Morphological Evolution of TiB2 and TiAl3 in Al–Ti–B Master Alloy Using Different Ti Adding Routes
Abstract
:1. Introduction
2. Experimental
3. Results
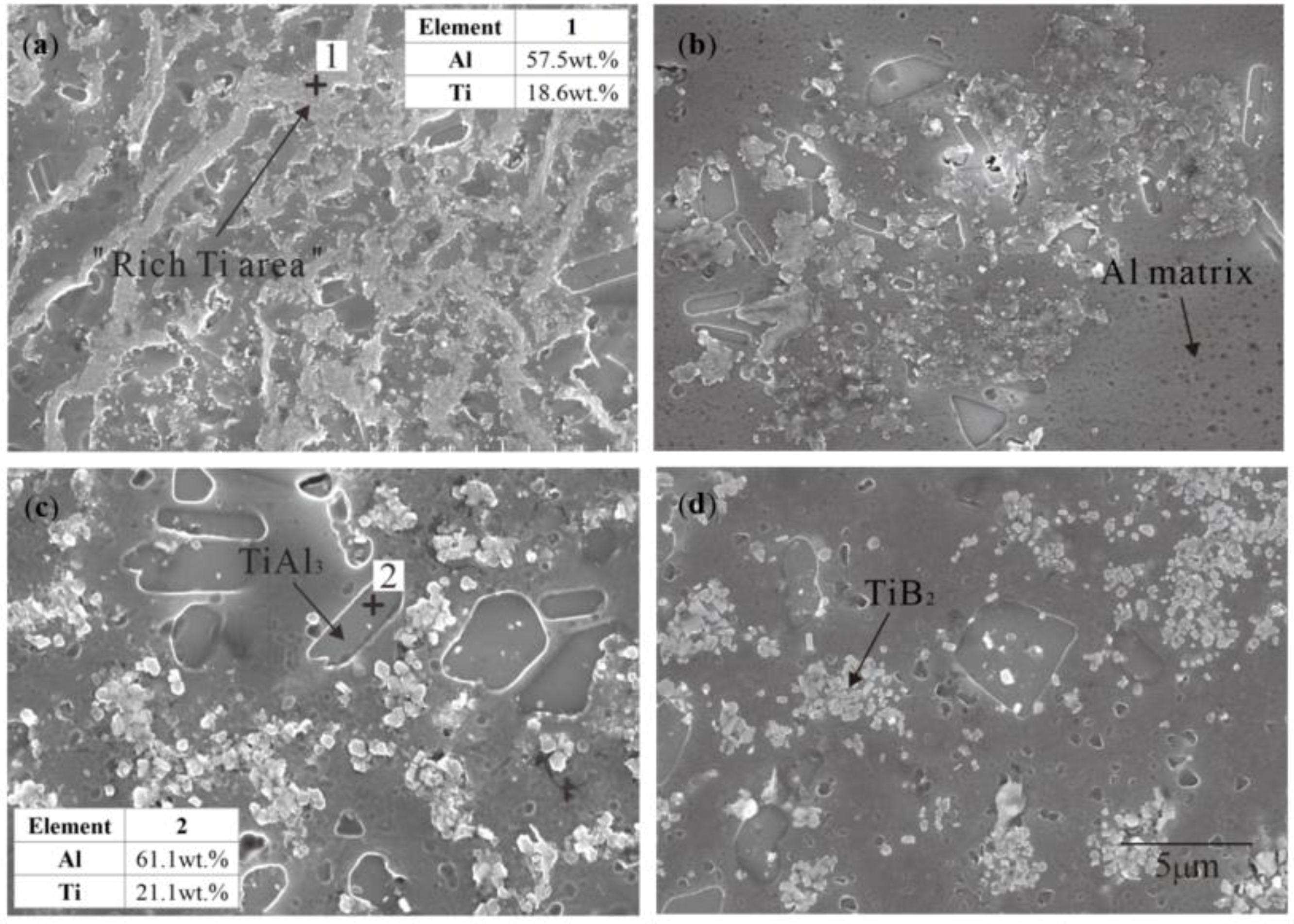
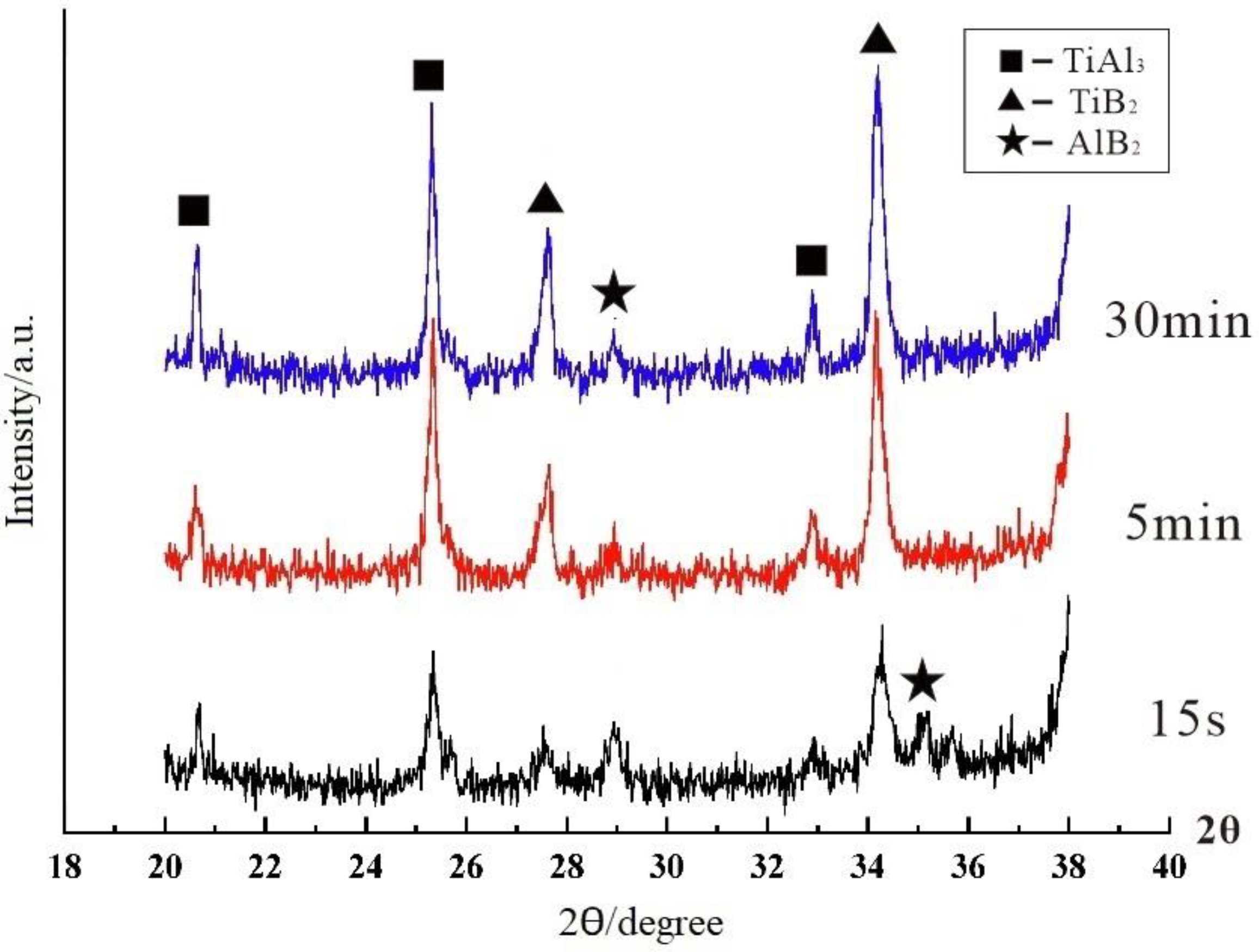
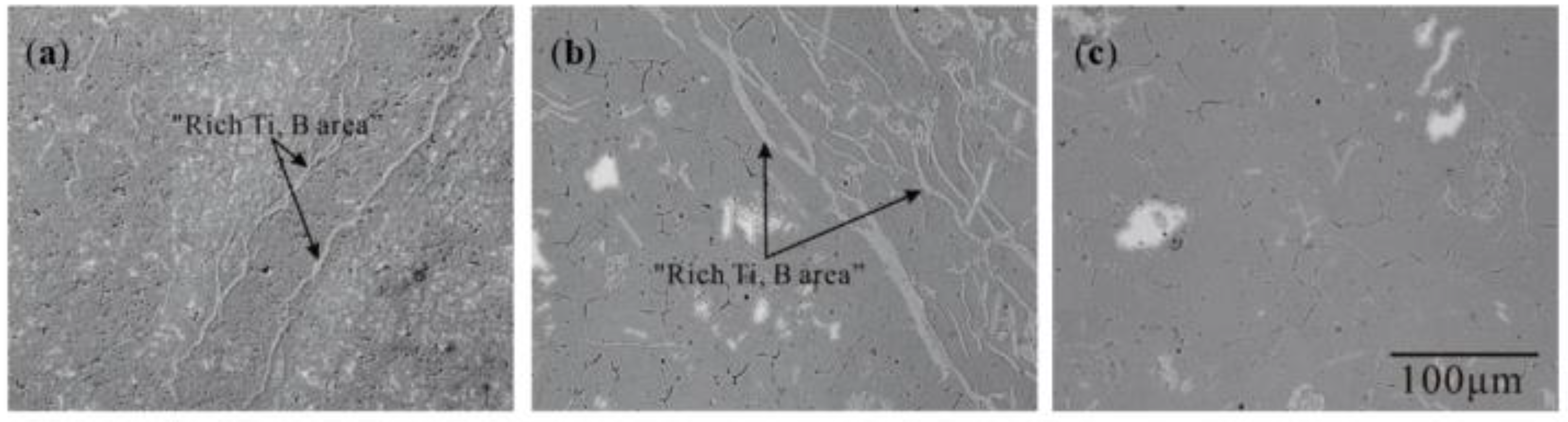
3.1. “Rich Ti, B Area” and Morphology of TiAl3, TiB2 in Halide Salt Route
