Does Current Knowledge Give a Variety of Possibilities for the Stabilization/Solidification of Soil Contaminated with Heavy Metals?—A Review
Abstract
:1. Introduction
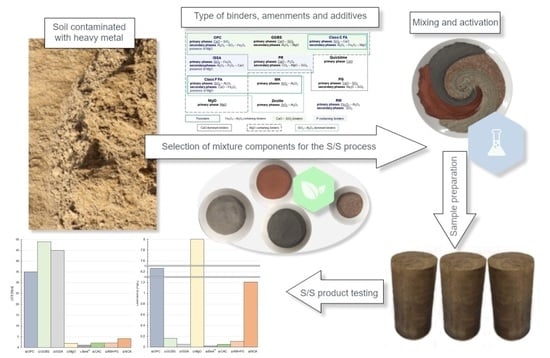
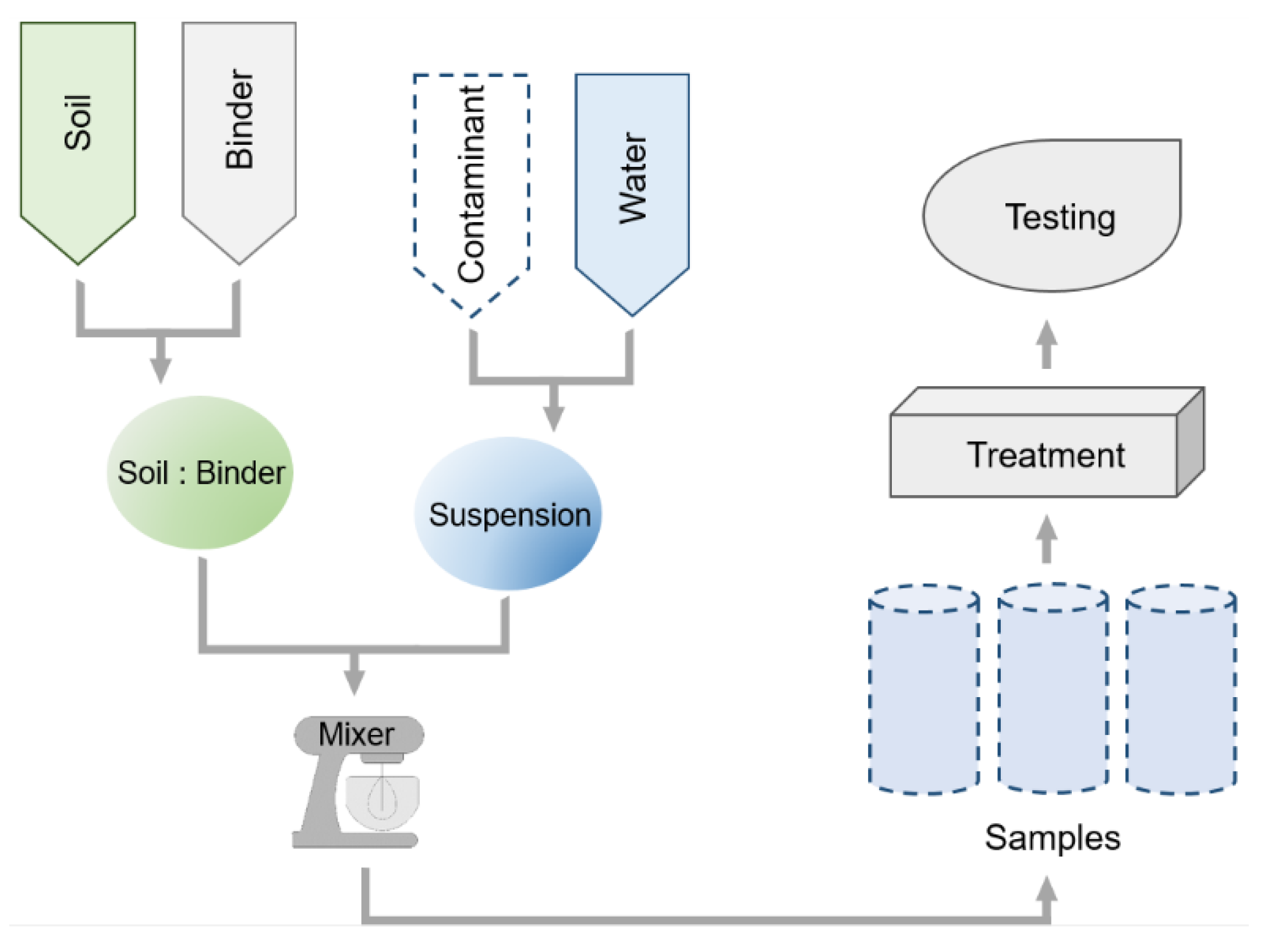
2. Samples Preparation and Curing Conditions
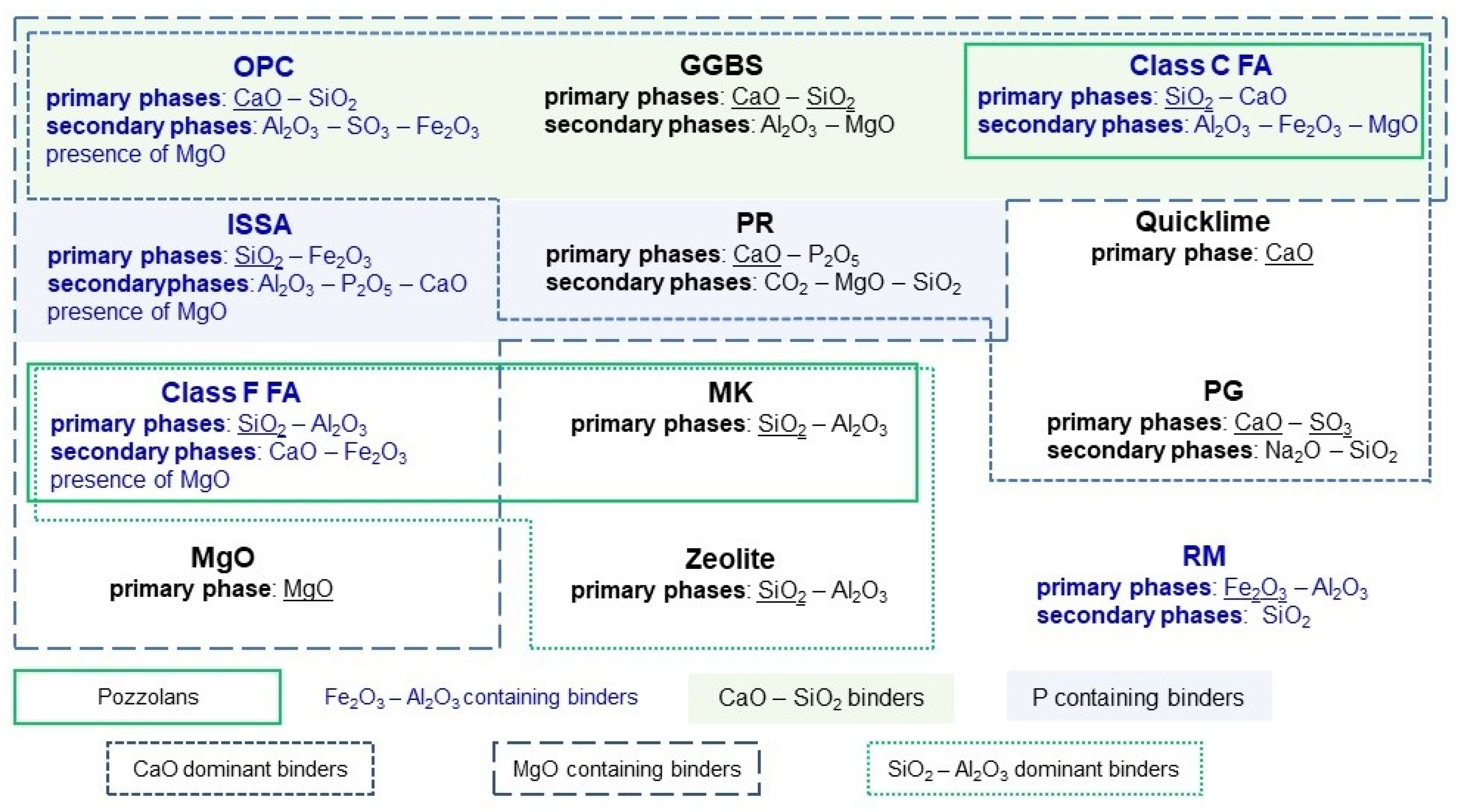
3. Characteristic of Binders, Amendments and Additives
3.1. Ordinary Portland Cement (OPC)
3.2. Ground Granulated Blast-Furnace Slag (GGBS)
3.3. Incinerated Sewage Sludge Ash (ISSA)
3.4. Fly Ash (FA) and Pulverized Fly Ash (PFA)
3.5. Magnesium Potassium Phosphate Cement (MPC)/(MKPC)
3.6. Red Gypsum (RG)
3.7. Phosphate Rock PR and Phosphoric Acid (PA)
3.8. Phosphogypsum (PG) and Potassium Dihydrogen Phosphate (KDP)
3.9. Red Mud (RM)
3.10. Calcium Aluminate Cement (CAC)
3.11. Bentonite
3.12. Lime (CaO), Quicklime (QL), Lime Production Waste (LPW)
3.13. Reactive Magnesia (MgO)
3.14. Geopolymers and Geopolymeric Binder Made of NaOH-Activated Metakaolin (MK)
3.15. Natural and Synthetic Zeolite
3.16. SPC Binder
3.17. EnvirOceMTM
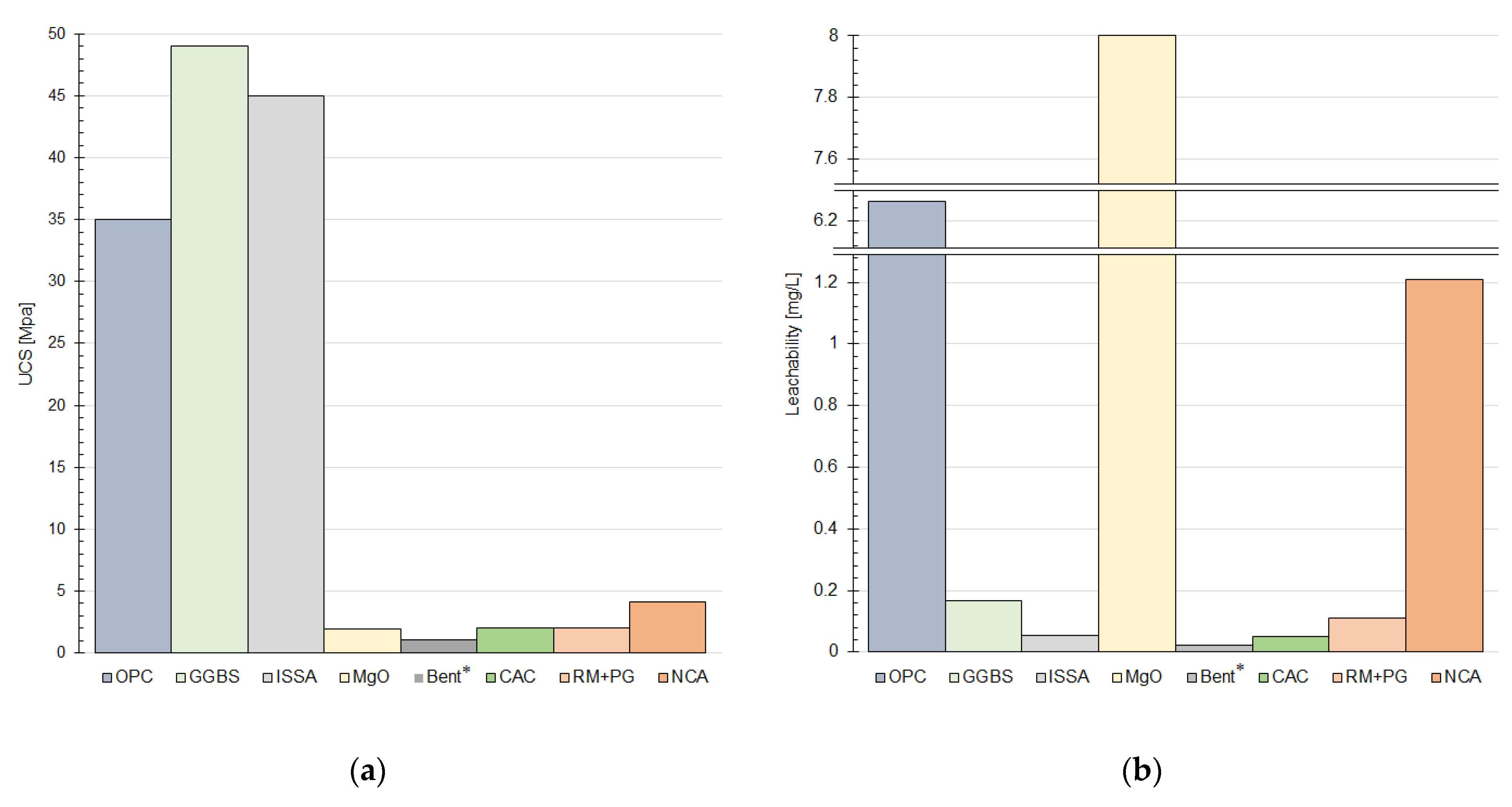
4. Effectiveness of Mixtures and Optimization Testing Methods
4.1. Unconfined Compression Strength
4.2. Leaching Behavior
4.3. Microstruture Investigation
4.4. Electrical Resistivity
4.5. Durability
4.5.1. Drying–Wetting Cycle
4.5.2. Sulfate and Acid Attack
4.5.3. Freeze—Thaw Impact
5. Conclusions
- The variety of proposed binders, additives and their mixtures and methods of activating the materials is very extensive in the literature, providing engineers with a wide range of options depending on the geochemical conditions of the treated site.
- Despite its many disadvantages, the most popular binder in the S/S process is Ordinary Portland Cement.
- Implementation of waste materials such as GGBS, FA, ISSA as amendments for part of the OPC for the stabilization/solidification process is becoming common practice, with many environmental and economic advantages.
- Replacing part of the cement with PFA or ISSA fly ash results in a significant decrease in the strength of the S/S product, but does not increase the leachability of the contaminants.
- The implementation of GGBS in place of part of the OPC results in an increase in strength, but significantly increases the leaching of contaminants when used in too large a quantity. The addition of an activator (e.g., MgO) significantly improves the ability of GGBS solidification.
- Considering the frequency of undertaking S/S process studies using red gypsum, red mud, calcium aluminate cement, bentonite, zeolites and superfine sulfate-resisting Portland cement, these materials should be considered niche products, effective for use only under specific conditions.
- In optimizing the mixture of binders and additives for the S/S process of heavy metal-contaminated soils, one of the main factors considered should remain the ecological aspect.
- The key studies assessing the effectiveness of S/S processes of contaminated soils are UCS and leachability studies. However, the scope of the latter varies widely and often does not take into account the actual conditions in the soil medium.
- The often-overlooked ageing tests, which take into account the effects of external factors on the mechanical and chemical stability of the resulting bonds when assessing durability, should be important in the evaluation of the S/S method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reethu, B.; Kumar, M.S.; Sharath, G.; Ramanjaneyulu, B.; Manchiryal, R.K. Stabilization of clayey soil using Gypsum. J. Stud. Res. 2020, 5, 886–888. [Google Scholar] [CrossRef]
- Consoli, N.C.; Prietto, P.D.M.; Lopes, L.d.S.; Winter, D. Control factors for the long term compressive strength of lime treated sandy clay soil. Transp. Geotech. 2014, 1, 129–136. [Google Scholar] [CrossRef]
- Al-Mukhtar, M.; Khattab, S.; Alcover, J.F. Microstructure and geotechnical properties of lime-treated expansive clayey soil. Eng. Geol. 2012, 139–140, 17–27. [Google Scholar] [CrossRef]
- Pakbaz, M.S.; Alipour, R. Influence of cement addition on the geotechnical properties of an Iranian clay. Appl. Clay Sci. 2012, 67–68, 1–4. [Google Scholar] [CrossRef]
- Maghous, S.; Consoli, N.C.; Fonini, A.; Pasa Dutra, V.F. A theoretical-experimental approach to elastic and strength properties of artificially cemented sand. Comput. Geotech. 2014, 62, 40–50. [Google Scholar] [CrossRef]
