Study on Effects of Refining Slag on Properties and Hydration of Cemented Solid Waste-Based Backfill
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Cementitious Material
2.1.2. Tailings Sand
2.2. Specimens Preparation
2.3. Methods
2.3.1. Macroscopic Properties Test
2.3.2. Microscopic Characterization
3. Results and Discussion
3.1. Single-Factor Test Results of RS Content
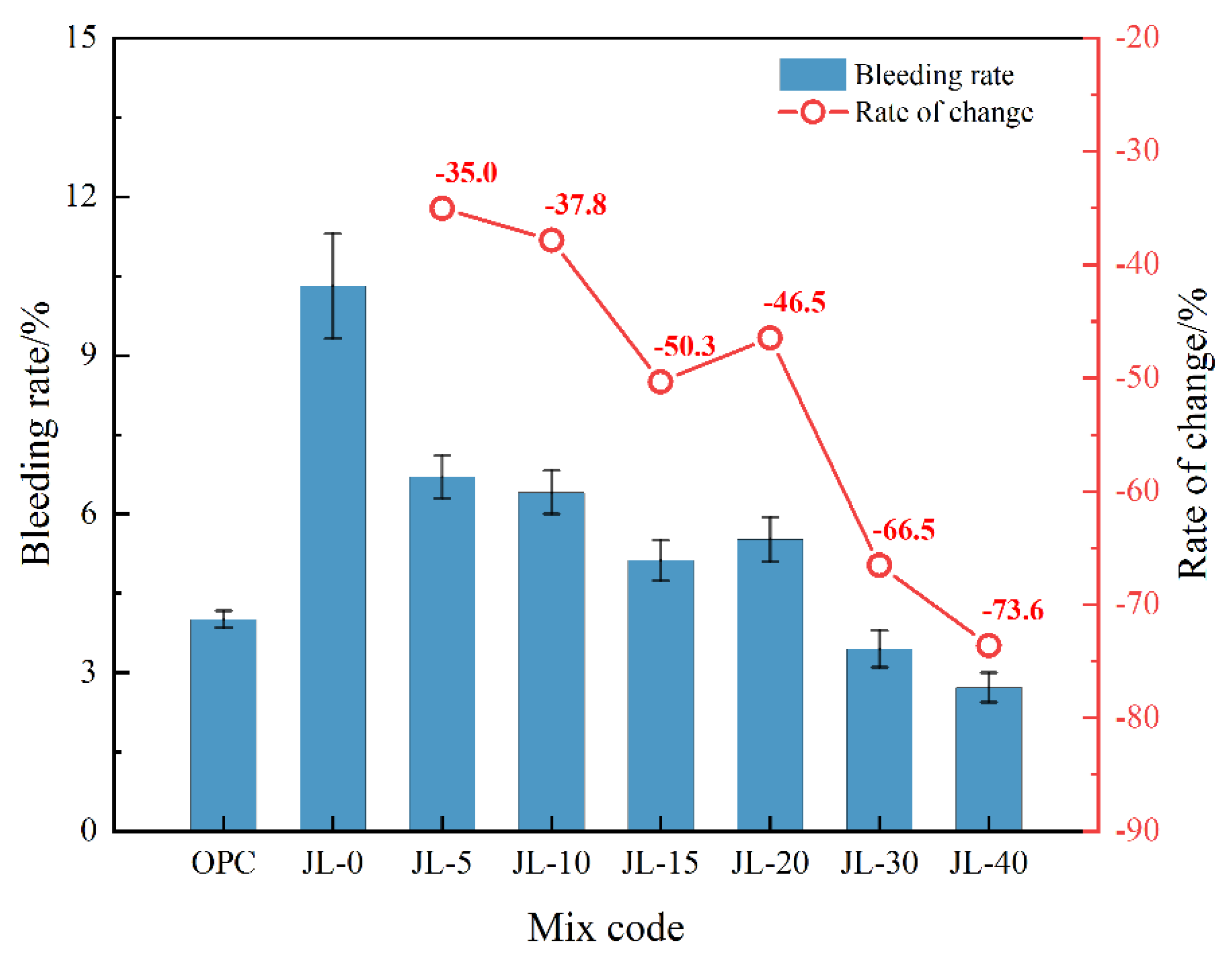
3.1.1. Bleeding Rate of Backfill Slurry
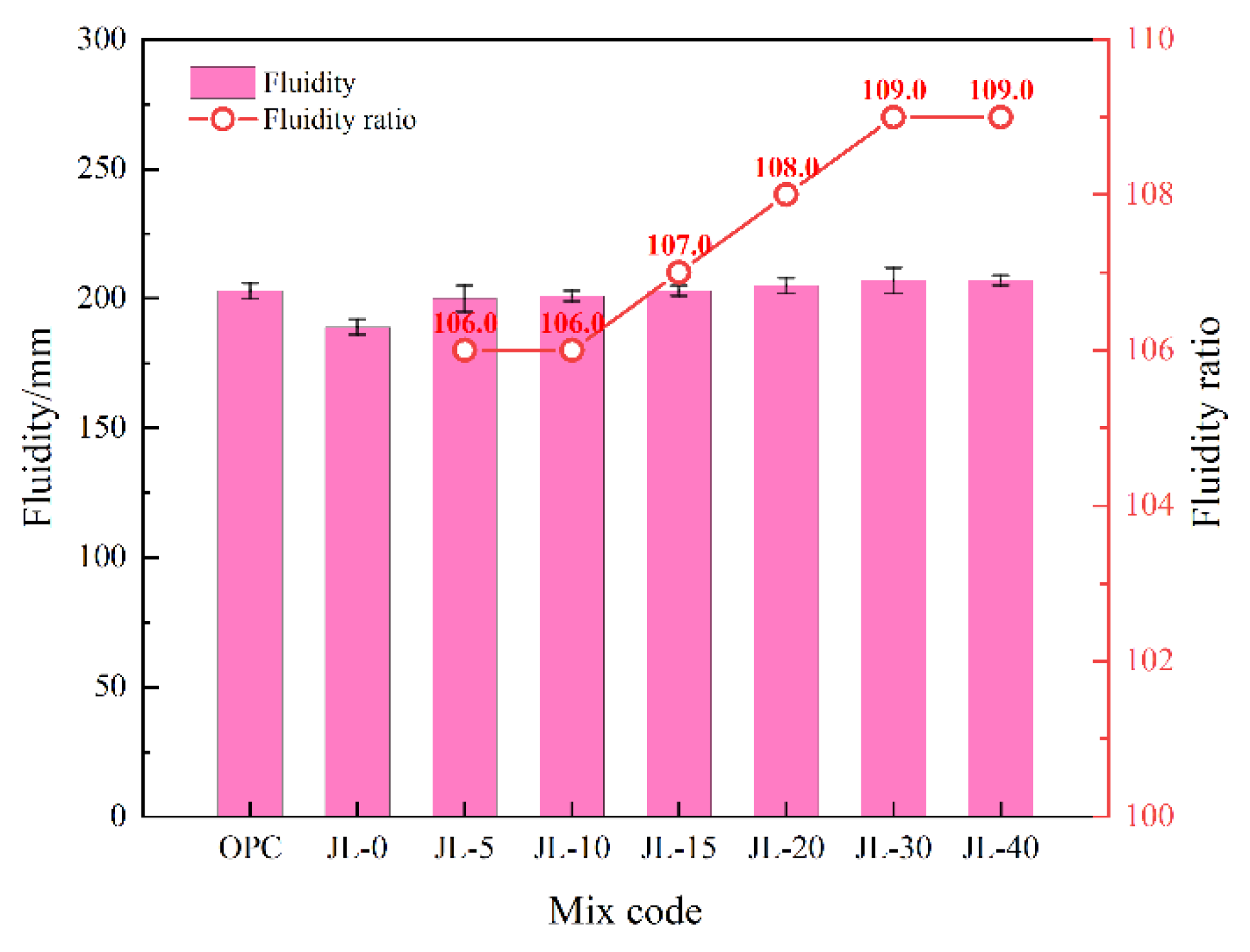
3.1.2. Fluidity of Backfill Slurry