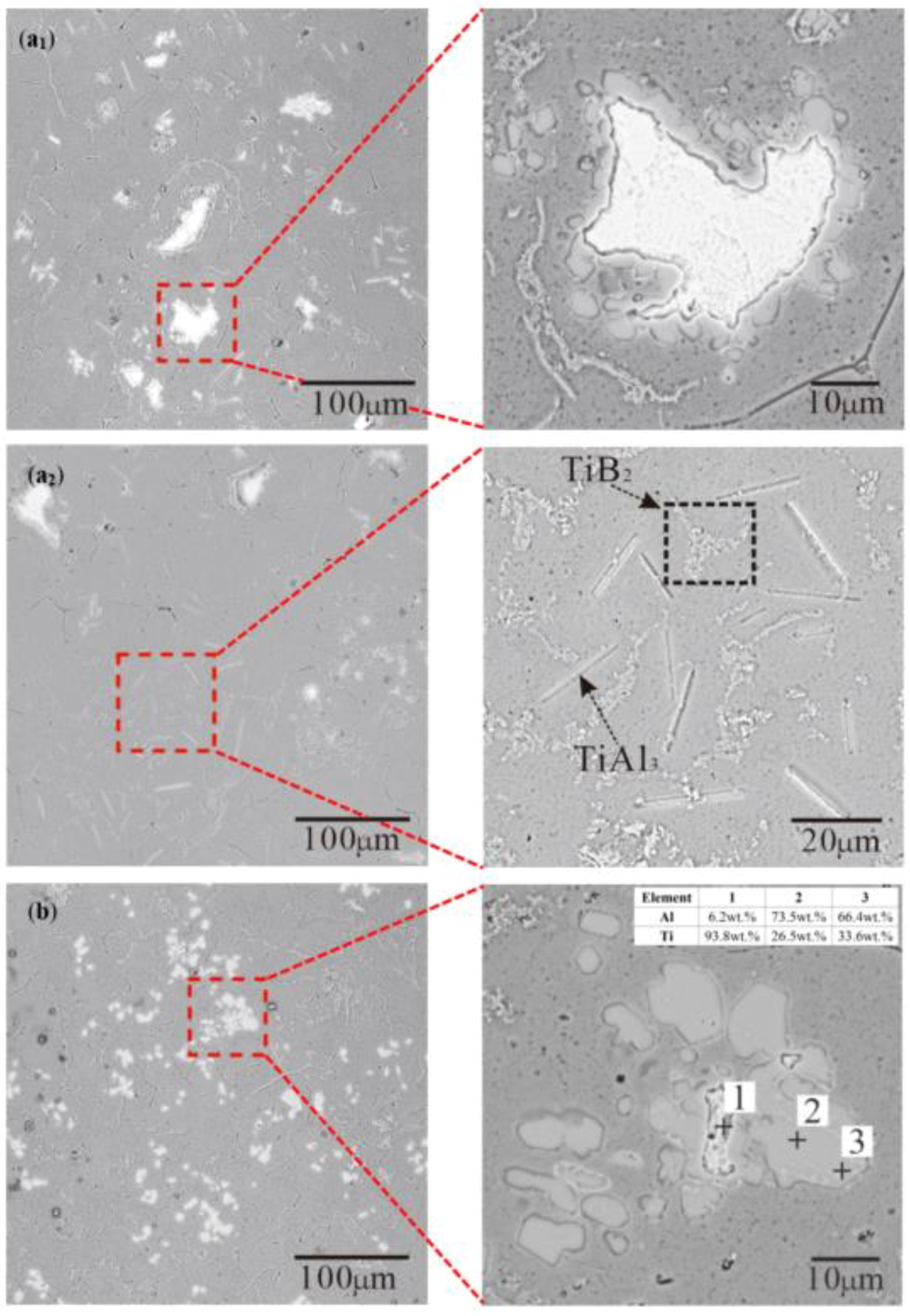
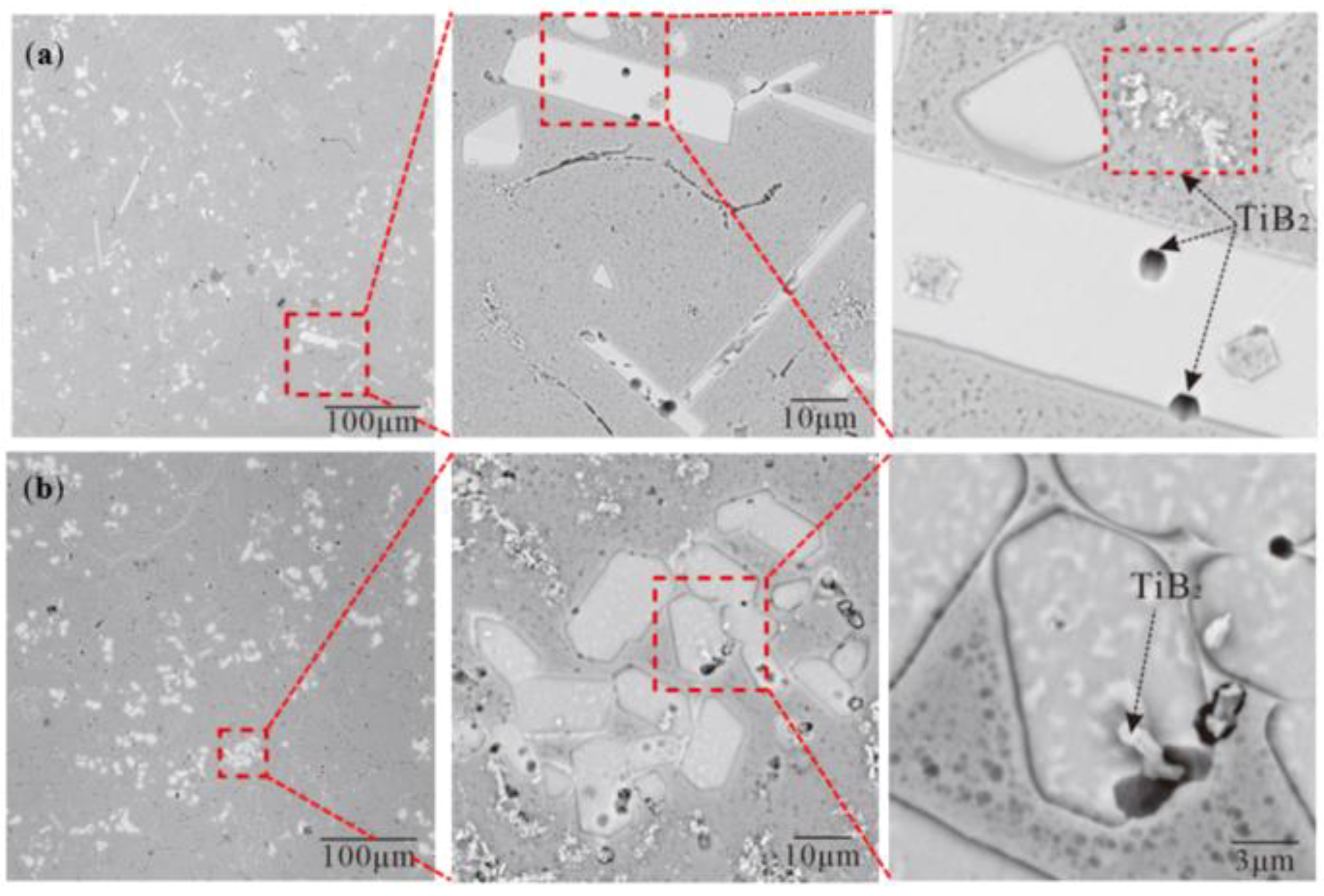
3.2. Morphology of TiAl3, TiB2 in the Ti-Sponge and Partial Ti-Sponge Route
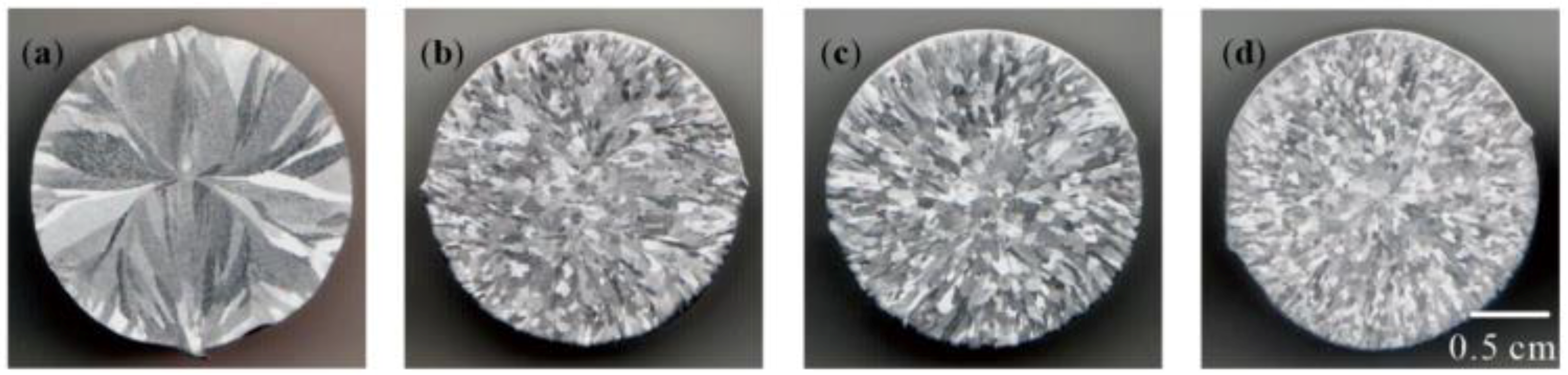
3.3. Refining Effect of Al–Ti–B Master Alloy
4. Discussion
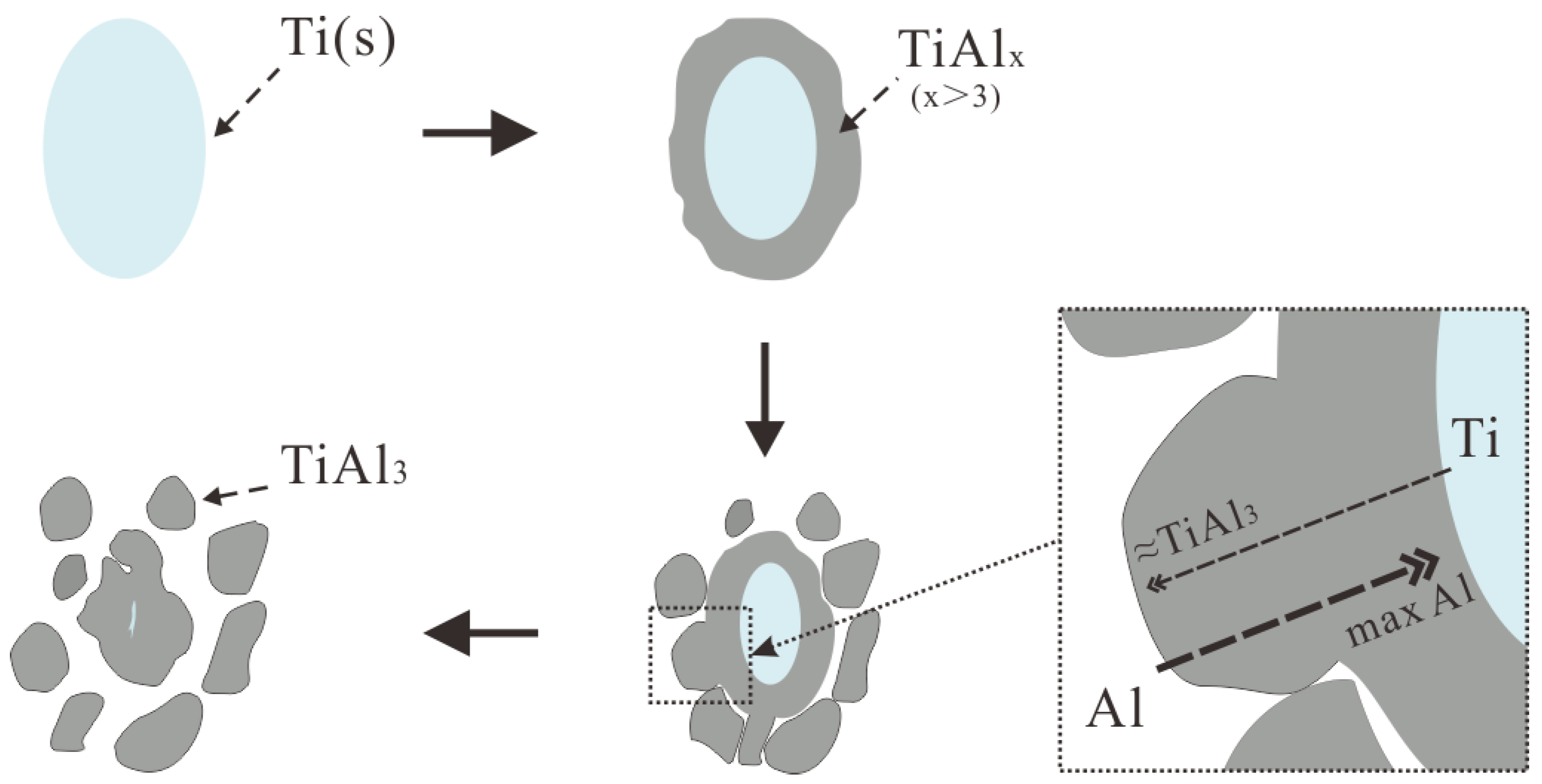