- Al-Jabban, W.; Knutsson, S.; Laue, J.; Al-Ansari, N. Stabilization of Clayey Silt Soil Using Small Amounts of Petrit, T. Engineering 2017, 9, 540–562. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.K.; Swain, S.K.; Sahoo, U.C. Stabilization of a Clayey Soil with Fly Ash and Lime: A Micro Level Investigation. Geotech. Geol. Eng. 2012, 30, 1197–1205. [Google Scholar] [CrossRef]
- Krishnakumari, M.B.; Swathika, A.; Subasree, M.; Irin Priscilla, P. Stabilization of Clayey Soil Using Various Additives. Int. Res. J. Eng. Technol. 2008, 6, 1177–1181. [Google Scholar]
- Fayaz, M.A. Laboratory Investigation for Stabilization of Clayey Soil in Kashmir Valley. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2909–2914. [Google Scholar] [CrossRef]
- Harbottle, M.J.; Al-Tabbaa, A.; Evans, C.W. A comparison of the technical sustainability of in situ stabilisation/solidification with disposal to landfill. J. Hazard. Mater. 2007, 141, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, S.; Bialobrzeski, T.; Kruszyńska, E.; Ciepiela, K. Przegląd metod oczyszczania gruntów i wód gruntowych in-situ. Prz. Geol. 2017, 65, 908–915. [Google Scholar]
- Khabidolda, S.M.; Abdiyussupov, G.G.; Omirzak, M.T. Development of Environmentally Clean Construction Materials Using Industrial Waste. Materials 2022, 15, 5726. [Google Scholar] [CrossRef]
- Navarro, A.; Cardellach, E.; Corbella, M. Immobilization of Cu, Pb and Zn in mine-contaminated soils using reactive materials. J. Hazard. Mater. 2011, 186, 1576–1585. [Google Scholar] [CrossRef]
- Saadeldin, R.; Siddiqua, S. Geotechnical characterization of a clay–cement mix. Bull. Eng. Geol. Environ. 2013, 72, 601–608. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, D.; Tao, H.; Yang, Y. The Effects of Portland and Sulphoaluminate Cements Solidification/Stabilization on Semi-Dynamic Leaching of Heavy Metal from Contaminated Sediment. Sustainability 2022, 14, 5681. [Google Scholar] [CrossRef]
- Jin, F.; Wang, F.; Al-Tabbaa, A. Three-year performance of in-situ solidified/stabilised soil using novel MgO-bearing binders. Chemosphere 2016, 144, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Voglar, G.E.; Leštan, D. Efficiency modeling of solidification/stabilization of multi-metal contaminated industrial soil using cement and additives. J. Hazard. Mater. 2011, 192, 753–762. [Google Scholar] [CrossRef]
- Scanferla, P.; Ferrari, G.; Pellay, R.; Volpi Ghirardini, A.; Zanetto, G.; Libralato, G. An innovative stabilization/solidification treatment for contaminated soil remediation: Demonstration project results. J. Soils Sediments 2009, 9, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Z.; Al-Tabbaa, A. PC-based and MgO-based binders stabilised/solidified heavy metal contaminated model soil: Strength and heavy metal speciation in early stage. Geotechnique 2018, 68, 1025–1030. [Google Scholar] [CrossRef]
- Means, J.; Nehring, K.; Smith, L. Application of Solidification and Stabilization to Waste Materials; CRC Press: Boca Raton, FA, USA, 1995. [Google Scholar]
- Bone, B.D.; Barnard, L.H.; Boardman, D.I.; Carey, P.J.; Hills, C.D.; Jones, H.M.; MacLeod, C.L.; Tyrer, M. Review of scientific literature on the use of stabilisation/solidification for the treatment of contaminated soil, solid waste and sludges (SC980003/SR2). In The Environment Agency, Bristol; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 1 844 323 196. [Google Scholar]
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Dasary, S.; Chittajallu, A.; Rekha, P.; Yerramilli, A. Stabilisation and solidification technologies for the remediation of contaminated soils and sediments: An overview. Land Contam. Reclam. 2005, 13, 23–48. [Google Scholar] [CrossRef]
- Tajudin, S.A.A.; Azmi, M.A.M.; Nabila, A.T.A. Stabilization/Solidification Remediation Method for Contaminated Soil: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 136, 012043. [Google Scholar] [CrossRef] [Green Version]
- Wiles, C.C. A review of solidification/stabilization technology. J. Hazard. Mater. 1987, 14, 5–21. [Google Scholar] [CrossRef]
- ASTM D1632-07; Standard Practice for Making and Curing Soil-Cement Compression and Flexure Test Specimens in the Laboratory. ANSI: NewYork, NY, USA, 2020.
