. J. Alloy. Compd. 2020, 845, 156328–156337. [Google Scholar] [CrossRef]
- Manu, K.S.; Raag, L.A.; Rajan, T.; Gupta, M.; Pai, B.C. Liquid Metal Infiltration Processing of Metallic Composites: A Critical Review. Metall. Mater. Trans. B 2016, 47, 1–21. [Google Scholar]
- Lin, Q.; Shen, P.; Qiu, F.; Zhang, D.; Jiang, Q. Wetting of polycrystalline B4C by molten Al at 1173–1473K. Scr. Mater. 2009, 60, 960–963. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, W.X.; Nie, H.H.; Zhou, J.; Li, Y.L.; Zhang, P. Microstructure and mechanical properties of B4C/6061Al laminar composites fabricated by power metallurgy. Vacuum 2017, 143, 363–370. [Google Scholar] [CrossRef]
- Chen, H.; Nie, H.; Wang, W.; Zhou, J.; Liu, R. A novel neutron shielding AA6061/B4C laminar composite fabricated by powder metallurgy: “SPS-HER”. J. Alloy. Compd. 2019, 806, 1445–1452. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, C.; Li, J.; Wang, W. Shielding composites for neutron and gamma-radiation with Gd2O3@W core-shell structured particles. Mater. Lett. 2020, 276, 128082–128091. [Google Scholar] [CrossRef]
- Cong, S.; Li, Y.; Ran, G.; Zhou, W.; Dong, S.; Feng, Q. High-temperature corrosion behavior of hot-pressed Al-based Gd2O3-W shielding materials used in spent fuel storage. Corros. Sci. 2021, 179, 109166–109178. [Google Scholar] [CrossRef]
- Pomaro, B.; Pandolfi, A. A Review on Radiation Damage in Concrete for Nuclear Facilities: From Experiments to Modeling. Model. Simul. Eng. 2016, 8, 4165746–4165756. [Google Scholar] [CrossRef]
- Huo, Z.; Zhao, S.; Zhong, G.; Zhang, H.; Hu, L. Surface modified-gadolinium/boron/polyethylene composite with high shielding performance for neutron and gamma-ray. Nucl. Mater. Energy 2021, 29, 101095–101107. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Taki, M.M.; Abdalsalam, A.H.; Mhareb, M.H.A.; Alajerami, Y.S.; Şakar, E.; Aygün, B.; KaKy, K.M. Fabrication, characterization of neutron and proton shielding investigation of tungsten oxide dispersed-ultra high Mw polyethylene. Chem. Phys. 2021, 548, 111227–111234. [Google Scholar] [CrossRef]
- Wang, P.; Tang, X.; Chai, H.; Chen, D.; Qiu, Y. Design, fabrication, and properties of a continuous carbon-fiber reinforced Sm2O3/polyimide gamma ray/neutron shielding material. Fusion Eng. Des. 2015, 101, 218–225. [Google Scholar] [CrossRef]
- Uddin, Z.; Yasin, T.; Shafiq, M. Development of novel silane modified boric acid/ high density polyethylene composites for radiation shielding applications. Radiat. Phys. Chem. 2022, 192, 109909–109919. [Google Scholar] [CrossRef]
- Ji, W.S.; Lee, J.W.; Yu, S.; Baek, B.K.; Chong, M.K. Polyethylene/boron-containing composites for radiation shielding. Thermochim. Acta 2014, 585, 5–9. [Google Scholar]
- Avcıoğlu, S.; Buldu, M.; Kaya, F.; Üstündağ, C.B.; Kam, E.; Menceloğlu, Y.Z.; Kaptan, H.Y.; Kaya, C. Processing and properties of boron carbide (B4C) reinforced LDPE composites for radiation shielding. Ceram. Int. 2020, 46, 343–352. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, W.; Lu, L.; Jiao, X.; Zhou, C. A “Sandwich” type of neutron shielding composite filled with boron carbide reinforced by carbon fiber. Chem. Eng. J. 2013, 220, 143–150. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, G.; Su, F.; Feng, Y.; Ji, Y.; Liu, D.; Yin, R.; Liu, C.; Shen, C. Multilayer polyethylene/ hexagonal boron nitride composites showing high neutron shielding efficiency and thermal conductivity. Compos. Commun. 2020, 19, 147–153. [Google Scholar] [CrossRef]
- Fu, X.; Hu, Y.; Li, H.; Tao, J. Study on the thermal and mechanical properties of novel neutron shielding composite laminates at elevated temperature. Mater. Sci. Eng. A 2017, 697, 101–110. [Google Scholar] [CrossRef]
- Tamayo, P.; Thomas, C.; Rico, J.; Cimentada, A.; Polanco, J.A. Thermoplastic micro- and nanocomposites for neutron shielding. In Micro and Nanostructured Composite Materials for Neutron Shielding Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 53–82. [Google Scholar]