4.1. Nucleation and Growth of TiAl3 and TiB2 in Different Ti Adding Routes—The Ti–TiAlx Mechanism
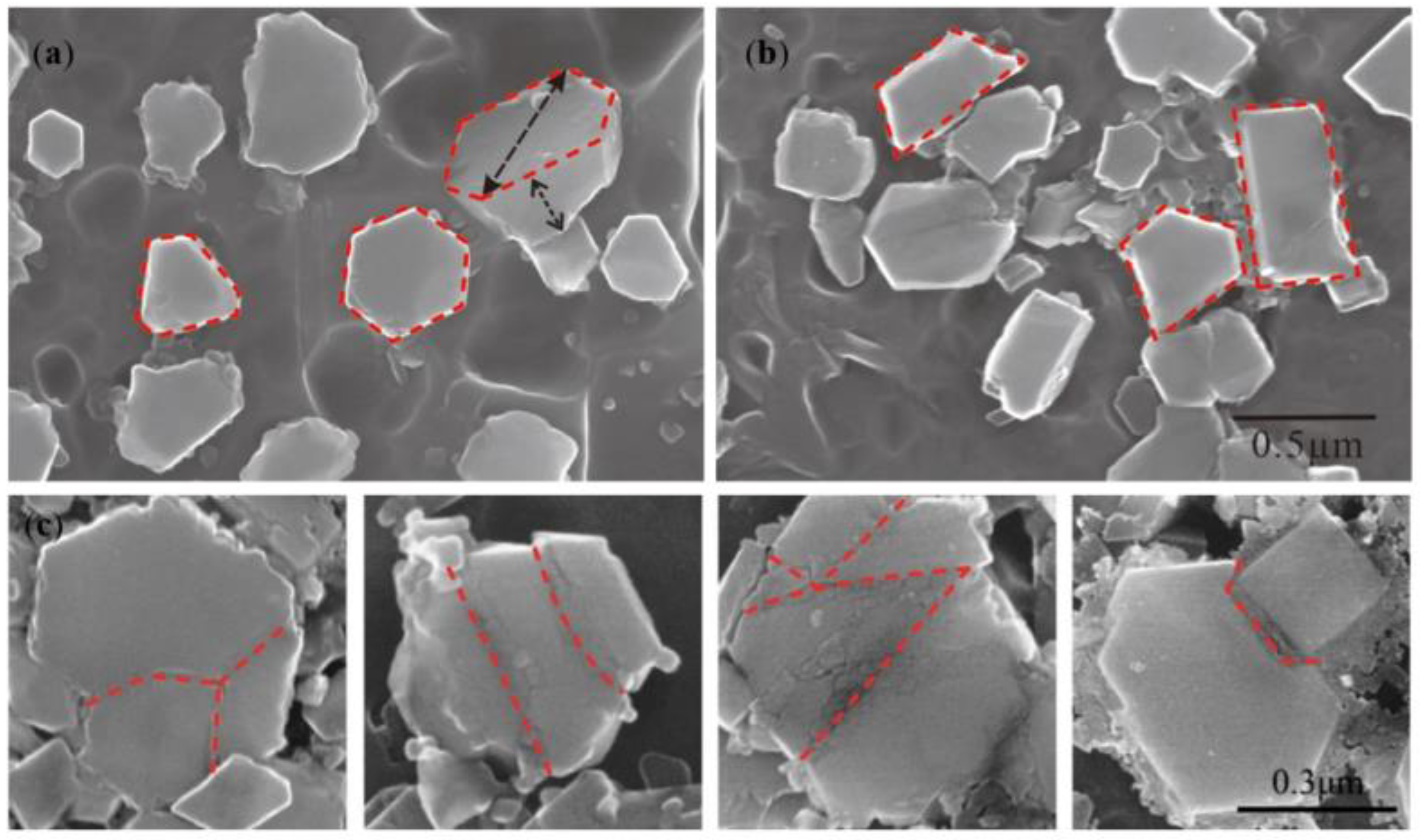
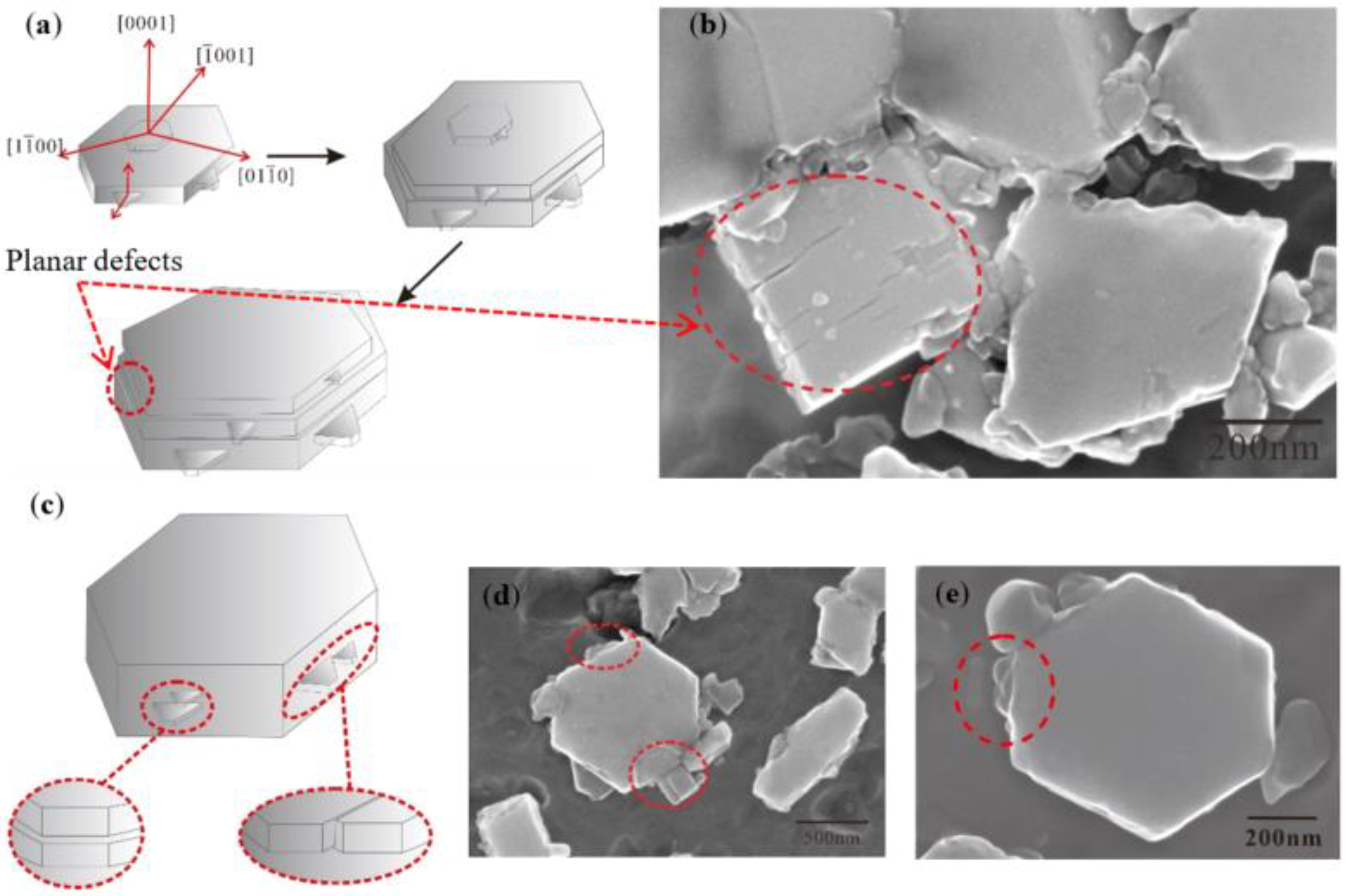
4.2. Evolution Mechanism of TiB2 with Irregular Polygon
5. Conclusions
- (1)
- The fluorine salt reaction occurred very fast when Al–Ti–B was prepared by the halide salt route (<10 S). A streamlined “rich Ti, B area” exists in the aluminum melt in the initial stage of the reaction ((800 °C, <30 S). This is a complex compound of (Tix, Al1−x) By. The “rich Ti, B area” is essential for the nucleation and growth of TiAl3 and TiB2.
- (2)
- The Ti addition route greatly influences the morphology of TiAl3. The formation of TiAl3 using the Ti-sponge route is based on the Ti–TiAlx mechanism. The nucleation of TiAl3 mainly depends on the mutual diffusion of Al and Ti powder, and TiAlx formed around the Ti particles. The TiAl3 formed based on the Ti–TiAlx mechanism is mainly block-shaped and the average size is 9.83 μm. In the halide salt route, TiAl3 formation is based on the reaction of Ti powder and KBF4 to form a rod-shaped TiAl3. The long, rod-shaped TiAl3 disappeared due to excess B and a lack of Ti in the aluminum melt. Finally, small blocks of TiAl3 formed with an average size of 4.7 μm.