- Li, J.; Poon, C.S. Innovative solidification/stabilization of lead contaminated soil using incineration sewage sludge ash. Chemosphere 2017, 173, 143–152. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Movahedrad, M. Stabilization/solidification of zinc-contaminated kaolin clay using ground granulated blast-furnace slag and different types of activators. Appl. Geochem. 2017, 81, 155–165. [Google Scholar] [CrossRef]
- Li, X.; Yang, R.; Li, H.; Yi, H.; Jing, H. Experimental study on solidification and stabilization of heavy-metal-contaminated soil using cementitious materials. Materials 2021, 14, 4999. [Google Scholar] [CrossRef]
- Wang, L.; Yu, K.; Li, J.S.; Tsang, D.C.W.; Poon, C.S.; Yoo, J.C.; Baek, K.; Ding, S.; Hou, D.; Dai, J.G. Low-carbon and low-alkalinity stabilization/solidification of high-Pb contaminated soil. Chem. Eng. J. 2018, 351, 418–427. [Google Scholar] [CrossRef]
- Vaidya, R.; Kodam, K.; Ghole, V.; Surya Mohan Rao, K.; Ghole, V.; Mohan, S. Validation of an in situ solidification/stabilization technique for hazardous barium and cyanide waste for safe disposal into a secured landfill. J. Environ. Manag. 2010, 91, 1821–1830. [Google Scholar] [CrossRef]
- Feng, Y.S.; Du, Y.J.; Zhou, A.; Zhang, M.; Li, J.S.; Zhou, S.J.; Xia, W.Y. Geoenvironmental properties of industrially contaminated site soil solidified/stabilized with a sustainable by-product-based binder. Sci. Total Environ. 2021, 765, 142778. [Google Scholar] [CrossRef]
- Wu, H.L.; Jin, F.; Bo, Y.L.; Du, Y.J.; Zheng, J.X. Leaching and microstructural properties of lead contaminated kaolin stabilized by GGBS-MgO in semi-dynamic leaching tests. Constr. Build. Mater. 2018, 172, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Li, J.S.; Xue, Q.; Wang, P.; Li, Z.Z.; Liu, L. Effect of drying-wetting cycles on leaching behavior of cement solidified lead-contaminated soil. Chemosphere 2014, 117, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Kogbara, R.B.; Yi, Y.; Al-Tabbaa, A. Process envelopes for stabilisation/solidification of contaminated soil using lime-slag blend. Environ. Sci. Pollut. Res. 2011, 18, 1286–1296. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y. Stabilization/solidification of lead- and zinc-contaminated soils using MgO and CO2. J. CO2 Util. 2019, 33, 215–221. [Google Scholar] [CrossRef]
- Tang, P.P.; Zhang, W.L.; Chen, Y.H.; Chen, G.; Xu, J. Stabilization/solidification and recycling of sediment from Taihu Lake in China: Engineering behavior and environmental impact. Waste Manag. 2020, 116, 1–8. [Google Scholar] [CrossRef]
- Al-Tabbaa, A.; Boes, N. Pilot in situ auger mixing treatment of a contaminated site. Part 4. Performance at five years. Proc. Inst. Civ. Eng. Geotech. Eng. 2002, 155, 187–202. [Google Scholar] [CrossRef]
- Xia, W.Y.; Du, Y.J.; Li, F.S.; Li, C.P.; Yan, X.L.; Arulrajah, A.; Wang, F.; Song, D.J. In-situ solidification/stabilization of heavy metals contaminated site soil using a dry jet mixing method and new hydroxyapatite based binder. J. Hazard. Mater. 2019, 369, 353–361. [Google Scholar] [CrossRef]
- Li, J.S.; Chen, L.; Zhan, B.; Wang, L.; Poon, C.S.; Tsang, D.C.W. Sustainable stabilization/solidification of arsenic-containing soil by blast slag and cement blends. Chemosphere 2021, 271, 129868. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Al-Tabbaa, A.; Yi, Y.; Stegemann, J.A. Cement–fly ash stabilisation/solidification of contaminated soil: Performance properties and initiation of operating envelopes. Appl. Geochem. 2013, 33, 64–75. [Google Scholar] [CrossRef]
- Arora, S.; Aydilek, A. Class F fly-ash-amended soils as highway base materials. J. Mater. Civ. Eng. 2005, 17, 640–649. [Google Scholar] [CrossRef] [Green Version]
- Boutouil, M.; Levacher, D. Effect of high initial water content on cement-based sludge solidification. Adv. Mater. Res. 2005, 9, 169–174. [Google Scholar] [CrossRef]
- Kogbara, R.B. Process Envelopes for and Biodegradation within Stabilised/Solidified Contaminated Soils. Ph.D. Thesis, Cambridge University, Cambridge, UK, 2011. [Google Scholar]
- Kogbara, R.B. A review of the mechanical and leaching performance of stabilised/solidified contaminated soils. Environ. Rev. 2014, 22, 66–86. [Google Scholar] [CrossRef]
- Cao, X.; Wahbi, A.; Ma, L.; Li, B.; Yang, Y. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Cho, D.-W.W.; Tsang, D.C.W.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q.; Poon, C.S. Sustainable stabilization/solidification of municipal solid waste incinerator fly ash by incorporation of green materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Yin, H.; Zhang, Y.; Pan, H.; Wang, L. Sustainable stabilization/solidification of the Pb, Zn, and Cd contaminated soil by red mud-derived binders. Environ. Pollut. 2021, 284, 117178. [Google Scholar] [CrossRef]
- Zhang, Y.; Labianca, C.; Chen, L.; De Gisi, S.; Notarnicola, M.; Guo, B.; Sun, J.; Ding, S.; Wang, L. Sustainable ex-situ remediation of contaminated sediment: A review. Environ. Pollut. 2021, 287, 117333. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.I. Utilization and efficiency of ground granulated blast furnace slag on concrete properties-A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Evaluation of novel reactive MgO activated slag binder for the immobilisation of lead and zinc. Chemosphere 2014, 117, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Tariq, A.; Yanful, E.K. A review of binders used in cemented paste tailings for underground and surface disposal practices. J. Environ. Manag. 2013, 131, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, J.; Zhang, Y.; Shen, Z.; Al-Tabbaa, A. MgO-GGBS Binder–Stabilized/Solidified PAE-Contaminated Soil: Strength and Leachability in Early Stage. J. Geotech. Geoenviron. Eng. 2021, 147, 04021059. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Wen, W.; Jia, L.; Xie, J.; Zhao, W.; Feng, H.; Cao, D.; Sun, F.; Han, P.; Bai, X.; He, B. Electrochemical response and effect evaluation of high belite sulphoaluminate cement combined with red mud-fly ash on solidification of Cu2+-contaminated kaolin. Case Stud. Constr. Mater. 2022, 17, e01497. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S.; Jin, F. Magnesia reactivity on activating efficacy for ground granulated blastfurnace slag for soft clay stabilisation. Appl. Clay Sci. 2016, 126, 57–62. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Zhou, Y.; Rinklebe, J.; Song, H.; Kwon, E.E.; Baek, K.; Ok, Y.S. Mechanistic insights into red mud, blast furnace slag, or metakaolin-assisted stabilization/solidification of arsenic-contaminated sediment. Environ. Int. 2019, 133, 105247. [Google Scholar] [CrossRef]
- Laforest, G.; Duchesne, J. Immobilization of chromium (VI) evaluated by binding isotherms for ground granulated blast furnace slag and ordinary Portland cement. Cem. Concr. Res. 2005, 35, 2322–2332. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zuo, X.B.; He, S.L.; Ayinde, O.; Yin, G.J. Influence of slag content and water-binder ratio on leaching behavior of cement pastes. Constr. Build. Mater. 2016, 129, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.J.; Jiang, N.J.; Liu, S.Y.; Jin, F.; Singh, D.N.; Puppala, A.J. Engineering properties and microstructural characteristics of cement-stabilized zinc-contaminated kaolin. Can. Geotech. J. 2014, 51, 289–302. [Google Scholar] [CrossRef]
- Amin, M.S.; Hashem, F.S.; Mohamed, M.R. Solidification/stabilisation of Zn2+ ions in metakaolin and homra- blended cement matrices. Adv. Cem. Res. 2012, 24, 239–248. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, G.; Wang, M.; Zhang, J.; Wang, Z.; Li, F.; Chen, H. Enhanced stabilization of Pb, Zn, and Cd in contaminated soils using oxalic acid-activated phosphate rocks. Environ. Sci. Pollut. Res. 2018, 25, 2861–2868. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, Q.; Chen, Z.; Xue, Q.; Chen, X.; Mu, Y.; Poon, C.S. Immobilization of high-Pb contaminated soil by oxalic acid activated incinerated sewage sludge ash. Environ. Pollut. 2021, 284, 117120. [Google Scholar] [CrossRef]