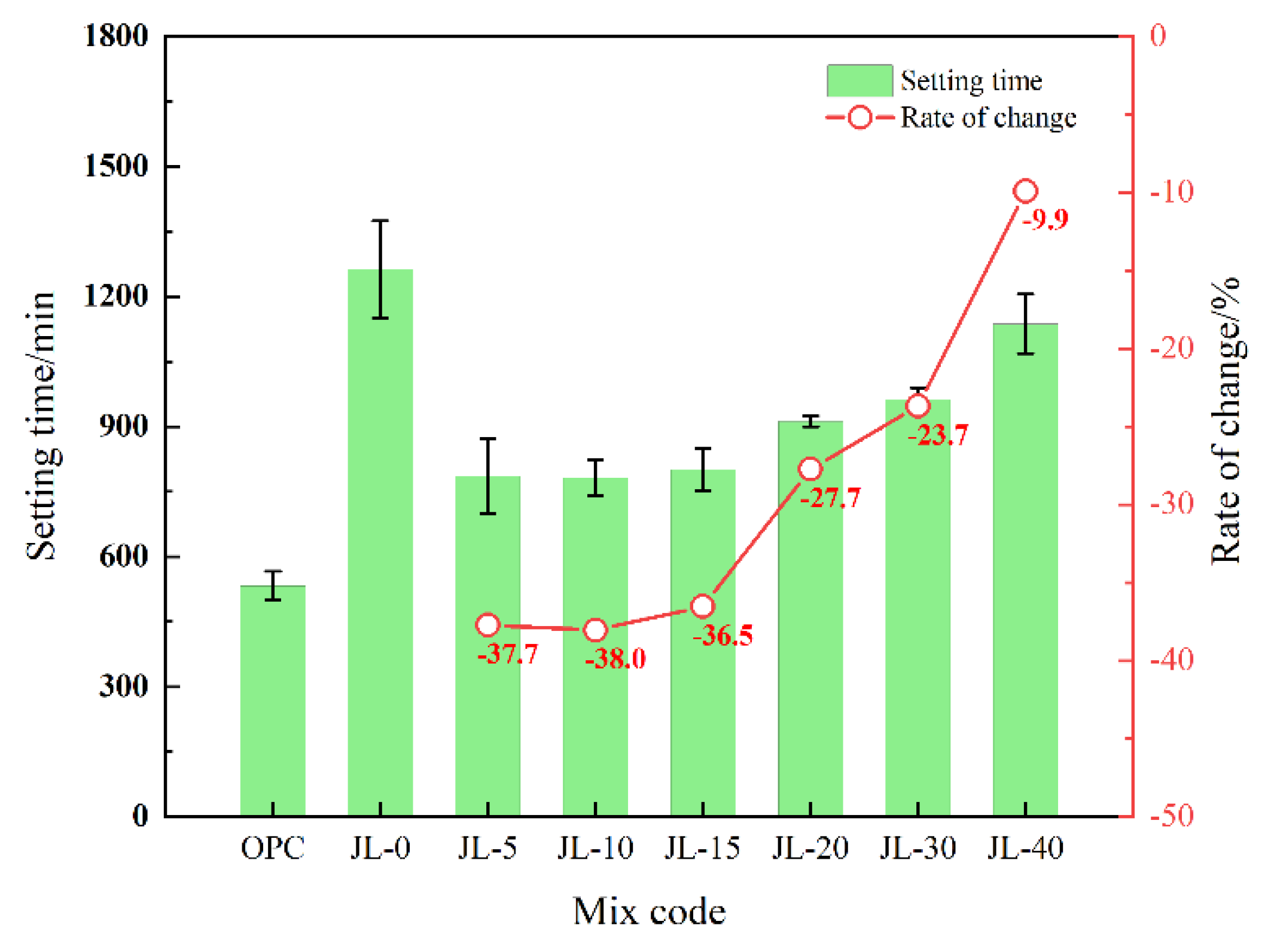
3.1.3. Setting Time of Backfill
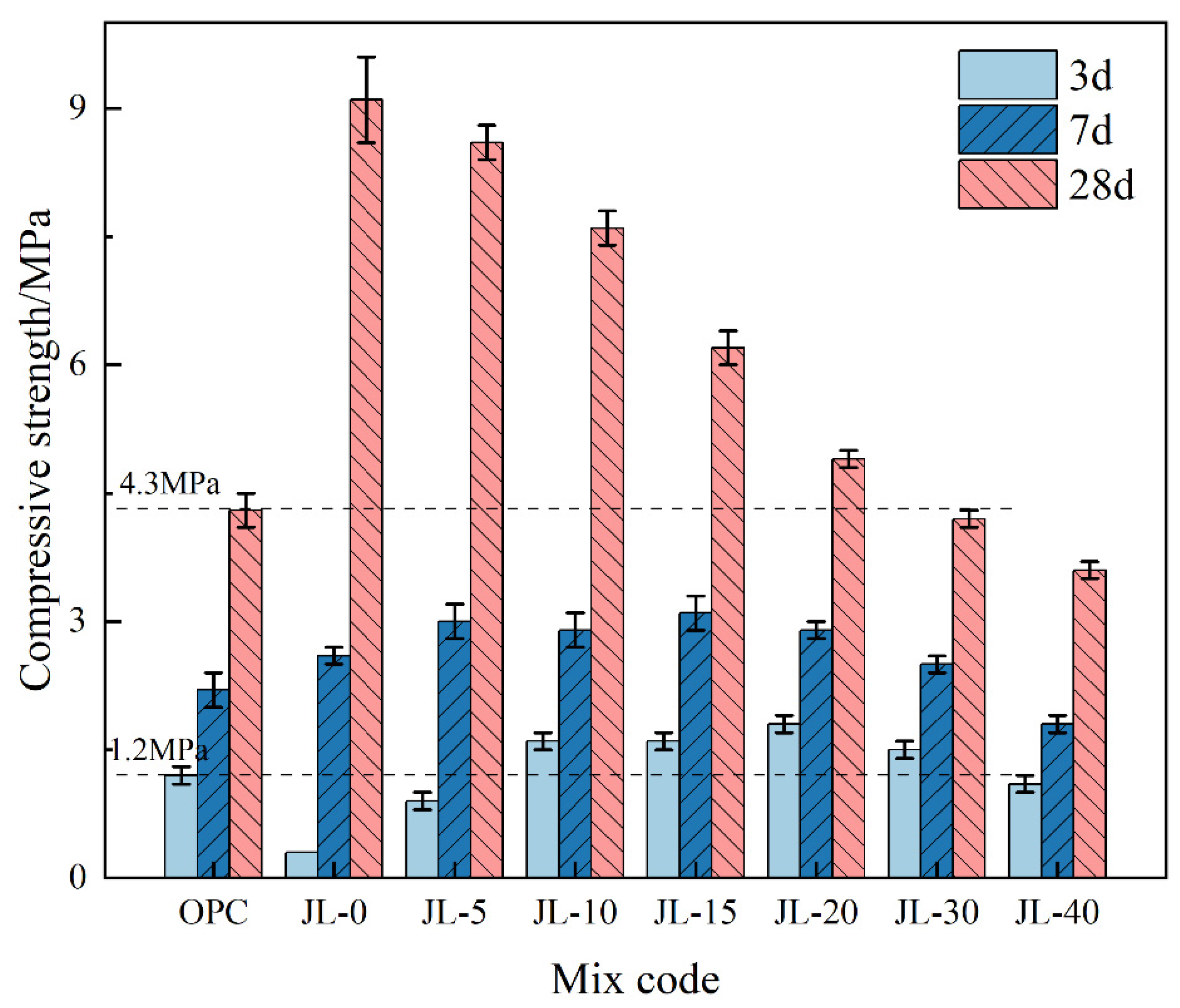
3.1.4. Compressive Strength of Backfill
3.2. Micro-Analysis Results of Solid Waste-Based Cementitious Materials with Different RS Contents
3.2.1. XRD Analysis
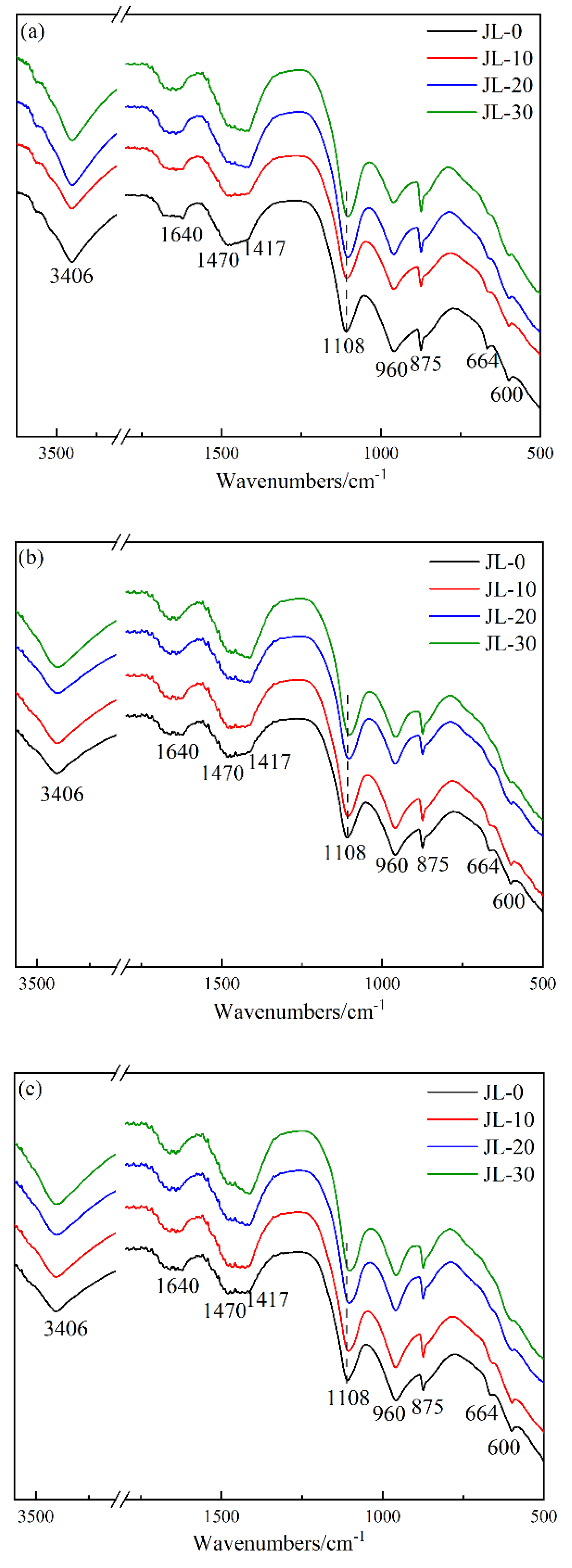
3.2.2. FT-IR Analysis
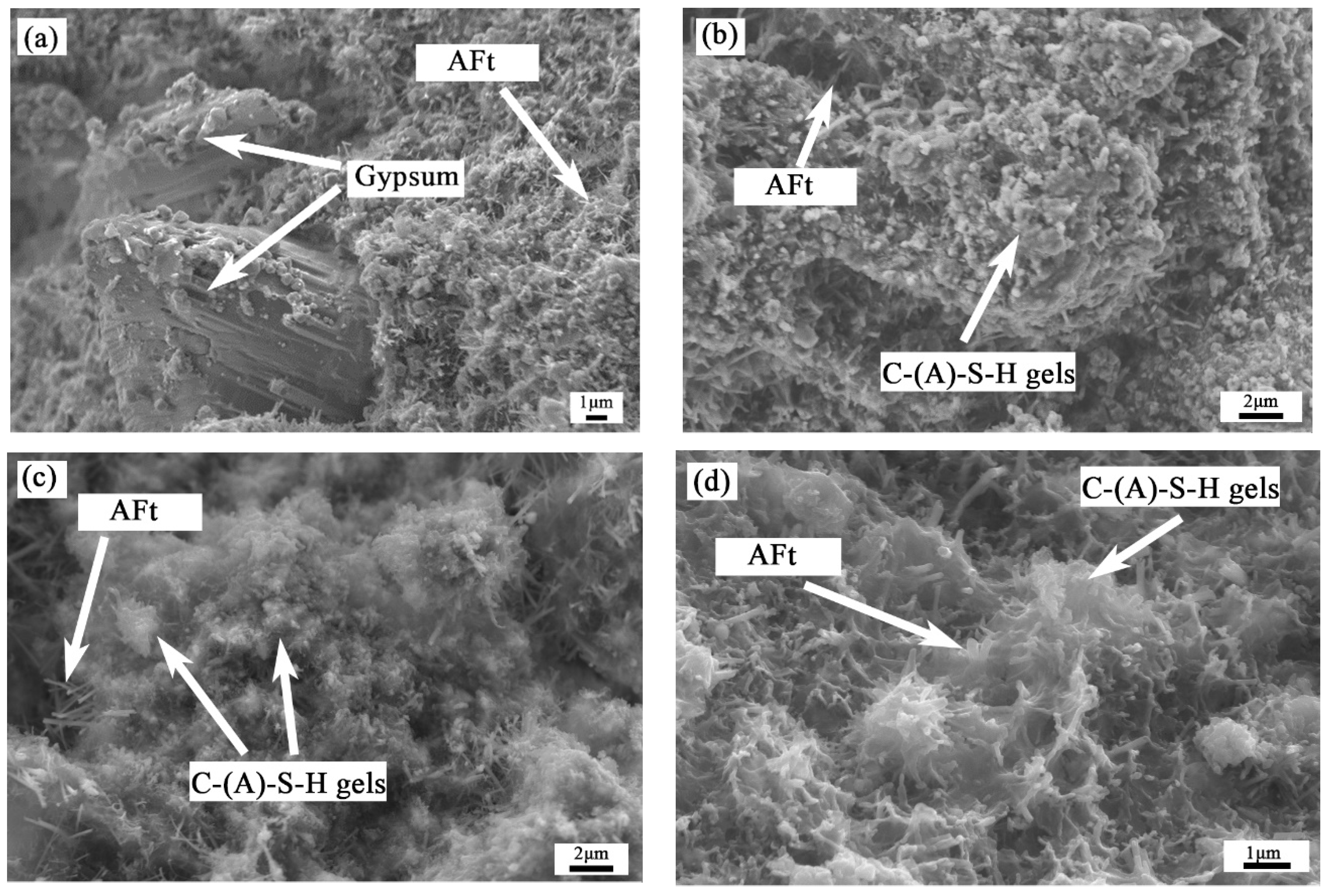
3.2.3. SEM Analysis
3.2.4. TG-DSC Analysis
- (1)
- The area of the endothermic peak of gypsum gradually decreases in all samples and completely disappears in the JL-20 sample, indicating that the addition of RS increases the consumption of desulfurized gypsum;
- (2)
- For the DSC curve of JL-20, there is a widened endothermic decomposition peak of C3AH6 at 364 °C after hydration for 3 d, indicating that the hydration of RS generates the intermediate product C3AH6. When the RS content is 20%, the generated C3AH6 is not entirely consumed. However, this endothermic decomposition peak is not found on the curve after hydration for 28 days, indicating that C3AH6 is wholly consumed at that time.