- (3)
- TiB2 particles showed different morphologies at different reaction times when the Master Alloy was prepared by the halide salt route. The TiB2 was an irregular and small particle at the initial stage of the reaction (10 S and 15 S). Regular hexagonal TiB2 particles appeared as the reaction time increased to 20 S and 30 S. The size of TiB2 was about 0.5 μm. The size of TiB2 particle continued to increase at 60 S. Some TiB2 collided together at 5 min. Both the crystal defects and the crowded growth environment caused by the “rich Ti, B area” are the fundamental reasons for the fragility and irregular shape of the TiB2.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.H.; Hage, F.S.; Ramasse, Q.M.; Schumacher, P. The nucleation sequence of α-Al on TiB2 particles in Al-Cu alloys. Acta Mater. 2021, 206, 116652. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F.; Jiang, B.; Que, Z. Impeding Nucleation for More Significant Grain Refinement. Sci. Rep. 2020, 10, 9448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, B.; Liu, B.; Nie, A.; Li, Q. Insight into Si poisoning on grain refinement of Al-Si/Al-5Ti-B system. Acta Mater. 2020, 187, 51–65. [Google Scholar] [CrossRef]
- Ding, W.W.; Zhao, X.Y.; Chen, T.L.; Zhang, H.X.; Liu, X.X.; Cheng, Y.; Lei, D.K. Effect of rare earth Y and Al-Ti-B master alloy on the microstructure and mechanical properties of 6063 aluminum alloy. J. Alloys Compd. 2020, 830, 154685. [Google Scholar] [CrossRef]
- Peeratatsuwan, C.; Chowwanonthapunya, T. Investigation of the grain refining performance of Al-5Ti-1B master alloy on the recycling process of A356 alloy. Materialwiss. Werkst. 2020, 51, 1346–1352. [Google Scholar] [CrossRef]
- Mi, L.; Wang, J.J.; Hu, Z.L. The Research Progress of TiB2 Impacts on the Refining Effect of Al-Ti-B Master Alloy. Appl. Mech. Mater. 2014, 574, 391–395. [Google Scholar] [CrossRef]
- Crossley, F.A.; Mondolfo, L.F. Mechanism of Grain Refinement in Aluminum Alloys. JOM 1951, 191, 1143–1148. [Google Scholar] [CrossRef]
- Backerud, L.; Gustafson, P.; Johnsson, M. Grain Refining Mechanisms in Aluminum as a Result of Additions of Titanium and Boron. Aluminium 1991, 67, 780. [Google Scholar]
- Wang, X.; Han, Q. Grain Refinement Mechanism of Aluminum by Al-Ti-B Master Alloys. In Light Metals; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Kashyap, K.T.; Chandrashekar, T. Effects and mechanisms of grain refinement in aluminium alloys. Bull. Mater. Sci. 2001, 24, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jiang, H.; Zhao, J.; He, J. Microstructure and grain refining efficiency of Al-5Ti-1B master alloys prepared by halide salt route. J. Mater. Process. Technol. 2017, 246, 205–210. [Google Scholar] [CrossRef]
- Yi, H.; Ma, N.; Zhang, Y.; Li, X.; Wang, H. Effective elastic moduli of Al-Si composites reinforced in situ with TiB2 particles. Scr. Mater. 2006, 54, 1093–1097. [Google Scholar] [CrossRef]
- Birol, Y. Production of Al-Ti-B master alloys from Ti sponge and KBF4. J. Alloys Compd. 2007, 440, 108–112. [Google Scholar] [CrossRef]
- Pourbagheri, H.; Aghajani, H. SHS-Produced Al-Ti-B Master Alloys: Performance in Commercial Al Alloy. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 245–254. [Google Scholar] [CrossRef]
- Chai, L.; Wang, H.; Chen, Z.; Cui, Y. Evaluation of Microstructure and Refining Effect of Al-TiB2 and Al-5Ti-1B Grain Refiners. Phys. Eng. Met. Mater. 2019, 217, 295–306. [Google Scholar]
- He, M.; Zhang, L.; Jiang, H.; He, J.; Zhao, J. Improved grain refinement in aluminium alloys by re-precipitated TiB2 particles. Mater. Lett. 2022, 312, 131657. [Google Scholar]
- Bian, X.F. Cast Metal Genetics; Shandong Science and Technology Press: Jinan, China, 1999. [Google Scholar]