- Dermatas, D.; Meng, X. Utilization of fly ash for stabilization/solidification of heavy metal contaminated soils. Eng. Geol. 2003, 70, 377–394. [Google Scholar] [CrossRef]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Jin, F.; Wu, H.L.; Liu, Z. Bin New phosphate-based binder for stabilization of soils contaminated with heavy metals: Leaching, strength and microstructure characterization. J. Environ. Manag. 2014, 146, 179–188. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.S.; Wang, L.; Zhang, Y.; Li, J.; Tong, L.; Hu, Q.; Dai, J.G.; Tsang, D.C.W. Stabilisation/solidification of municipal solid waste incineration fly ash by phosphate-enhanced calcium aluminate cement. J. Hazard. Mater. 2021, 408, 124404. [Google Scholar] [CrossRef]
- Cadar, O.; Dinca, Z.; Senila, M.; Torok, A.I.; Todor, F.; Levei, E.A. Immobilization of Potentially Toxic Elements in Contaminated Soils Using Thermally Treated Natural Zeolite. Materials 2021, 14, 3777. [Google Scholar] [CrossRef]
- Xia, W.Y.; Feng, Y.S.; Jin, F.; Zhang, L.M.; Du, Y.J. Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr. Build. Mater. 2017, 156, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Gardner, L.J.; Bernal, S.A.; Walling, S.A.; Corkhill, C.L.; Provis, J.L.; Hyatt, N.C. Characterisation of magnesium potassium phosphate cements blended with fly ash and ground granulated blast furnace slag. Cem. Concr. Res. 2015, 74, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Zhu, J.; Fu, Q.; Zuo, J.; Liu, Y.; Hu, H. Immobilization of lead in anthropogenic contaminated soils using phosphates with/without oxalic acid. J. Environ. Sci. 2015, 28, 64–73. [Google Scholar] [CrossRef]
- Oprčkal, P.; Mladenovič, A.; Zupančič, N.; Ščančar, J.; Milačič, R.; Serjun, V.Z. Remediation of contaminated soil by red mud and paper ash. J. Clean. Prod. 2020, 256, 120440. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Akbari, H.R.; Salimi, M. Enhanced stabilization of highly expansive clays by mixing cement and silica fume. Appl. Clay Sci. 2016, 132–133, 675–684. [Google Scholar] [CrossRef]
- Nedunuri, S.S.S.A.; Sertse, S.G.; Muhammad, S. Microstructural study of Portland cement partially replaced with fly ash, ground granulated blast furnace slag and silica fume as determined by pozzolanic activity. Constr. Build. Mater. 2020, 238, 117561. [Google Scholar] [CrossRef]
- Namarak, C.; Satching, P.; Tangchirapat, W.; Jaturapitakkul, C. Improving the compressive strength of mortar from a binder of fly ash-calcium carbide residue. Constr. Build. Mater. 2017, 147, 713–719. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Dhoury, M.; Champenois, J.-B.; Mercier, C.; Damidot, D. Combined effects of lithium and borate ions on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 97, 50–60. [Google Scholar] [CrossRef]
- Sharma, H.D.; Reddy, K.R. Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Technologies. Environ. Int. 2004, 35, 50–55. [Google Scholar]
- Batchelor, B. Overview of waste stabilization with cement. Waste Manag. 2006, 26, 689–698. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Al-Tabbaa, A.; Yi, Y.; Stegemann, J.A. PH-dependent leaching behaviour and other performance properties of cement-treated mixed contaminated soil. J. Environ. Sci. 2012, 24, 1630–1638. [Google Scholar] [CrossRef]
- Roy, A.; Eaton, H.C.; Cartledge, F.K.; Tittlebaum, M.E. Solidification/stabilization of a heavy metal sludge by a Portland cement/fly ash binding mixture. Waste Hazard. Mater. 1991, 8, 33–41. [Google Scholar] [CrossRef]
- Lin, S.L.; Cross, W.H.; Chian, E.S.K.; Lai, J.S.; Giabbai, M.; Hung, C.H. Stabilization and solidification of lead in contaminated soils. J. Hazard. Mater. 1996, 48, 95–110. [Google Scholar] [CrossRef]
- Sanchez, F.; Gervais, C.; Garrabrants, A.C.; Barna, R.; Kosson, D.S. Leaching of inorganic contaminants from cement-based waste materials as a result of carbonation during intermittent wetting. Waste Manag. 2002, 22, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Çokça, E.; Ünlü, K. Comparison of Two Leaching Tests to Assess the Effectiveness of Cement-Based Hazardous Waste Solidification/Stabilization. J. Eng. Environ. Sci. 2003, 27, 201–212. [Google Scholar]
- Shawabkeh, R.A. Solidification and stabilization of cadmium ions in sand–cement–clay mixture. J. Hazard. Mater. 2005, 125, 237–243. [Google Scholar] [CrossRef]
- Moon, D.H.; Lee, J.R.; Grubb, D.G.; Park, J.H. An assessment of Portland cement, cement kiln dust and class C fly ash for the immobilization of Zn in contaminated soils. Environ. Earth Sci. 2010, 61, 1745–1750. [Google Scholar] [CrossRef]
- Voglar, G.E.; Leštan, D. Solidification/stabilisation of metals contaminated industrial soil from former Zn smelter in Celje, Slovenia, using cement as a hydraulic binder. J. Hazard. Mater. 2010, 178, 926–933. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Al-Tabbaa, A.; Stegemann, J.A. Process Envelopes For Stabilised/Solidified Contaminated Soils: Initiation work. In Proceedings of the Fifth International Conference on Environmental Science and Technology, Bangkok, Thailand, 11–13 November 2019; American Science Press: Houston, TN, USA, 2019. [Google Scholar]
- He, H.; Suito, H. Immobilization of hexavalent chromium in aqueous solution through the formation of 3CaO·(Al, Fe)2O3 Ca (OH)2·xH2O phase, ettringite and C–S–H gel. ISIJ Int. 2002, 42, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Gougar, M.L.D.; Scheetz, B.E.; Roy, D.M. Ettringite and C-S-H Portland Cement Phases for waste ion immobilization: A Review. Waste Manag. 1996, 16, 295–303. [Google Scholar] [CrossRef]
- Spence, R.D.; Shi, C. Stabilization and Solidification of Hazardous, Radioactive, and Mixed Wastes; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781420032789. [Google Scholar]
- Su, Y.; Yang, J.; Liu, D.; Zhen, S.; Lin, N.; Zhou, Y. Solidification/stabilization of simulated cadmium-contaminated wastes with magnesium potassium phosphate cement. Environ. Eng. Res. 2016, 21, 15–21. [Google Scholar] [CrossRef]
- Bakhshi, N.; Sarrafi, A.; Ramezanianpour, A.A. Immobilization of hexavalent chromium in cement mortar: Leaching properties and microstructures. Environ. Sci. Pollut. Res. 2019, 26, 20829–20838. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Bai, Y.; Ma, B.; Wang, G.; Tan, H. Stabilization/solidification on chromium (III) wastes by C3A and C3A hydrated matrix. J. Hazard. Mater. 2014, 268, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Min, X.; Ke, Y.; Fei, J.; Liu, D.; Tang, C. Immobilization potential and immobilization mechanism of arsenic in cemented paste backfill. Miner. Eng. 2019, 138, 101–107. [Google Scholar] [CrossRef]
- Contessi, S.; Calgaro, L.; Dalconi, M.C.; Bonetto, A.; Bellotto, M.P.; Ferrari, G.; Marcomini, A.; Artioli, G. Stabilization of lead contaminated soil with traditional and alternative binders. J. Hazard. Mater. 2020, 382, 120990. [Google Scholar] [CrossRef] [PubMed]
- Contessi, S.; Dalconi, M.C.; Pollastri, S.; Calgaro, L.; Meneghini, C.; Ferrari, G.; Marcomini, A.; Artioli, G. Cement-stabilized contaminated soil: Understanding Pb retention with XANES and Raman spectroscopy. Sci. Total Environ. 2021, 752, 141826. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, Y.J.; Liu, S.-Y.; Jin, F. Evaluation of Cement Hydration Properties of Cement-Stabilized Lead-Contaminated Soils Using Electrical Resistivity Measurement. J. Hazard. Toxic, Radioact. Waste 2011, 15, 312–320. [Google Scholar] [CrossRef]
- Vandaperre, L.J.; Liska, M.; Al-tabbaa, A. Hydration and Mechanical Properties of Magnesia, Pulverized Fuel Ash, and Portland Cement Blends. J. Mater. Civ. Eng. 2008, 20, 375–383. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dai, J.G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of lead on stabilization/solidification by ordinary Portland cement and magnesium phosphate cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Use of recycled CRT funnel glass as fine aggregate in dry-mixed concrete paving blocks. J. Clean. Prod. 2014, 68, 209–215. [Google Scholar] [CrossRef]