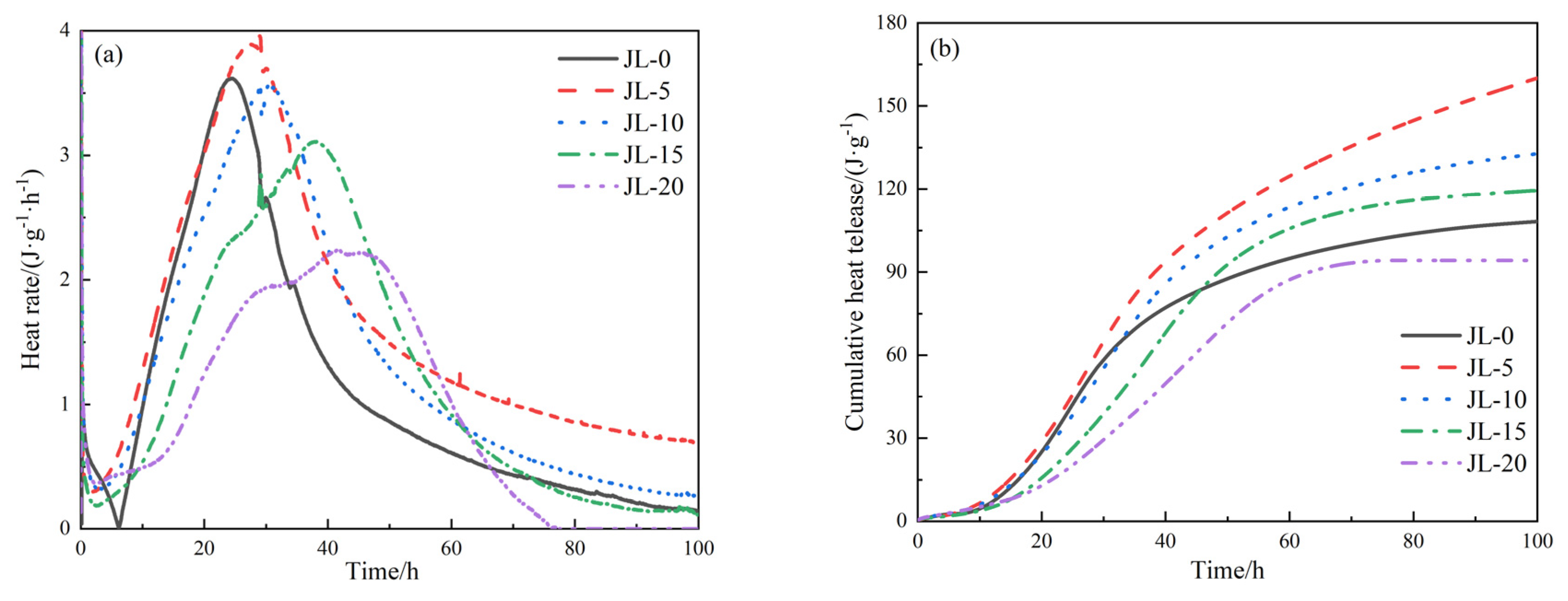
3.2.5. Heat of Hydration
4. Discussion
4.1. Hydration Mechanism of Solid Waste-Based RS-GGBS-SS-DG Quaternary Cementitious Materials
- (1)
- Dissolution of raw materials
- (2)
- Formation of hydration products
- (3)
- Cementation and condensation of hydration products
4.2. Effects of RS on Properties of Cemented Solid Waste-Based Backfill
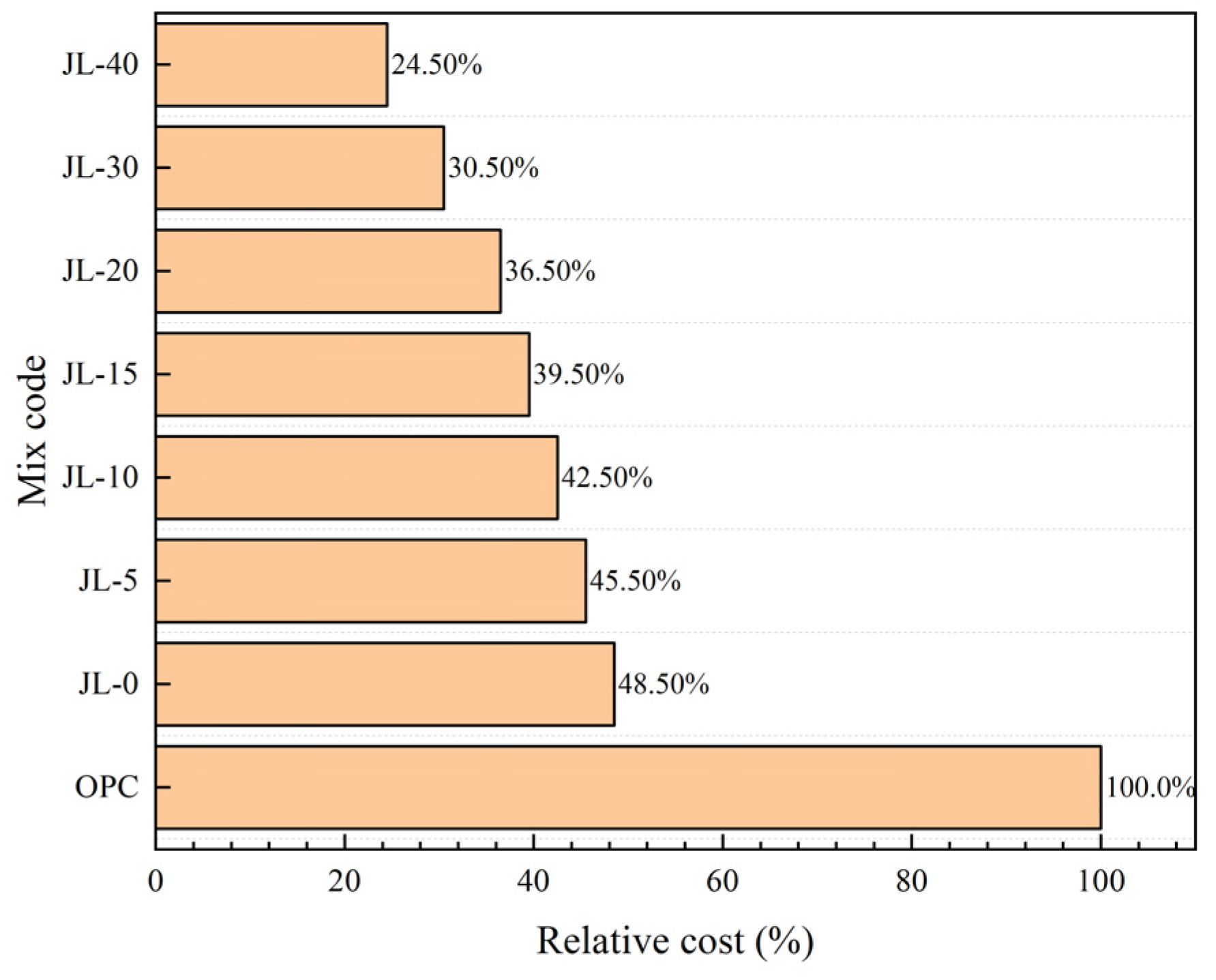
4.3. Cost Analysis of Solid Waste-Based Cementitious Material
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cao, H.; Gao, Q.; Zhang, X.; Guo, B. Research Progress and Development Direction of Filling Cementing Materials for Filling Mining in Iron Mines of China. Gels 2022, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, X.; Qiao, L.; Zhang, S.; Su, K.; Qiu, Z.; Li, X.; Zhao, Q.; Yu, C. Study On the Spatial Distribution of Ureolytic Microorganisms in Farmland Soil Around Tailings with Different Heavy Metal Pollution. Sci. Total Environ. 2021, 775, 144946. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yilmaz, E.; Cao, S. Influence of Industrial Solid Waste as Filling Material On Mechanical and Microstructural Characteristics of Cementitious Backfills. Constr. Build. Mater. 2021, 299, 124288. [Google Scholar] [CrossRef]
- Qi, W.; Ren, Q.; Zhao, Q.; Feng, Y.; Qi, W.; Han, Y.; Huang, Y. Effectiveness of Soda Residue-Activated Ggbs as Alternative Binder On Compressive Strength and Workability of Cemented Paste Backfills: Reuse of Multi-Source Solid Wastes. Constr. Build. Mater. 2022, 348, 128594. [Google Scholar] [CrossRef]
- Zhang, M.; Li, K.; Ni, W.; Zhang, S.; Liu, Z.; Wang, K.; Wei, X.; Yu, Y. Preparation of Mine Backfilling From Steel Slag-Based Non-Clinker Combined with Ultra-Fine Tailing. Constr. Build. Mater. 2022, 320, 126248. [Google Scholar] [CrossRef]
- Behera, S.K.; Mishra, D.P.; Singh, P.; Mishra, K.; Mandal, S.K.; Ghosh, C.N.; Kumar, R.; Mandal, P.K. Utilization of Mill Tailings, Fly Ash and Slag as Mine Paste Backfill Material: Review and Future Perspective. Constr. Build. Mater. 2021, 309, 125120. [Google Scholar] [CrossRef]
- Chen, S.; Du, Z.; Zhang, Z.; Yin, D.; Feng, F.; Ma, J. Effects of Red Mud Additions On Gangue-Cemented Paste Backfill Properties. Powder Technol. 2020, 367, 833–840. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Sun, Q.; Liu, L.; Yang, J.; Xue, H. Preparation of Cemented Oil Shale Residue-Steel Slag-Ground Granulated Blast Furnace Slag Backfill and its Environmental Impact. Materials 2021, 14, 2052. [Google Scholar] [CrossRef]
- Cavusoglu, I.; Yilmaz, E.; Yilmaz, A.O. Sodium Silicate Effect On Setting Properties, Strength Behavior and Microstructure of Cemented Coal Fly Ash Backfill. Powder Technol. 2021, 384, 17–28. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Li, Y.; Xu, D. Hydration Mechanism and Orthogonal Optimisation of Mix Proportion for Steel Slag–Slag-Based Clinker-Free Prefabricated Concrete. Constr. Build. Mater. 2019, 228, 117036. [Google Scholar] [CrossRef]