- Auradi, V.; Kori, S.A. Preparation and Characterization of Al-1Ti-3B Master Alloys by Salt Route and Evaluation of their Grain Refining Performance on Al-7Si Alloys. Mater. Sci. Forum 2014, 790–791, 173–178. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, J.; Zhu, Z.; Guo, P. Melt reaction mechanism of Al-Ti-B system. Spec. Cast. Nonferrous Alloys 2006, 26, 12–14. [Google Scholar]
- Liu, X.F.; Bian, X.F.; Yang, Y. The formation law of TiAl3 morphologies in AlTi5B master alloy. Spec. Cast. Nonferrous Alloys 1997, 5, 4–6. [Google Scholar]
- Xie, H.; Lv, J. Precipation of TiAl3 in remelting Al-5Ti-1B and the grain refinement of 7050 alloy. Mater. Res. Express 2021, 8, 066513. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Nie, J.; Liu, X. Influence of forming process on three-dimensional morphology of TiB2 particles in Al-Ti-B alloys. Trans. Nonferrous Met. Soc. China 2012, 22, 564–570. [Google Scholar] [CrossRef]
- Zhang, L.L.; Jiang, H.X.; Jie, H.E.; Zhao, J.Z. Kinetic behaviour of TiB2 particles in Al melt and their effect on grain refinement of aluminium alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 2035–2044. [Google Scholar] [CrossRef]
- Wang, X.; Song, J.; Vian, W.; Ma, H.; Han, Q. The Interface of TiB2 and Al3Ti in Molten Aluminum. Metall. Mater. Trans. B 2016, 47, 3285. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al-Ti-B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Dai, W.; Han, Q. On the Understanding of Aluminum Grain Refinement by Al-Ti-B Type Master Alloys. Metall. Mater. Trans. B 2015, 46, 1620–1625. [Google Scholar] [CrossRef]
- Hong, M.A.; Jian, L.I.; Zhang, B.; Fang, H. Analysis of microstructures in Al-Ti-B alloy. Chin. J. Nonferrous Met. 2001, 5, 801–805. [Google Scholar]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Emamy, M.; Mahta, M.; Rasizadeh, J. Formation of TiB2 particles during dissolution of TiAl3 in Al-TiB2 metal matrix composite using an in situ technique. Compos. Sci. Technol. 2006, 66, 1063–1066. [Google Scholar] [CrossRef]
- Sun, D.; Wang, L. Calculation of Interdiffusion Coefficient between a Ti Substrate and an A1 Film. Shanghai Nonferrous Met. 1992, 2, 1–5. [Google Scholar]
- Hou, B. Pay Attention to Some Problem in Using Al-Ti-B Refiner during Aluminium Continuous Cast-rolling Process. Light Alloy Fabr. Technol. 2004, 32, 14–17. [Google Scholar]
- Ramesh, C.S.; Pramod, S.; Keshavamurthy, R. A study on microstructure and mechanical properties of Al 6061-TiB2 in-situ composites. Mater. Sci. Eng. A 2011, 528, 4125–4132. [Google Scholar] [CrossRef]
- Fjellstedt, J.; Jarfors, A.E.W. On the precipitation of TiB2 in aluminum melts from the reaction with KBF4 and K2TiF6. Mater. Sci. Eng. A 2005, 413–414, 527–532. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, W.L.; Zhang, E.L.; Zeng, S.Y. Morphology evolution and growth mechanism of TiB2 in Ti-54Al-xB alloys. Acta Metall. Sin.-Chin. Ed. 2002, 38, 699–702. [Google Scholar]


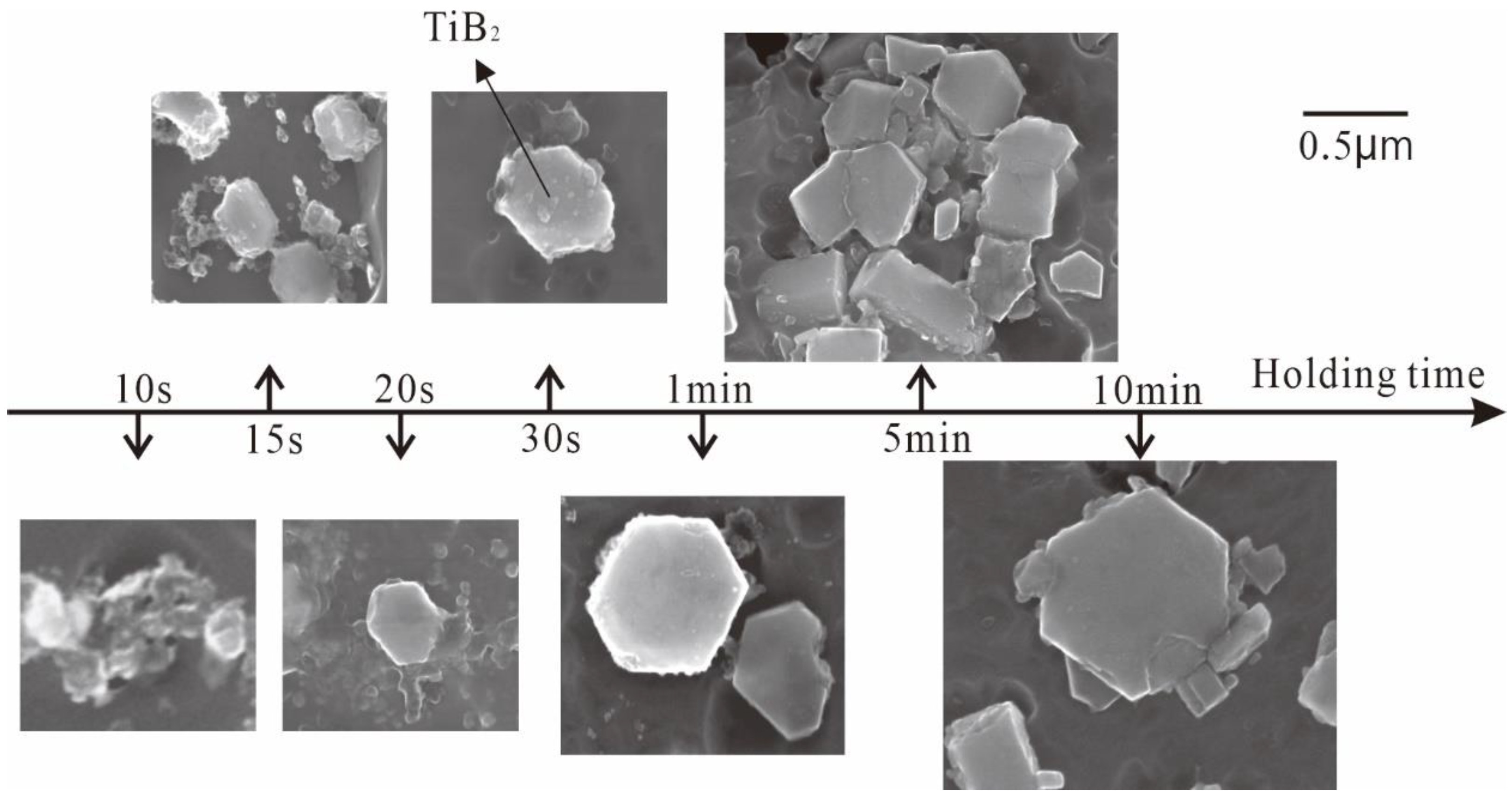

| Temperature | Reaction Time | |||||||
|---|---|---|---|---|---|---|---|---|
| 800 °C | 10 S | 15 S | 20 S | 30 S | 60 S | 300 S | 600 S | 1800 S |
| Content of Ti | Reaction Time | ||
|---|---|---|---|
| 30% Ti-sponge route | 15 S | 300 S | 1800 S |
| 60% Ti-sponge route | |||
| Ti-sponge route | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Lu, Z.; Mi, L.; Hu, Z.; Yang, W. Morphological Evolution of TiB2 and TiAl3 in Al–Ti–B Master Alloy Using Different Ti Adding Routes. Materials 2022, 15, 1984. https://doi.org/10.3390/ma15061984
Zhao Y, Lu Z, Mi L, Hu Z, Yang W. Morphological Evolution of TiB2 and TiAl3 in Al–Ti–B Master Alloy Using Different Ti Adding Routes. Materials. 2022; 15(6):1984. https://doi.org/10.3390/ma15061984
Chicago/Turabian StyleZhao, Yanjun, Zepeng Lu, Li Mi, Zhiliu Hu, and Wenchao Yang. 2022. "Morphological Evolution of TiB2 and TiAl3 in Al–Ti–B Master Alloy Using Different Ti Adding Routes" Materials 15, no. 6: 1984. https://doi.org/10.3390/ma15061984