- Ling, T.C.; Poon, C.S. Spent fluorescent lamp glass as a substitute for fine aggregate in cement mortar. J. Clean. Prod. 2017, 161, 646–654. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, P.; Li, J.S.; Zhang, T.T.; Wang, S.Y. Investigation of the leaching behavior of lead in stabilized/solidified waste using a two-year semi-dynamic leaching test. Chemosphere 2017, 166, 1–7. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Yong, R.N.; Deiranlou, M. Enhancement of Cement-Based Solidification/Stabilization of a lead-contaminated smectite clay. J. Hazard. Mater. 2021, 403, 123969. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.S.; Tsang, D.C.W.; Poon, C.S.; Shih, K. Recycling contaminated wood into eco-friendly particleboard using green cement and carbon dioxide curing. J. Clean. Prod. 2016, 137, 861–870. [Google Scholar] [CrossRef]
- Morales, L.; Garzón, E.; Romero, E.; Sánchez-Soto, P.J. Microbiological induced carbonate (CaCO3) precipitation using clay phyllites to replace chemical stabilizers (cement or lime). Appl. Clay Sci. 2019, 174, 15–28. [Google Scholar] [CrossRef]
- Mujah, D.; Cheng, L.; Shahin, M.A. Microstructural and Geomechanical Study on Biocemented Sand for Optimization of MICP Process. J. Mater. Civ. Eng. 2019, 31, 04019025. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in use and research on cementitious material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Lijun, H.; Jiangshan, L.; Qiang, X.; Zhen, C.; Yaoyu, Z.; Chi, S.P. Bacterial-induced mineralization (BIM) for soil solidification and heavy metal stabilization: A critical review. Sci. Total Environ. 2020, 746, 140967. [Google Scholar] [CrossRef]
- Li, J.S.; Beiyuan, J.; Tsang, D.C.W.; Wang, L.; Poon, C.S.; Li, X.D.; Fendorf, S. Arsenic-containing soil from geogenic source in Hong Kong: Leaching characteristics and stabilization/solidification. Chemosphere 2017, 182, 31–39. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; O’Connor, D.; Hou, D. Solidification/Stabilization for Soil Remediation: An Old Technology with New Vitality. Environ. Sci. Technol. 2019, 53, 11615–11617. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Liu, Z.P.; Jin, F. Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef]
- Wei, M.-L.; Du, Y.J.; Reddy, K.R.; Wu, H.-L. Effects of freeze-thaw on characteristics of new KMP binder stabilized Zn- and Pb-contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 19473–19484. [Google Scholar] [CrossRef]
- Perera, A.; Al-Tabbaa, A. Stabilisation/Solidification Treatment and Remediation; CRC Press: Cambridge, UK, 2005; pp. 181–191. [Google Scholar]
- Cheah, C.B.; Chung, K.Y.; Ramli, M.; Lim, G.K. The engineering properties and microstructure development of cement mortar containing high volume of inter-grinded GGBS and PFA cured at ambient temperature. Constr. Build. Mater. 2016, 122, 683–693. [Google Scholar] [CrossRef]
- Li, S. Microstructure and composition characterisation of three 20-year-old GGBS-OPC blended pastes. Constr. Build. Mater. 2016, 123, 226–234. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhao, L.Y.; McCabe, B.A.; Chen, Y.H.; Morrison, L. Dredged marine sediments stabilized/solidified with cement and GGBS: Factors affecting mechanical behaviour and leachability. Sci. Total Environ. 2020, 733, 138551. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Li, J.S.; Yeung, T.L.Y.; Ding, S.; Poon, C.S. Green remediation of contaminated sediment by stabilization/solidification with industrial by-products and CO2 utilization. Sci. Total Environ. 2018, 631, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.L.; Kukacka, L.E. Blast furnace slag-modified grouts for in situ stabilization of chromium-contaminated soil. Waste Manag. 1995, 15, 193–202. [Google Scholar] [CrossRef]
- Miturski, M.; Głuchowski, A.; Sas, W. Influence of dispersed reinforcement on mechanical properties of stabilized soil. Materials 2021, 14, 5982. [Google Scholar] [CrossRef]
- Wang, L.; Yu, I.K.M.; Tsang, D.C.W.; Li, S.; Poon, C.S. Mixture Design and Reaction Sequence for Recycling Construction Wood Waste into Rapid-Shaping Magnesia-Phosphate Cement Particleboard. Ind. Eng. Chem. Res. 2017, 56, 6645–6654. [Google Scholar] [CrossRef]
- Ren, P.; Ling, T.C. Roles of chlorine and sulphate in MSWIFA in GGBFS binder: Hydration, mechanical properties and stabilization considerations. Environ. Pollut. 2021, 284, 117175. [Google Scholar] [CrossRef]
- Knapik, K. Analizy Doświadczalne i Numeryczne Wybranego Gruntu Wzmo Cnionego Popiołami Lotnymi z Procesu Fluidalnego Spalania Węgla. Ph.D Thesis, Silesian University of Technology, Gliwice, Poland, 2016. [Google Scholar]
- Sargent, P.; Hughes, P.N.; Rouainia, M.; White, M.L. The use of alkali activated waste binders in enhancing the mechanical properties and durability of soft alluvial soils. Eng. Geol. 2013, 152, 96–108. [Google Scholar] [CrossRef]
- Deja, J. Immobilization of Cr6+, Cd2+, Zn2+ and Pb2+ in alkali-activated slag binders. Cem. Concr. Res. 2002, 32, 1971–1979. [Google Scholar] [CrossRef]
- Roy, A.; Eaton, H.C.; Cartledge, F.K.; Tittlebaum, M.E. The effect of sodium sulfate on solidification/stabilization of a synthetic electroplating sludge in cementitious binders. J. Hazard. Mater. 1992, 30, 297–316. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Jin, F.; Al-Tabbaa, A. Mechanism of reactive magnesia-ground granulated blastfurnace slag (GGBS) soil stabilization. Can. Geotech. J. 2015, 53, 773–782. [Google Scholar] [CrossRef]
- Du, Y.J.; Bo, Y.L.; Jin, F.; Liu, C.Y. Durability of reactive magnesia-activated slag-stabilized low plasticity clay subjected to drying-wetting cycle. Eur. J. Environ. Civ. Eng. 2016, 20, 215–230. [Google Scholar] [CrossRef]
- Limbachiya, V.; Ganjian, E.; Claisse, P. Strength, durability and leaching properties of concrete paving blocks incorporating GGBS and SF. Constr. Build. Mater. 2016, 113, 273–279. [Google Scholar] [CrossRef]
- Pomakhina, E.; Deneele, D.; Gaillot, A.C.; Paris, M.; Ouvrard, G. 29Si solid state NMR investigation of pozzolanic reaction occurring in lime-treated Ca-bentonite. Cem. Concr. Res. 2012, 42, 626–632. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, L.; Cui, J.L.; Poon, C.S.; Beiyuan, J.; Tsang, D.C.W.; Li, X.D. Effects of low-alkalinity binders on stabilization/solidification of geogenic As-containing soils: Spectroscopic investigation and leaching tests. Sci. Total Environ. 2018, 631–632, 1486–1494. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Zhang, B.; Fang, C.; Li, L. Physicochemical properties of alkali activated GGBS and fly ash geopolymeric recycled concrete. Constr. Build. Mater. 2019, 204, 384–398. [Google Scholar] [CrossRef]
- Shah, S.J.; Shroff, A.V.; Patel, J.V.; Tiwari, K. Stabilization of fuel oil contaminated soil—A case study. Geotech. Geol. Eng. 2003, 21, 415–427. [Google Scholar] [CrossRef]
- Li, X.D.; Poon, C.S.; Sun, H.; Lo, I.M.C.; Kirk, D.W. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef]
- Al-Tabbaa, A.; Evans, C.W.; Wallace, C.J. Pilot in situ auger mixing treatment of a contaminated site. Part 2. Site trial. Proc. Inst. Civ. Eng. Geotech. Eng. 1998, 131, 89–95. [Google Scholar] [CrossRef]
- Chitambira, B. Accelerated ageing of cement stabilised/solidified contaminated soils with elevated temperatures. Ph.D. Thesis, Cambridge University, Cambridge, UK, 2004. [Google Scholar]
- Antemir, A. Performance assessment of S/S waste-forms: Initial results from site characterisation, sampling and testing. In Proceedings of the International Conference on Stabilisation/Solidification Treatment and Remediation; CRC Press: Cambridge, UK, 2005. [Google Scholar]
- Wang, L.; Kwok, J.S.H.; Tsang, D.C.W.; Poon, C.S. Mixture design and treatment methods for recycling contaminated sediment. J. Hazard. Mater. 2015, 283, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Vukićević, M.; Pujević, V.; Marjanowić, M.; Jocković, S. Maraš-Dragojević Fine grained soil stabilization using class F fly ash with and without cement. In Proceedings of the XVI ECSMGE Geotechnical Engineering for Infrastructure and Development, Edinburgh, UK, 13–17 September 2015; Volume 1, pp. 2671–2676. Available online: https://www.icevirtuallibrary.com/doi/abs/10.1680/ecsmge.60678.vol5.413 (accessed on 15 November 2022).
- ASTM C618; Standard Specification for Coalfly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete. ASTM Standards: West Conshohocken, PA, USA, 2003.