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Li, K. Ammonia-Soda Residue and Metallurgical Slags From Iron and Steel Industries as Cementitious Materials for Clinker-Free Concretes. J. Clean Prod. 2021, 307, 127262. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.; Ni, W. Green Concrete with Ground Granulated Blast-Furnace Slag Activated by Desulfurization Gypsum and Electric Arc Furnace Reducing Slag. J. Clean Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, W.; Li, Y.; Ba, H.; Li, N.; Ju, Y.; Zhao, B.; Jia, G.; Hu, W. The Mechanism of Hydration Reaction of Granulated Blast Furnace Slag-Steel Slag-Refining Slag-Desulfurization Gypsum-Based Clinker-Free Cementitious Materials. J. Build. Eng. 2021, 44, 103289. [Google Scholar] [CrossRef]
- Adesanya, E.; Sreenivasan, H.; Kantola, A.M.; Telkki, V.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Ladle Slag Cement—Characterization of Hydration and Conversion. Constr. Build. Mater. 2018, 193, 128–134. [Google Scholar] [CrossRef]
- Nguyen, H.; Adesanya, E.; Ohenoja, K.; Kriskova, L.; Pontikes, Y.; Kinnunen, P.; Illikainen, M. Byproduct-Based Ettringite Binde—A Synergy Between Ladle Slag and Gypsum. Constr. Build. Mater. 2019, 197, 143–151. [Google Scholar] [CrossRef]
- Baltakys, K.; Eisinas, A.; Vasiliauskiene, K.; Palou, M.T.; Dambrauskas, T. The Effect of Calcined Mayenite On the Hydration of Ordinary Portland Cement. Ceram. Int. 2022, in press. [Google Scholar]
- Du, Y.; Yi, Y.; He, B. Physicochemical Properties of Lf Refining Slag Andcomprehensive Utilization of Slag. Environ. Sci. Technol. 2019, 42, 88–94. [Google Scholar]
- Li, Y.; Wang, K.; Liang, W.; Zhang, S.; Lu, X.; Xu, C.; Ni, W. Hydration Characteristics of Lf Refining Slag From Handaniron & Steel Group. J. Cent. South Univ. (Sci. Technol.) 2021, 52, 339–349. [Google Scholar]
- Nguyen, H.; Kinnunen, P.; Carvelli, V.; Mastali, M.; Illikainen, M. Strain Hardening Polypropylene Fiber Reinforced Composite From Hydrated Ladle Slag and Gypsum. Compos. Part B Eng. 2019, 158, 328–338. [Google Scholar] [CrossRef]
- Setién, J.; Hernández, D.; González, J.J. Characterization of Ladle Furnace Basic Slag for Use as a Construction Material. Constr. Build. Mater. 2009, 23, 1788–1794. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Experimental Evaluation of Eaf Ladle Steel Slag as a Geo-Fill Material: Mineralogical, Physical & Mechanical Properties. Constr. Build. Mater. 2017, 154, 23–33. [Google Scholar]
- Adolfsson, D.; Robinson, R.; Engström, F.; Björkman, B. Influence of Mineralogy On the Hydraulic Properties of Ladle Slag. Cem. Concr. Res. 2011, 41, 865–871. [Google Scholar] [CrossRef]
- Mason, B. The Constitution of some Open-Heart Slag. J. Iron Steel Inst. 1994, 11, 69–80. [Google Scholar]
- GB/T 50080; Standard for Test Method of Performance on Ordinary Fresh Concrete. China Architecture & Building Press: Beijing, China, 2016.