- Bhatia, S.; Angra, S.; Khan, S. A review on mechanical and tribological characterization of boron carbide reinforced epoxy composite. Adv. Compos. Mater. 2020, 30, 307–337. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Wu, Z.; Shen, H.; Weng, H.; Chen, H.; Huang, W.; Wang, M.; Ge, X.; Lin, M. Effect of gamma and neutron irradiation on properties of boron nitride/epoxy resin composites. Polym. Degrad. Stab. 2021, 190, 109643–109667. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, J.K.; Kim, J.W.; Lee, G.J. Properties of B4C–PbO–Al(OH)3-epoxy nanocomposite prepared by ultrasonic dispersion approach for high temperature neutron shields. J. Nucl. Mater. 2014, 445, 63–71. [Google Scholar] [CrossRef]
- Abenojar, J.; Martínez, M.A.; Velasco, F.; Pascual-Sánchez, V.; Martín-Martínez, J.M. Effect of Boron Carbide Filler on the Curing and Mechanical Properties of an Epoxy Resin. J. Adhes. 2009, 85, 216–238. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, J.; Mao, X.; Song, L.; Yang, X.; Liu, S. Toughening mechanisms of epoxy resin using aminated metal-organic framework as additive. Mater. Lett. 2019, 240, 113–116. [Google Scholar] [CrossRef]
- Hu, C.; Zhai, Y.T.; Song, L.L.; Mao, X.D. Structure-thermal activity relationship in a novel polymer/MOF-based neutron-shielding material. Polym. Compos. 2020, 41, 1–10. [Google Scholar] [CrossRef]
- Hu, G.; Shi, G.; Hu, H.; Yang, Q.; Yu, B.; Sun, W. Development of gradient composite shielding material for shielding neutrons and gamma rays. Nucl. Eng. Technol. 2020, 52, 2387–2393. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Q.; Zhai, Y. Thermal, mechanical investigation and neutron shielding analysis for Gd-MOF/polyimide materials. RSC Adv. 2021, 11, 40148–40158. [Google Scholar] [CrossRef]
- Weng, G.; Huang, G.; Qu, L.; Zhang, P.; Nie, Y.; Wu, J. Natural rubber with low heat generation achieved by the inclusion of boron carbide. J. Appl. Polym. Sci. 2010, 118, 2050–2055. [Google Scholar] [CrossRef]
- Barala, S.S.; Manda, V.; Jodha, A.S.; Meghwal, L.R.; Ajay, C.; Gopalani, D. Ethylene, ropylene diene monomer-based polymer composite for attenuation of high energy radiations. J. Appl. Polym. Sci. 2020, 138, 50334–50342. [Google Scholar] [CrossRef]
- Salimi, M.; Ghal-Eh, N.; Amirabadi, E.A. Characterization of a new shielding rubber for use in neutron–gamma mixed fields. Nucl. Sci. Technol. 2018, 29, 36–43. [Google Scholar] [CrossRef]
- Prochon, P.; Piotrowski, T. The effect of cement and aggregate type and w/c ratio on the bound water content and neutron shielding efficiency of concretes. Constr. Build. Mater. 2020, 264, 120210–120218. [Google Scholar] [CrossRef]
- Chidiac, S.E.; El-Samrah, M.G.; Reda, M.A.; Abdel-Rahman, M.A.E. Mechanical and radiation shielding properties of concrete containing commercial boron carbide powder. Constr. Build. Mater. 2021, 313, 125466–125476. [Google Scholar] [CrossRef]
- Zalegowski, K.; Piotrowski, T.; Garbacz, A.; Adamczewski, G. Relation between microstructure, technical properties and neutron radiation shielding efficiency of concrete. Constr. Build. Mater. 2020, 138, 117389–117408. [Google Scholar] [CrossRef]
- Mesbahi, A.; Ghiasi, H. Shielding properties of the ordinary concrete loaded with micro- and nano-particles against neutron and gamma radiations. Appl. Radiat. Isot. 2018, 136, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Uher, C. The thermal conductivity of fibre-reinforced concrete. Cem. Concr. Res. 1974, 4, 497–509. [Google Scholar] [CrossRef]
- Thomas, C.; Rico, J.; Tamayo, P.; Setién, J.; Ballester, F.; Polanco, J.A. Neutron shielding concrete incorporating B4C and PVA fibers exposed to high temperatures. J. Build. Eng. 2019, 26, 100859–100869. [Google Scholar] [CrossRef]








| Z | Symbol | Name | A (u) | σcs (barn) | σis (barn) | σs (barn) | σa (barn) |
|---|---|---|---|---|---|---|---|
| 64 | Gd | Gadolinium | 157.3 | 29.388 | 151.222 | 180.22 | 49,700 |
| 62 | Sm | Samarium | 150.4 | 0.422 | 39.333 | 39.33 | 5922 |
| 63 | Eu | Europium | 152.0 | 6.754 | 2.544 | 9.24 | 4530 |