- Akhter, H.; Butler, L.G.; Branz, S.; Cartledge, F.K.; Tittlebaum, M.E. Immobilization of As, Cd, Cr and PB-containing soils by using cement or pozzolanic fixing agents. J. Hazard. Mater. 1990, 24, 145–155. [Google Scholar] [CrossRef]
- Kaniraj, S.R.; Havanagi, V.G. Compressive strength of cement stabilized fly ash-soil mixtures. Cem. Concr. Res. 1999, 29, 673–677. [Google Scholar] [CrossRef]
- Kostarelos, K.; Reale, D.; Dermatas, D.; Rao, E.; Moon, D.H. Optimum dose of lime and fly ash for treatment of hexavalent chromium-contaminated soil. Water Air Soil Pollut. Focus 2006, 6, 171–189. [Google Scholar] [CrossRef]
- Wang, D.L.; Chen, M.L.; Tsang, D.D.C.W. Green Remediation by Using Low-Carbon Cement-Based Stabilization/Solidification Approaches; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128179826. [Google Scholar]
- Wang, A.J.; Zhang, J.; Li, J.M.; Ma, A.B.; Liu, L.T. Effect of liquid-to-solid ratios on the properties of magnesium phosphate chemically bonded ceramics. Mater. Sci. Eng. C 2013, 33, 2508–2512. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of magnesium potassium phosphate cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Li, J.-S.; Xue, Q.; Wang, P.; Zhang, T.-T.; Zhao, Y. Comparison of Solidification/Stabilization of Lead Contaminated Soil between Magnesia–Phosphate Cement and Ordinary Portland Cement under the Same Dosage. Environ. Prog. Sustain. Energy 2016, 35, 88–94. [Google Scholar] [CrossRef]
- Singh, D.; Mandalika, V.R.; Parulekar, S.J.; Wagh, A.S. Magnesium potassium phosphate ceramic for 99Tc immobilization. J. Nucl. Mater. 2006, 348, 272–282. [Google Scholar] [CrossRef]
- Buj, I.; Torras, J.; Rovira, M.; de Pablo, J. Leaching behaviour of magnesium phosphate cements containing high quantities of heavy metals. J. Hazard. Mater. 2010, 175, 789–794. [Google Scholar] [CrossRef]
- Buj, I.; Torras, J.; Casellas, D.; Rovira, M.; de Pablo, J. Effect of heavy metals and water content on the strength of magnesium phosphate cements. J. Hazard. Mater. 2009, 170, 345–350. [Google Scholar] [CrossRef]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Hughes, P.N.; Glendinning, S.; Manning, D.A.C.; White, M.L. Use of red gypsum in soil mixing engineering applications. Proc. Inst. Civ. Eng.Geotech. Eng. 2011, 164, 223–234. [Google Scholar] [CrossRef]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, adsorption and rhizosphere effect: The mechanisms for Phosphate-induced Pb immobilization in soils—A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, Y.; Huang, L.; Fu, Q.; Deng, Y.; Hu, H. Mechanism of lead immobilization by oxalic acid-activated phosphate rocks. J. Environ. Sci. 2012, 24, 919–925. [Google Scholar] [CrossRef]
- Fang, Y.; Cao, X.; Zhao, L. Effects of phosphorus amendments and plant growth on the mobility of Pb, Cu, and Zn in a multi-metal-contaminated soil. Environ. Sci. Pollut. Res. 2012, 19, 1659–1667. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.Q.; Rhue, D.R.; Appel, C.S. Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 2004, 131, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Wu, H. liang Effect of carbonation on leachability, strength and microstructural characteristics of KMP binder stabilized Zn and Pb contaminated soils. Chemosphere 2016, 144, 1033–1042. [Google Scholar] [CrossRef]
- Fernández Bertos, M.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Yoo, J.C.; Beiyuan, J.; Wang, L.; Tsang, D.C.W.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.D. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci. Total Environ. 2018, 616, 572–582. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Jeong Choi, Y.; Kim, J.G. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 2009, 77, 1069–1075. [Google Scholar] [CrossRef]
- Bougharraf, N.; Louati, D.; Mosbahi, M.; Rouis, M.J.; Rigane, H. Comparison of the effectiveness of different binders in solidification/stabilization of a contaminated soil. Arab. J. Geosci. 2018, 11, 348. [Google Scholar] [CrossRef]
- Navarro-Blasco, I.; Duran, A.; Sirera, R.; Fernández, J.M.; Alvarez, J.I. Solidification/stabilization of toxic metals in calcium aluminate cement matrices. J. Hazard. Mater. 2013, 260, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, R.C.; Angulski da Luz, C.; Zorel, H.E.; Pereira Filho, J.I. Behavior of calcium aluminate cement (CAC) in the presence of hexavalent chromium. Cem. Concr. Compos. 2016, 73, 114–122. [Google Scholar] [CrossRef]
- Voglar, G.E.; Leštan, D. Equilibrium leaching of toxic elements from cement stabilized soil. J. Hazard. Mater. 2013, 246, 18–25. [Google Scholar] [CrossRef]
- Kraszewski, C.; Dreger, M.; Mitrut, M.; Przygoda, M. Stabilizacja gruntów wapnem palonym w inżynierii komunikacyjnej-teoria i praktyka. Inżynieria Morska I Geotech. 2020, 1, 82–86. [Google Scholar]
- Ma, Y.; Chen, W. Study on the Mechanism of Stabilizing Loess with Lime: Analysis of Mineral and Microstructure Evolution. Adv. Civ. Eng. 2021, 2021, 6641496. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Jin, F.; Al-Tabbaa, A. The performance of blended conventional and novel binders in the in-situ stabilisation/solidification of a contaminated site soil. J. Hazard. Mater. 2015, 285, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.H.; Dermatas, D. An evaluation of lead leachability from stabilized/solidified soils under modified semi-dynamic leaching conditions. Eng. Geol. 2006, 85, 67–74. [Google Scholar] [CrossRef]
- Vandaperre, L.J.; Liska, M.; Al-Tabbaa, A. Reactive magnesium oxide cements: Properties and applications. In Proceedings of the Sustainable Construction Materials and Technologies-International Conference on Sustainable Construction Materials and Technologies; CRC Press: Coventry, UK, 2007; pp. 397–410. [Google Scholar]
- Al-Tabbaa, A. Reactive magnesia cement. Eco-Efficient Concr. 2013, 2013, 523–543. [Google Scholar] [CrossRef]
- Wang, L.; Yeung, T.L.K.; Lau, A.Y.T.; Tsang, D.C.W.; Poon, C.S. Recycling contaminated sediment into eco-friendly paving blocks by a combination of binary cement and carbon dioxide curing. J. Clean. Prod. 2017, 164, 1279–1288. [Google Scholar] [CrossRef]
- Yi, Y.; Lu, K.; Liu, S.; Al-Tabbaa, A. Properties change of reactive magnesia-stabilised soil subjected to forced carbonation. Can. Geotech. J. 2016, 53, 314–325. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Unluer, C.; Al-Tabbaa, A. Carbonating magnesia for soil stabilization. Can. Geotech. J. 2013, 50, 899–905. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Resistance of alkali-activated slag concrete to acid attack. Cem. Concr. Res. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Guo, B.; Pan, D.; Liu, B.; Volinsky, A.A.; Fincan, M.; Du, J.; Zhang, S. Immobilization mechanism of Pb in fly ash-based geopolymer. Constr. Build. Mater. 2017, 134, 123–130. [Google Scholar] [CrossRef]
- Nikolić, V.; Komljenović, M.; Marjanović, N.; Baščarević, Z.; Petrović, R. Lead immobilization by geopolymers based on mechanically activated fly ash. Ceram. Int. 2014, 40, 8479–8488. [Google Scholar] [CrossRef]
- Ji, Z.; Pei, Y. Bibliographic and visualized analysis of geopolymer research and its application in heavy metal immobilization: A review. J. Environ. Manag. 2019, 231, 256–267. [Google Scholar] [CrossRef]
- Liu, F.; Tang, R.; Wang, B.; Yuan, X. Experimental study on solidification of PB2+ in fly ash-based geopolymers. Sustainability 2021, 13, 12621. [Google Scholar] [CrossRef]
- Luna Galiano, Y.; Fernández Pereira, C.; Vale, J. Stabilization/solidification of a municipal solid waste incineration residue using fly ash-based geopolymers. J. Hazard. Mater. 2011, 185, 373–381. [Google Scholar] [CrossRef]
- Mao, Y.; Muhammad, F.; Yu, L.; Xia, M.; Huang, X.; Jiao, B.; Shiau, Y.; Li, D. The Solidification of Lead-Zinc Smelting Slag through Bentonite Supported Alkali-Activated Slag Cementitious Material. Int. J. Environ. Res. Public Heal. 2019, 16, 1121. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.K.; Kumar, S. Influence of iron making slags on strength and microstructure of fly ash geopolymer. Constr. Build. Mater. 2013, 38, 924–930. [Google Scholar] [CrossRef]
- Guzmán-Carrillo, H.R.; Gasca-Tirado, J.R.; López-Romero, J.M.; Apátiga-Castro Luis, M.; Rivera-Muñoz Eric, M.; Pineda-Piñón, J.; Pérez-Bueno, J.J.; Feregrino-Montes, C.; López-Naranjo, E.J.; Manzano-Ramírez, A. Encapsulation of toxic heavy metals from waste CRT using calcined kaolin base-geopolymer. Mater. Chem. Phys. 2021, 257, 123745. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2021, 283, 119173. [Google Scholar] [CrossRef]
- Napia, C.; Sinsiri, T.; Jaturapitakkul, C.; Chindaprasirt, P. Leaching of heavy metals from solidified waste using Portland cement and zeolite as a binder. Waste Manag. 2012, 32, 1459–1467. [Google Scholar] [CrossRef]
- Feng, N.; Xing, F.; Leng, F.-G. Zeolite ceramsite cellular concrete. Mag. Concr. Res. 2000, 52, 117–122. [Google Scholar] [CrossRef]
- Allagoa, M. Solidification/Stabilisation of contaminated site soil: Field versus Laboratory results. Appl. Sci. 2011, 11, 7474. [Google Scholar]
- Antemir, A.; Hills, C.D.; Carey, P.J.; Magnié, M.C.; Polettini, A. Investigation of 4-year-old stabilised/solidified and accelerated carbonated contaminated soil. J. Hazard. Mater. 2010, 181, 543–555. [Google Scholar] [CrossRef]
- Li, W.; Ni, P.; Yi, Y. Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci. Total Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef]
- ASTM D4219-08; Standard Test Method for Unconfined Compressive Strength Index of Chemical-Grouted Soils. ASTM International: Philadelphia, PA, USA, 2008.
- Jalal, F.E.; Mulk, S.; Memon, S.A.; Jamhiri, B.; Naseem, A. Strength, Hydraulic, and Microstructural Characteristics of Expansive Soils Incorporating Marble Dust and Rice Husk Ash. Adv. Civ. Eng. 2021, 2021. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Prohibition on the Disposal of Bulk Liquid Hazardous Waste in LandfillseStatutory Interpretive Guidance; EPA: Washington, DC, USA, 1996. [Google Scholar]
- US EPA, E. Method 1311: Toxicity characteristic leaching procedure; United States Environmental Protection Agency: Washington, DC, USA, 1992; Volume 1–35, pp. 1–35. [Google Scholar]
- Sima, J.; Cao, X.; Zhao, L.; Luo, Q. Toxicity characteristic leaching procedure over- or underestimates leachability of lead in phosphate-amended contaminated soils. Chemosphere 2015, 138, 744–750. [Google Scholar] [CrossRef]
- US EPA, E. Method 1312: Synthetic Precipitation Leaching Procedure. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846; United States Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- ASTM C1308-08; Standard Test Method for Accelerated Leach Test for Diffusive Releases from Solidified Waste and a Computer Program to Model Diffusive, Fractional Leaching from Cylindrical Waste Forms. ASTM International: West Conshohocken, PA, USA, 1992.