- JGJ/T 70; Standard for Test Method of Performance on Building Mortar. China Architecture & Building Press: Beijing, China, 2009.
- GB/T 2419; Test Method for Fluidity of Cement Mortar. China Architecture & Building Press: Beijing, China, 2005.
- Li, Y.; Ni, W.; Li, J.; Li, N.; Chen, X.; Zhang, Y. Study On Basic Performance of Roller Steel Slag and Hot Smoldering Steel Slag. J. Harbin Inst. Technol. 2020, 52, 132–139. [Google Scholar]
- Wen, N.; Zhao, Y.; Yu, Z.; Liu, M. A Sludge and Modified Rice Husk Ash-Based Geopolymer: Synthesis and Characterization Analysis. J. Clean Prod. 2019, 226, 805–814. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Zhao, Y.; Liu, X. Hydration Characteristics and Environmental Friendly Performance of a Cementitious Material Composed of Calcium Silicate Slag. J. Hazard. Mater. 2016, 306, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.; Prashant, S.; Kumar, M. Micro-Characterisation of Alkali Activated Paste with Fly Ash-Ggbs-Metakaolin Binder System with Ambient Setting Characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of Steel Slag and Gypsum for Building Materials and Associated Reaction Mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Jia, Y.; Jin, Z. Composition and Structure of Hardened Geopolymerproducts Using Infrared Ray Analysis Methods. J. Wuhan Univ. Technol. 2005, 27, 31–34. [Google Scholar]
- Stoch, A.; Zdaniewicz, M.; Paluszkiewicz, C. The Effect of Polymethylsiloxanes On Hydration of Clinker Phases. J. Mol. Struct. 1999, 511–512, 319–325. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility Studies between N-a-S-H and C-a-S-H Gels. Study in the Ternary Diagram Na2O–Cao–Al2O3–Sio2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Trezza, M.A.; Lavat, A.E. Analysis of the System 3Cao·Al2O3–Caso4·2H2O–Caco3–H2O by Ft-Ir Spectroscopy. Cem. Concr. Res. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Sáez Del Bosque, I.F.; Medina, J.M.; Frías, M.; Sánchez De Rojas, M.I.; Medina, C. Use of Biomass-Fired Power Plant Bottom Ash as an Addition in New Blended Cements: Effect On the Structure of the C-S-H Gel Formed During Hydration. Constr. Build. Mater. 