| 48 | Cd | Cadmium | 112.4 | 3.046 | 3.461 | 6.50 | 2520 |
| 66 | Dy | Dysprosium | 162.5 | 35.988 | 54.412 | 90.39 | 994 |
| 5 | B | Boron | 10.80 | 3.545 | 1.701 | 5.24 | 767 |
| 74 | W | Tungsten | 183.85 | 2.97 | 1.63 | 4.6 | 18.3 |
| 28 | Ni | Nickel | 58.69 | 13.3 | 5.2 | 18.50 | 4.49 |
| 24 | Cr | Chromium | 51.996 | 1.66 | 1.83 | 3.49 | 3.05 |
| 26 | Fe | Iron | 55.8 | 11.225 | 0.401 | 11.62 | 2.5633 |
| 13 | Al | Aluminum | 27.0 | 1.495 | 0.008 | 1.50 | 0.2313 |
| Compound | Neutron Absorption | Behavior under High Temperatures | Chemical Resistance | Mechanical Strength | Usage | Ref. |
|---|---|---|---|---|---|---|
| B4C | High | Excellent | Excellent | Excellent | Low density; very expensive; can be added to any matrix; low elastic modulus and tensile strength | [10] |
| h-BN | Medium–high | Excellent | Excellent | Poor | Low density; very expensive; an ideal additive for high energy neutron decay polyethylene and polyimide | [11] |
| H3BO3 | Medium | Very poor | Good | Poor | Lowest density; cheap; usually used in combination with polymer matrix composites | [12] |
| Gd/Gd2O3 | Highest | Excellent | Very good | Good | High density; commonly used in metal-based shielding materials | [13] |
| Sm2O3 | Very high | Excellent | Very good | Good | High density; medium; commonly used in polymer groups | [14] |
| W/WO3 | Very low | Excellent | Very good | Good | Very high density; expensive; auxiliary absorption of gamma rays | [15] |
| Carbon Fiber | Very low | Excellent | Excellent | Excellent | Very low density; very expensive; excellent nuclear reflective material | [16] |
| Carbon Nanotubes | Very low | Excellent | Excellent | Excellent | Very low density; very expensive; commonly used in metal-based shielding materials | [17] |
| Fe–B | Medium | Medium | Good | Good | Added to concrete as a promising shield material for the fast neutron application | [18] |
| Element Content (wt%) | B | Cu | Si | Fe | Mg | Mn | Zn | Ti | Others |
|---|---|---|---|---|---|---|---|---|---|
| AA1100 | 1.25–4.5 | 0.05–0.20 | 1.0 (Si + Fe) | - | 0.05 | 0.01 | - | 0.10 total | |
| AA6351 | 2.0 | 0.10 | 0.7–1.3 | 0.50 | 0.4–0.8 | 0.4–0.8 | 0.02 | 2.4 × B | 0.15 total |
| Temperature (°C) | Elongation (%) | Yield Strength (MPa) | Tensile Strength (MPa) | ||||||
| 25 | 10 | 276 | 310 | ||||||
| 200 | 12 | 193 | 214 | ||||||
| Phase | Microhardness (kg/mm3) | Density (g/mm3) | Formation Temperature (°C) |
|---|---|---|---|
| A1 | 19 | 2.70 | - |
| B4C | 2750~4950 | 2.52 | - |
| Al3BC | 1400 | - | 450 |
| AlB2 | 980 | 3.16 | 600 |
| AlB24C4 | 2530~2650 | 2.54 | 1000 |
| Al4C3 | 1250 | 2.93 | 1000+ |
| Material | Attenuation Coefficient Σ (cm−1) | Density (g/cm3) | Coefficient of Thermal Expansion (10−5/°C) | Melting Point (°C) | Tensile Yield Strength (MPa) | Tg (°C) |
|---|---|---|---|---|---|---|
| HDPE | 0.145 | 0.96 | 15.3 | 130 | 26.3 | −110 |
| LDPE | 0.330 | 0.91 | 2.0 | 114 | 11.5 | −110 |
| Shielding Materials | Decomposition Temperature (°C) | Tensile Strength (MPa) | Neutron Permeability/% (Thickness/cm) | Ref. |
|---|---|---|---|---|
| Cf reinforced 21 wt% Sm2O3/PI | 300 | 200 | 50% (1 cm) | [78] |
| (3 wt% h-BN + 3 wt% Gd2O3)/PI | - | 78 | 70% (1 cm) | [74] |
| 3 wt% Gd–MOF/PI | 568 | 75 | 14% (1 cm) | [93] |
| 30 wt% B4C/PI (films) | 622 | 406 | 24% (0.08 cm) | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Yang, Z.; Li, J.; Guo, Y.; Yang, G.; Yu, Y.; Zhang, J. The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel. Materials 2022, 15, 3255. https://doi.org/10.3390/ma15093255
Qi Z, Yang Z, Li J, Guo Y, Yang G, Yu Y, Zhang J. The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel. Materials. 2022; 15(9):3255. https://doi.org/10.3390/ma15093255
Chicago/Turabian StyleQi, Zhengdong, Zhong Yang, Jianping Li, Yongchun Guo, Guichun Yang, Yang Yu, and Jiachen Zhang. 2022. "The Advancement of Neutron-Shielding Materials for the Transportation and Storage of Spent Nuclear Fuel" Materials 15, no. 9: 3255. https://doi.org/10.3390/ma15093255