- US EPA (United States Environmental Protection Agency). US EPA Method 1315. Mass Transfer Rates of Constituents in Monolithic or Compacted Granular Materials Using a Semi-dynamic Tank Leaching Procedure; US EPA (United States Environmental Protection Agency): Washington, DC, USA, 2013. [Google Scholar]
- ASTM D 5233-92 (Reapproved 1999); Standard Test Method for Single Batch Extraction Method for Wastes. Annual Book of ASTM Standards. American Society for Testing and Materials: Philadelphia, PA, USA, 1999.
- Davidson, C.M.; Duncan, A.L.; Littlejohn, D.; Ure, A.M.; Garden, L.M. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal. Chim. Acta 1998, 363, 45–55. [Google Scholar] [CrossRef]
- Leiva, C.; Arroyo, F.; Villegas, R.; Vilches, L.F. Immobilization of heavy metals (Cd, Ni or Pb) using aluminate geopolymers. Mater. Lett. 2018, 227, 184–186. [Google Scholar] [CrossRef]
- Boardman, D.I. Lime Stabilisation: Clay-Metal-Lime Interactions; Electronic Theses Online Service: London, UK, 1999. [Google Scholar]
- Wilk, C.M. Stabilization of Heavy Metals with Portland Cement; Portland Cement Association: Skokie, IL, USA, 1997. [Google Scholar]
- Wei, X.; Li, Z. Early hydration process of portland cement paste by electrical measurement. J. Mater. Civ. Eng. 2006, 18, 99–105. [Google Scholar] [CrossRef]
- Damasceno, V.M.; Fratta, D.; Bosscher, P.J. Development and validation of a low-cost electrical resistivity tomographer for soil process monitoring. Can. Geotech. J. 2009, 46, 842–854. [Google Scholar] [CrossRef]
- Giao, P.H.; Chung, S.G.; Kim, D.Y.; Tanaka, H. Electric imaging and laboratory resistivity testing for geotechnical investigation of Pusan clay deposits. J. Appl. Geophys. 2003, 52, 157–175. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Mehdizadeh, H. Estimation of Osmotic Suction and Electrical Conductivity in Unsaturated Soils Using Filter Paper Method. Geotech. Test. J. 2008, 31, 142–148. [Google Scholar] [CrossRef]
- Liu, S.Y.; Du, Y.J.; Han, L.H.; Gu, M.F. Experimental study on the electrical resistivity of soil–cement admixtures. Environ. Geol. 2008, 54, 1227–1233. [Google Scholar] [CrossRef]
- Liu, J.; Zha, F.; Xu, L.; Kang, B.; Tan, X.; Deng, Y.; Yang, C. Mechanism of stabilized/solidified heavy metal contaminated soils with cement-fly ash based on electrical resistivity measurements. Meas. J. Int. Meas. Confed. 2019, 141, 85–94. [Google Scholar] [CrossRef]
- Liu, J.; Xu, D.; Xiong, L.; Hills, C.; Carey, P.; Gardner, K. Comparison of properties of traditional and accelerated carbonated solidified/stabilized contaminated soils. J. Environ. Sci. 2008, 20, 593–598. [Google Scholar] [CrossRef]
- ASTM D4843-88; Standard Test Method for Wetting and Drying Test of Solid Wastes. ASTM International: West Conshohocken, PA, USA, 2016.
- Yi, Y.; Li, C.; Liu, S.; Jin, F. Magnesium sulfate attack on clays stabilised by carbide slag- and magnesia-ground granulated blast furnace slag. Geotech. Lett. 2015, 5, 306–312. [Google Scholar] [CrossRef]
- Du, Y.-J.; Jiang, N.-J.; Shen, S.-L.; Jin, F. Experimental investigation of influence of acid rain on leaching and hydraulic characteristics of cement-based solidified/stabilized lead contaminated clay. J. Hazard. Mater. 2012, 225, 195–201. [Google Scholar] [CrossRef] [PubMed]
- ASTM D560-03; Standard Test Methods for Freezing and Thawing Compacted Soil-Cement Mixtures. ASTM International: West Conshohocken, PA, USA, 2003.
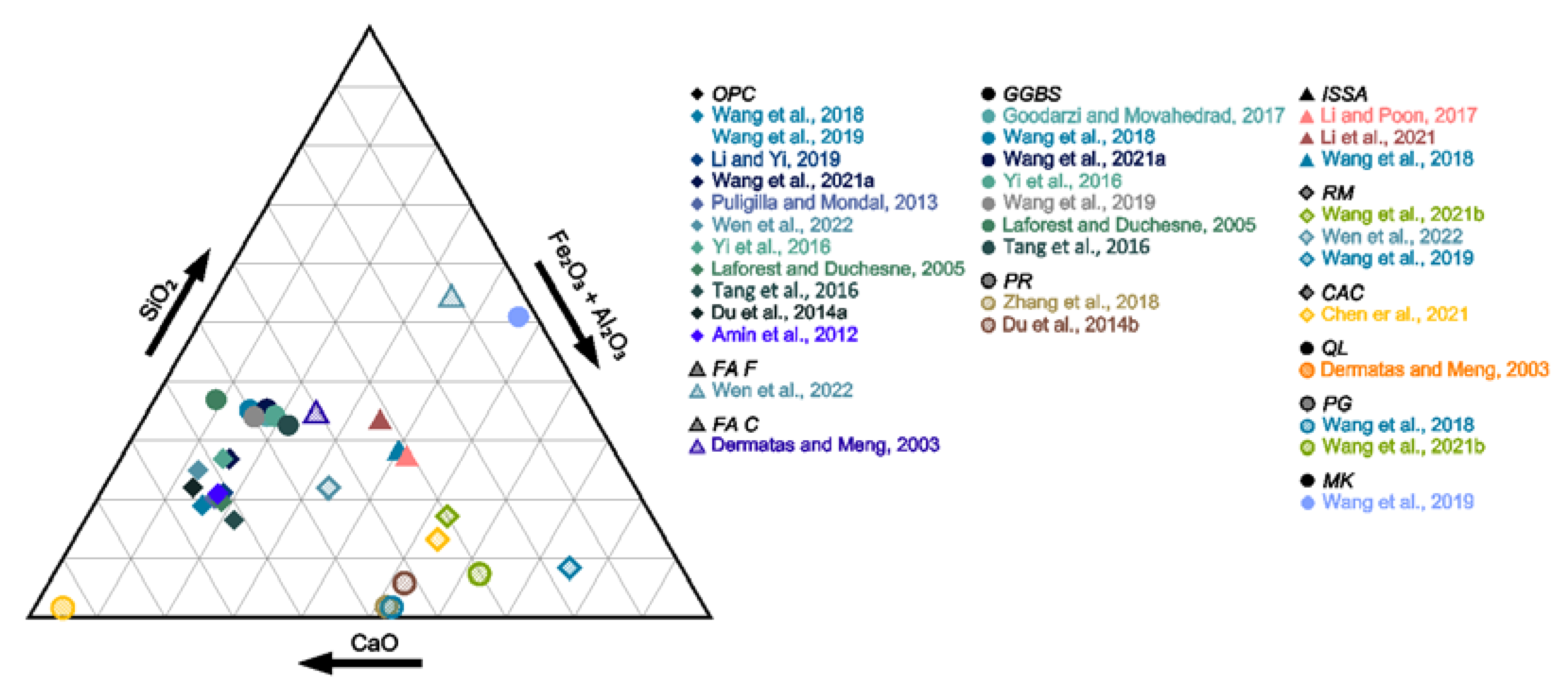
| Binder/Additive | SiO2 | Al2O3 | SO3 | CaO | TiO2 | Fe2O3 | MgO | K2O | P2O5 | Na2O | CO2 | MnO | F | LOI | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 18.99–27.4 | 4.41–11.5 | 2.25–4.52 | 46.6–65.72 | 0.17–0.51 | 2.31–4.03 | 1.02–3.71 | 0.24–1.31 | – | 0.08–0.23 | 0.17–0.48 | – | 0.05–0.06 | – | 0–6.19 | [31,37,57,58,59,60,61,62,63,64,65] |
| GGBS | 32.7–36.77 | 7.77–29.43 | 1.46–2.37 | 31.49–40 | 0.36–1.63 | 0.31–5.54 | 5.5–13.91 | 0.43–0.85 | – | −0.01–0.04 | 0–0.36 | – | 0.28–1.02 | – | −1.49–2.67 | [29,31,57,60,61,62,63,66] |
| ISSA | 27.24–31.7 | 13.72–17.2 | 2.07–3.45 | 6.34–10.96 | 0.7–5.04 | 17.8–27.35 | 2.9–3.52 | 2.0–2.77 | – | 9.23–12.28 | 4.4–6.52 | – | – | – | nt–0.99 | [28,31,67] |
| Class F FA | 53.97 | 31.15 | 0.727 | 4.01 | – | 4.16 | 1.01 | 2.04 | – | 0.89 | – | – | – | – | [59] | |
| Class C FA | 34.2 | 19.3 | 2.2 | 25.8 | – | 5.64 | 5.07 | 0.52 | – | 2.4 | – | – | – | 0.11 | [68] | |
| PR | 1.15–6.14 | 0.27–1.23 | – | 45.93–48.4 | – | nt–0.16 | nt–6.96 | – | – | 21.9 *–25.10 | – | nt–13 | – | 2.23–2.41 | nt–13.12 | [66,69] |
| PG | 1.26–8.8 | nt–0.72 | 39.64–55.3 | 37.5–47.05 | – | – | nt–0.32 | nt–0.32 | – | nt–0.03 | nt–10.03 | – | – | – | – | [31,50] |
| RM | 9.11–21.43 | 4.57–26.1 | nt-0.67 | nt–45.15 | nt–3.98 | 9.98–59.37 | nt–0.33 | nt–1.56 | – | nt–0.37 | nt–11.51 | – | 0.2–6 | – | nt–13.41 | [12,50,59,61] |
| CAC | 7.38 | 52.9 | 0.31 | 34.1 | 2.23 | 1.83 | 0.37 | 0.45 | – | 0.19 | – | – | – | – | – | [70] |
| QL | 1.2 | – | 0.012 | 95.4 | – | – | 0.85 | – | – | – | – | – | – | 0.55 | [68] | |
| MgO | 0.9–1.1 | 0.12–0.41 | 0.05–0.28 | 0.5–1.39 | – | 0.03–0.7 | 95.8–89.5 | 0.01–3.57 | – | – | – | – | nt–0.02 | – | 0–2.76 | [29,37,54,55,57] |
| MK | 49.55–50.30 | 35.22–47 | 0.05–0.59 | 0.18–0.2 | – | 0.52–1.05 | 0–0.36 | 0.19–0.28 | – | nt–0.28 | 0–0.01 | – | – | – | nt–4.23 | [61,65] |