2019, 228, 117081. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Thermal Analysis to Assess Pozzolanic Activity of Calcined Kaolinitic Clays. J. Therm. Anal. Calorim. 2014, 117, 547–556. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Zhou, Q.; Peng, Z.; Qi, T.; Li, X.; Liu, G. Reaction of Tricalcium Aluminate Hexahydrate(C3Ah6) with Carbon Dioxide. J. Cent. South Univ. (Sci. Technol.) 2011, 42, 595–599. [Google Scholar]
- Li, Y.; Wu, B.; Ni, W.; Mu, X. Synergies in Early Hydration Reaction of Slag-Steel Slag-Gypsum System. J. Northeast. Univ. (Nat. Sci.) 2020, 41, 581–586. [Google Scholar]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation Mechanisms of Three Types of Industrial by-Product Gypsums On Steel Slag–Granulated Blast Furnace Slag-Based Binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Du, H.; Li, J.; Ni, W.; Xu, D.; Li, N.; Mu, X.; Hou, Y.; Li, Y.; Fu, P. Optimization of the Whole-Waste Binder Containing Molten Iron Desulfurization Slag From Kambara Reactor for Concrete Production. J. Build. Eng. 2022, 54, 104594. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Liu, X. Hydration Mechanism and Leaching Behavior of Bauxite-Calcination-Method Red Mud-Coal Gangue Based Cementitious Materials. J. Hazard. Mater. 2016, 314, 172–180. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, C.; Chen, Y. Properties and Mechanism of Hydration of Fly Ash Belite Cement Prepared From Low-Quality Fly Ash. Appl. Sci. 2020, 10, 7026. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Ettringite Formation in Concrete. Build. Sci. 2007, 23, 105–110. [Google Scholar]
- Yan, S.; Cai, A.; Zhou, J. Study On the Hydration Mechanism of C3S-C12a7-H2O and C3S-C12a7-Caso4·2H2O-H2O System and its Performances. J. Chin. Ceram. Soc. 2003, 31, 199–204. [Google Scholar]
- Zhen, G.; Lu, X.; Cheng, X.; Chen, H.; Yan, X.; Zhao, Y. Hydration Process of the Aluminate 12Cao·7Al2O3-Assisted Portland Cement-Based Solidification/Stabilization of Sewage Sludge. Constr. Build. Mater. 2012, 30, 675–681. [Google Scholar] [CrossRef]