| Zeolite | 69.96 | 13.61 | – | 3.61 | 0.02 | 1.38 | 0.51 | 1.79 | – | 1.61 | – | 0.03 | – | 7.47 | [71] | |
| CaO | – | – | – | 98.9 | – | – | 0.2 | – | – | nt | – | – | – | – | – | [72] |
| No | Symbol * | S/W | Binder/Additive | WC | Heavy Metal | UCS | Leaching | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [%] | [%] | [%] | Type | [mg/kg] | [MPa] | mg/L | |||||
| 1 | PC | GGBS | ISSA | 20 | Pb | 1941 | TCLP | [31] | |||
| S/PC | 50 | 50 | – | – | 35 | 6.26 | |||||
| S/BC | 50 | 25 | 25 | – | 49 | 0.168 | |||||
| Pb/ISSA = 1:2 | 50 | 18.10 | 18.10 | 13.8 | 45 | 0.055 | |||||
| 2 | SC | OPC | ISSA | 24–50% | Ba | 168,000 | Leachate pH | [32] | |||
| C1 | 80 | 20 | – | – | 7.9 | 2.2 | |||||
| H3 | 50 | – | 37.5 | 12.5 | 8.6 | 4.3 | |||||
| 3 | CAC/OPC/GGBS | TWEEN 80 | BENTONIT | 22 | Pb | 96.7 | AFNOR NF X31-211 | [164] | |||
| M1 | 73 | 5/–/– | 0.025 | – | 0.65 | 0.101 | |||||
| M2 | 68 | 10/–/– | 0.05 | – | 2.0 | 0.05 | |||||
| M3 | 68 | 5/–/– | 0.05 | 5 | 0.45 | 0.197 | |||||
| M4 | 63 | 10/–/– | 0.075 | 5 | 0.95 | 0.143 | |||||
| M5 | 63 | 5/–/– | 0.075 | 10 | 0.15 | 0.290 | |||||
| M6 | 58 | 10/–/– | 0.1 | 10 | 1.1 | 0.022 | |||||
| M7 | 73 | –/3/2 | 0.025 | – | 0.4 | 0.290 | |||||
| M8 | 68 | –/6/4 | 0.05 | – | 1 | 0.068 | |||||
| M9 | 68 | –/3/2 | 0.05 | 5 | 0.22 | 0.300 | |||||
| M10 | 63 | –/6/4 | 0.075 | 5 | 0.95 | 0.105 | |||||
| M11 | 63 | –/3/2 | 0.075 | 10 | 0.25 | 0.190 | |||||
| M12 | 58 | –/6/4 | 0.1 | 10 | 1 | 0.068 | |||||
| 4 | PC | MgO | CO2 | 7.5 | Pb | Leached Pb [mg/kg] | [37] | ||||
| Pb + PC | 95 | 5 | – | – | 4000 | 2.0 | 100 | ||||
| Pb + PC | 95 | 5 | – | – | 16,000 | 0.9 | 1100 | ||||
| Pb + MgO + CO2 | 95 | – | 5 | used | 4000 | 1.9 | 8 | ||||
| Pb + MgO + CO2 | 95 | – | 5 | used | 16,000 | 1.95 | 9.8 | ||||
| 5 | Ca(OH)2 | MgO | GGBS | 40 | Zn/Pb | TCLP | [54] | ||||
| CGZn0.25 | 75 | 3.75 | – | 21.25 | 0.25 | 4.1 | 0.264 | ||||
| CGZn0.5 | 75 | 3.75 | – | 21.25 | 0.5 | 1.9 | 0.220 | ||||
| CGZn1 | 75 | 3.75 | – | 21.25 | 1 | 0.45 | 0.178 | ||||
| CGPb0.25 | 75 | 3.75 | – | 21.25 | 0.25 | 5.9 | ND | ||||
| CGPb0.5 | 75 | 3.75 | – | 21.25 | 0.5 | 7.9 | 0.072 | ||||
| CGPb1 | 75 | 3.75 | – | 21.25 | 1 | 7.9 | 0.18 | ||||
| MGZn0.25 | 75 | – | 3.75 | 21.25 | 0.25 | 4.9 | 0.091 | ||||
| MGZn0.5 | 75 | – | 3.75 | 21.25 | 0.5 | 5.0 | 0.082 | ||||
| MGZn1 | 75 | – | 3.75 | 21.25 | 1 | 3.2 | 0.076 | ||||
| MGPb0.25 | 75 | – | 3.75 | 21.25 | 0.25 | 5.0 | 0.066 | ||||
| MGPb0.5 | 75 | – | 3.75 | 21.25 | 0.5 | 5.9 | 0.062 | ||||
| MGPb1 | 75 | – | 3.75 | 21.25 | 1 | 7.1 | 0.166 | ||||
| 6 | OPC | ISSA | 20 | Pb | 5000 | SBET [mg/kg] | [28] | ||||
| H0 | 90 | 10 | – | 10 | 36 | ||||||
| H0.2 | 90 | 8 | 2 | 4.6 | 35 | ||||||
| H0.5 | 90 | 5 | 5 | 2.6 | 31.5 | ||||||
| 7 | 8 | soil | OPC | GGBS | 20 | As | 170.4 | TCLP | [41] | ||
| O5 | 96 | 4 | – | 3.2 | 0.011 | ||||||
| O4G1 | 96 | 3 | 1 | 3.7 | 0.015 | ||||||
| O2.5G2.5 | 96 | 2 | 2 | 6.0 | 0.021 | ||||||
| O10 | 92 | 8 | – | 7.5 | 0.06 | ||||||
| O8G2 | 92 | 6 | 2 | 7.8 | 0.065 | ||||||
| O5G5 | 92 | 4 | 4 | 9.5 | 0.012 | ||||||
| 8 | RM | PG | OPC | nt | Zn/Pb/Cd | 5000 | TCLP | [50] | |||
| RPPC7.5 | 92.5 | 4.3 | 1.1 | 2.1 | 0.726 | 4.2/0.8/8 | |||||
| RPPC10 | 90 | 5.7 | 1.4 | 2.9 | 1.1 | 0.8/0.3/0.9 | |||||
| RPPC15 | 85 | 8.6 | 2.2 | 4.2 | 2.013 | 0.3/0.11/0.7 | |||||
| PC10 | 90 | – | – | 10 | 3.3 | 0.4/0.9/0.8 | |||||
| 9 | CCR | PG | 16.5–17.6 | Ni/Zn | 6352/5352 | China HJ/T 299 | [33] | ||||
| 10% bin/dos | 90 | 6 | 3 | 1 | 0.46 | 0.01/0.12 | |||||
| <10%bin-dos | >90 | <6 | <3 | <1 | <0.3 | >0.06/>0.7 | |||||
| 10 | KSil | FA | KOH | Zn | 15,900 | TCLP | [183] | ||||
| KSil0.46KOH | 21 | 16.8 | 58.8 | 3.4 | 18.8 | 0.75 | 8.67 | ||||
| OPC | FA | lime | 22.2 | 2.0 | <0.001 | ||||||
| OPC lime | 35.8 | 7.1 | 50 | 7.1 | |||||||
| 11 | NCA | 13 | Pb | 10,000 | *** 7d | *** 7d | [30] | ||||
| NCA ** 10% | 90 | 10 | – | – | 4.1 | 1.21 | |||||
| NCA ** 20% | 80 | 20 | – | – | 6.32 | 0.318 | |||||
| NCA ** 30% | 70 | 30 | – | – | 10.12 | 0.075 | |||||
| NCA ** 40% | 60 | 40 | – | – | 11.16 | 0.027 | |||||
| 12 | SPC | – | – | Pb | 9710 | TCLP | [72] | ||||
| 92 | 8 | – | – | 22 | 0.352 | 1.8 | |||||
| 90 | 10 | – | – | 22 | 0.432 | 0.9 | |||||
| 13 | OPC | GGBS | – | – | As | 1985 | TCLP | [134] | |||
| O4G1 | 95 | 4 | 1 | – | – | 1.1 | 4 | ||||
| O2.5G2.5 | 95 | 2.5 | 2.5 | – | – | 1.05 | 5.3 | ||||
| 14 | OPC | CaO | MgO | 7.5 | Pb | 16,000 | SBLT | [192] | |||
| Pb + OPC | 95 | 5 | – | – | 1.0 | 1000 | |||||
| Pb + CaO | 95 | – | 5 | – | 0.18 | 8000 | |||||
| Pb + MgO | 95 | – | – | 5 | 0.05 | 2 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lal, A.; Fronczyk, J. Does Current Knowledge Give a Variety of Possibilities for the Stabilization/Solidification of Soil Contaminated with Heavy Metals?—A Review. Materials 2022, 15, 8491. https://doi.org/10.3390/ma15238491
Lal A, Fronczyk J. Does Current Knowledge Give a Variety of Possibilities for the Stabilization/Solidification of Soil Contaminated with Heavy Metals?—A Review. Materials. 2022; 15(23):8491. https://doi.org/10.3390/ma15238491
Chicago/Turabian StyleLal, Agnieszka, and Joanna Fronczyk. 2022. "Does Current Knowledge Give a Variety of Possibilities for the Stabilization/Solidification of Soil Contaminated with Heavy Metals?—A Review" Materials 15, no. 23: 8491. https://doi.org/10.3390/ma15238491