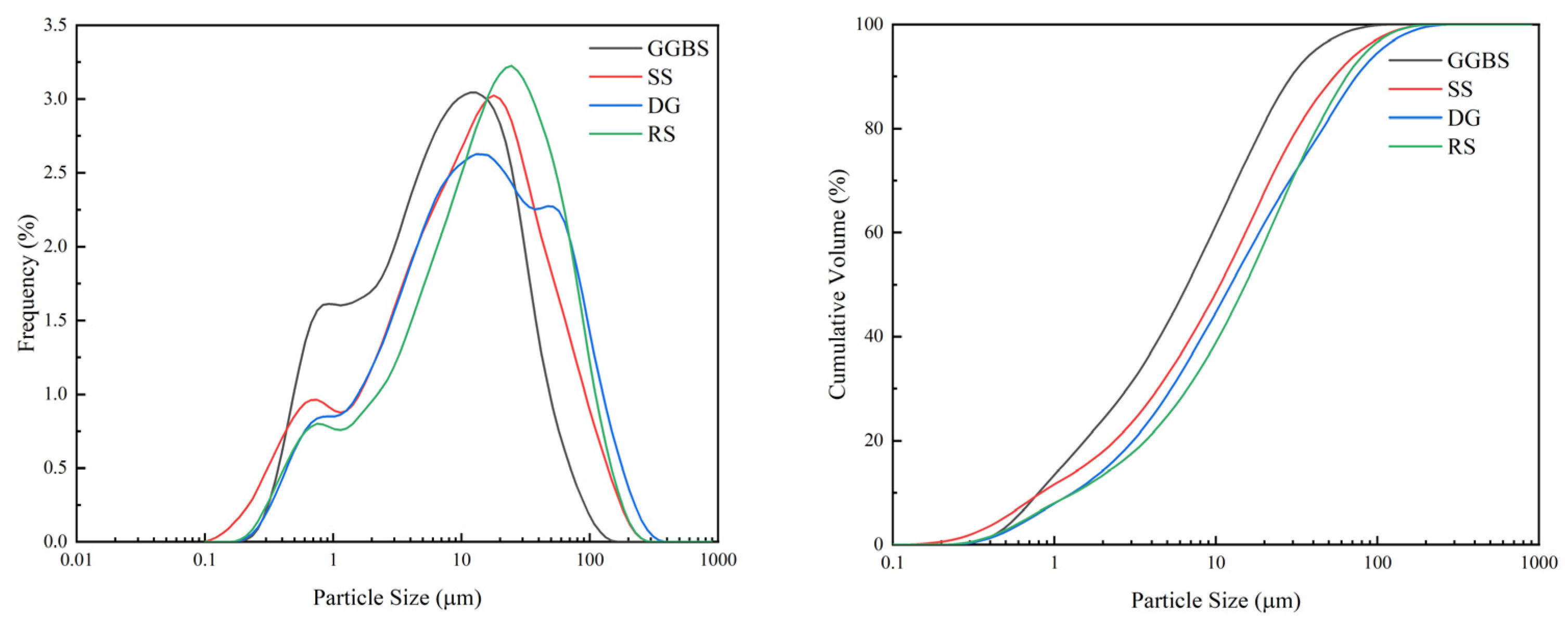
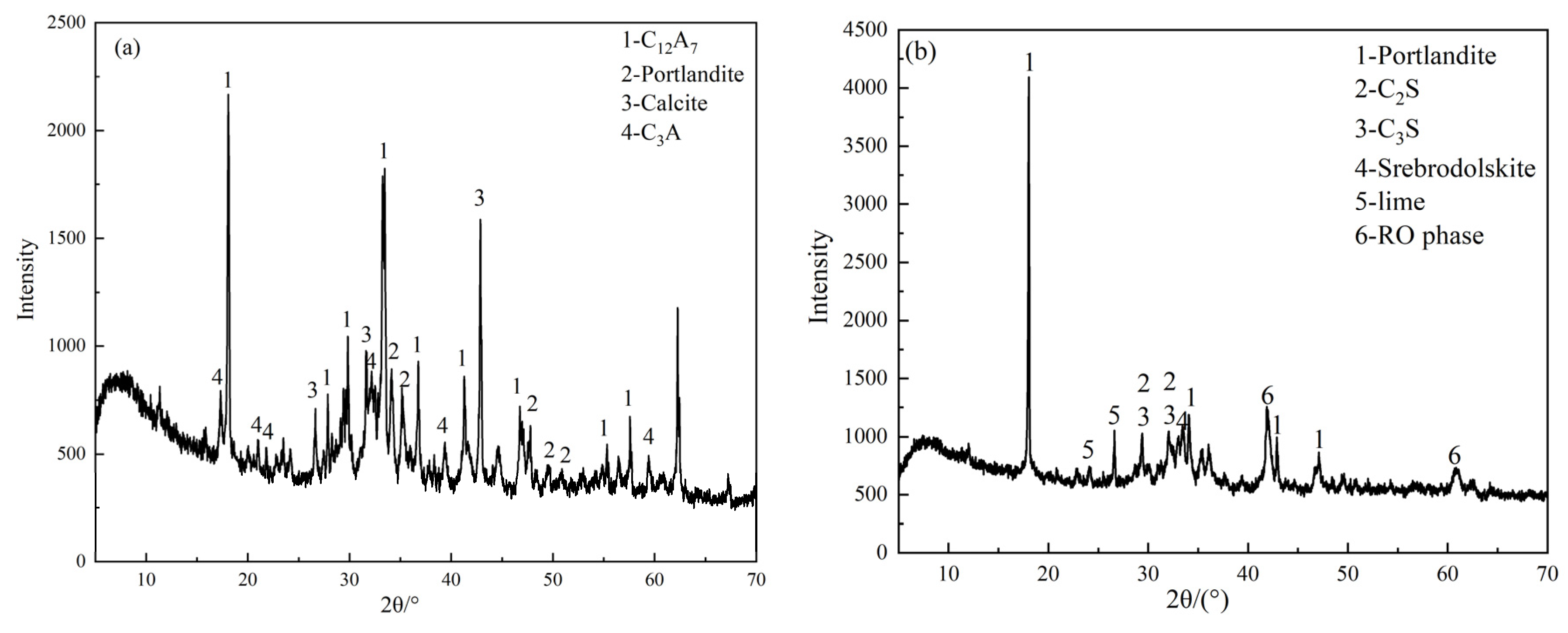

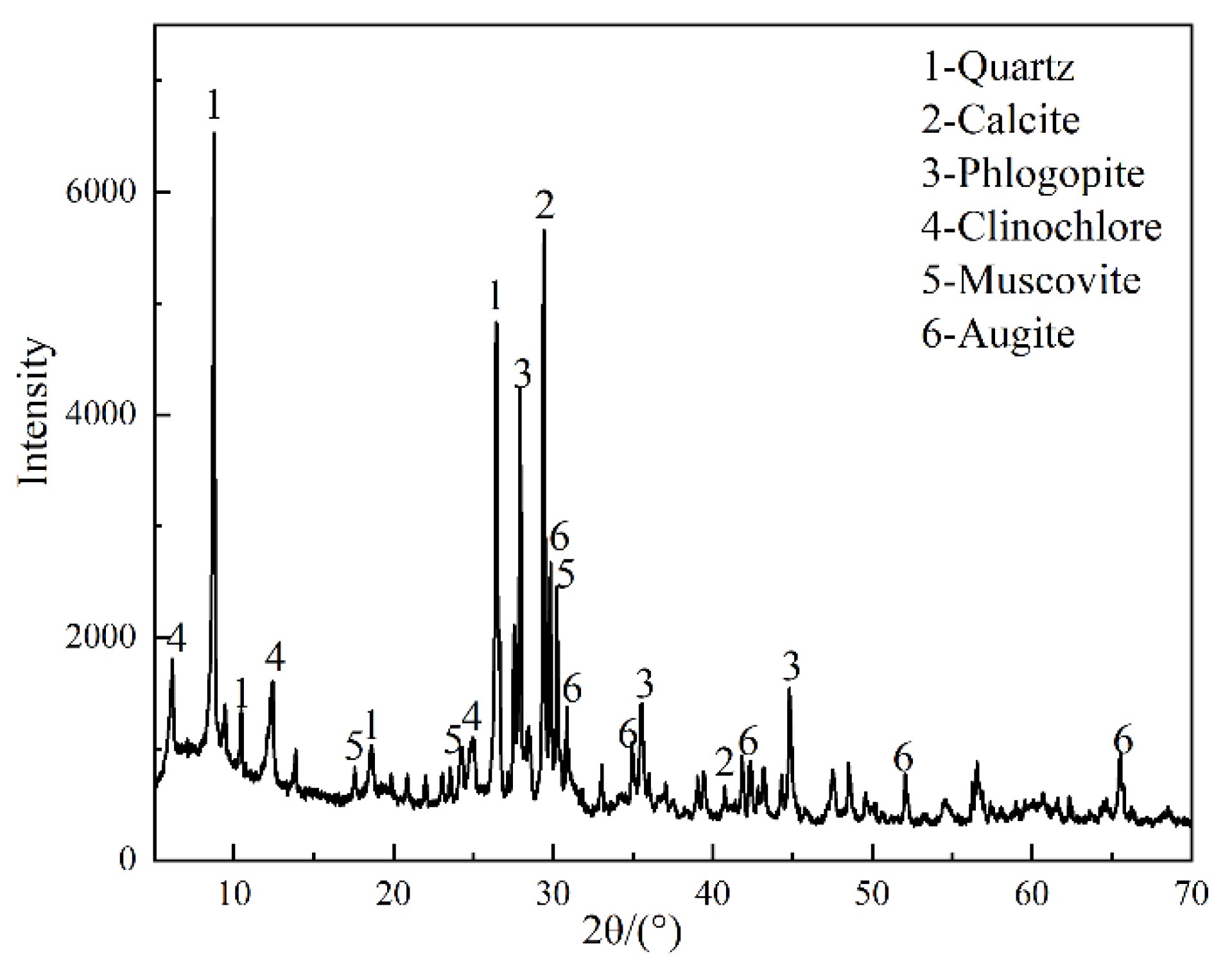
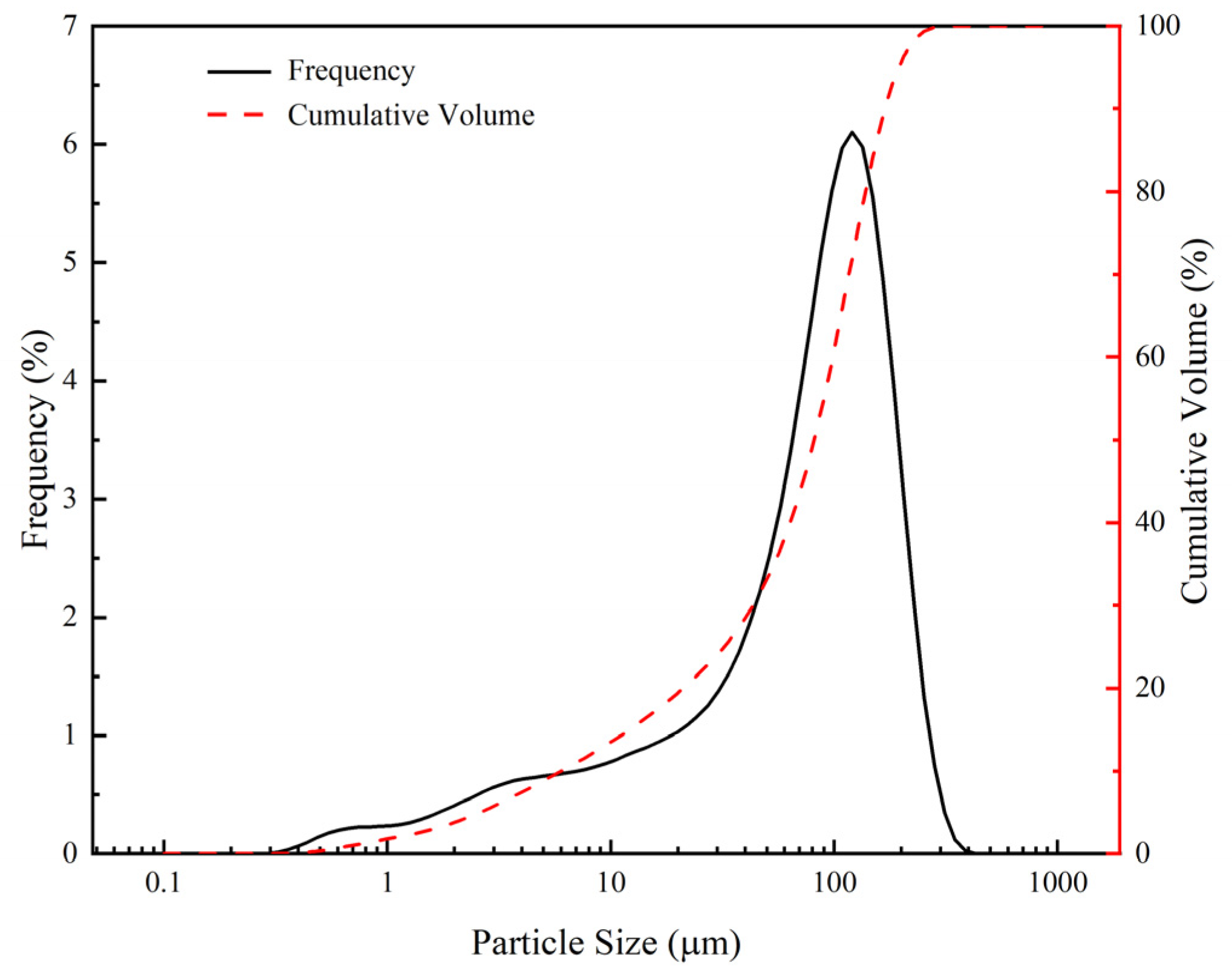


| Materials | CaO | SiO2 | MgO | Fe2O3 | Al2O3 | MnO | SO3 | TiO2 | K2O | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RS | 49.05 | 10.7 | 5.09 | 9.92 | 18.20 | 3.11 | 1.51 | - | 0.08 | 0.16 | - |
| SS | 38.80 | 16.30 | 7.53 | 22.70 | 3.26 | 2.93 | 0.51 | - | 0.09 | 0.11 | 1.75 |
| GGBS | 40.90 | 29.20 | 8.18 | 0.51 | 15.20 | 1.13 | 1.92 | 1.55 | 0.46 | 0.41 | - |
| DG | 41.20 | 3.65 | 6.03 | 1.34 | 0.68 | 0.04 | 44.80 | - | 0.07 | 0.11 | - |
| Composition | CaO | SiO2 | MgO | Fe2O3 | Al2O3 | MnO | SO3 | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|
| Content | 23.1 | 36.6 | 13.3 | 7.9 | 7.88 | 0.17 | 2.05 | 1.75 | 1.45 |
| Mix Code | RS | GGBS | SS | DG | Cement |
|---|---|---|---|---|---|
| OPC | 0 | 0 | 0 | 0 | 100 |
| JL-0 | 0 | 65 | 20 | 15 | 0 |
| JL-5 | 5 | 60 | 20 | 15 | 0 |
| JL-10 | 10 | 55 | 20 | 15 | 0 |
| JL-15 | 15 | 50 | 20 | 15 | 0 |
| JL-20 | 20 | 45 | 20 | 15 | 0 |
| JL-30 | 30 | 35 | 20 | 15 | 0 |
| JL-40 | 40 | 25 | 20 | 15 | 0 |
| Raw Material | Pozzolanic Activity | Dissolved Products | Reaction Conditions |
|---|---|---|---|
| RS | low | Al3+,OH−,Ca2+ | N/A |
| GGBS | high | active SiO2, AlO2− | basic environment (OH−) |
| SS * | low | Ca2+, OH−, M2+ | N/A |
| DG | N/A | SO42−, Ca2+ | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Mu, X.; Ni, W.; Xu, D.; Li, K. Study on Effects of Refining Slag on Properties and Hydration of Cemented Solid Waste-Based Backfill. Materials 2022, 15, 8338. https://doi.org/10.3390/ma15238338
Tang C, Mu X, Ni W, Xu D, Li K. Study on Effects of Refining Slag on Properties and Hydration of Cemented Solid Waste-Based Backfill. Materials. 2022; 15(23):8338. https://doi.org/10.3390/ma15238338
Chicago/Turabian StyleTang, Chang, Xinli Mu, Wen Ni, Dong Xu, and Keqing Li. 2022. "Study on Effects of Refining Slag on Properties and Hydration of Cemented Solid Waste-Based Backfill" Materials 15, no. 23: 8338. https://doi.org/10.3390/ma15238338





