Utilization of Waste Glass in Autoclaved Silica–Lime Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Characterization
2.1.1. Lime
2.1.2. Quartz Sand
2.1.3. Water
2.1.4. Container Glass
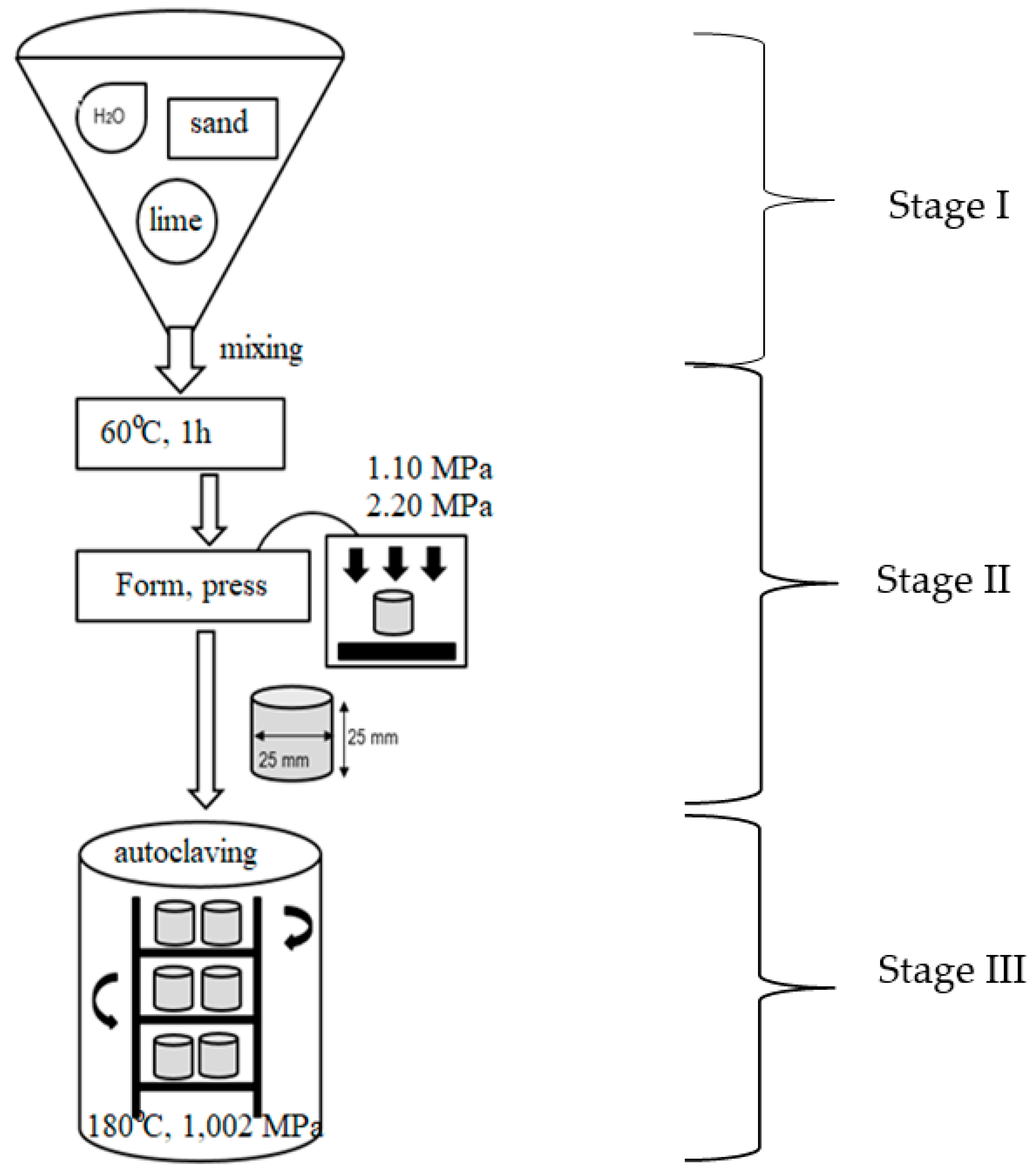
2.2. Preparation of Silica–Lime Samples with the Addition of Coloured Container Glass
2.3. Testing Methods
3. Results and Discussion
4. Conclusions
- a complete substitution of quartz sand with glass waste aggregate in silica–lime products is possible and has a beneficial effect on selected physical properties of the finished products;
- the use of different colours of waste glass has a variable impact on the physical properties of the autoclaved silica–lime products;
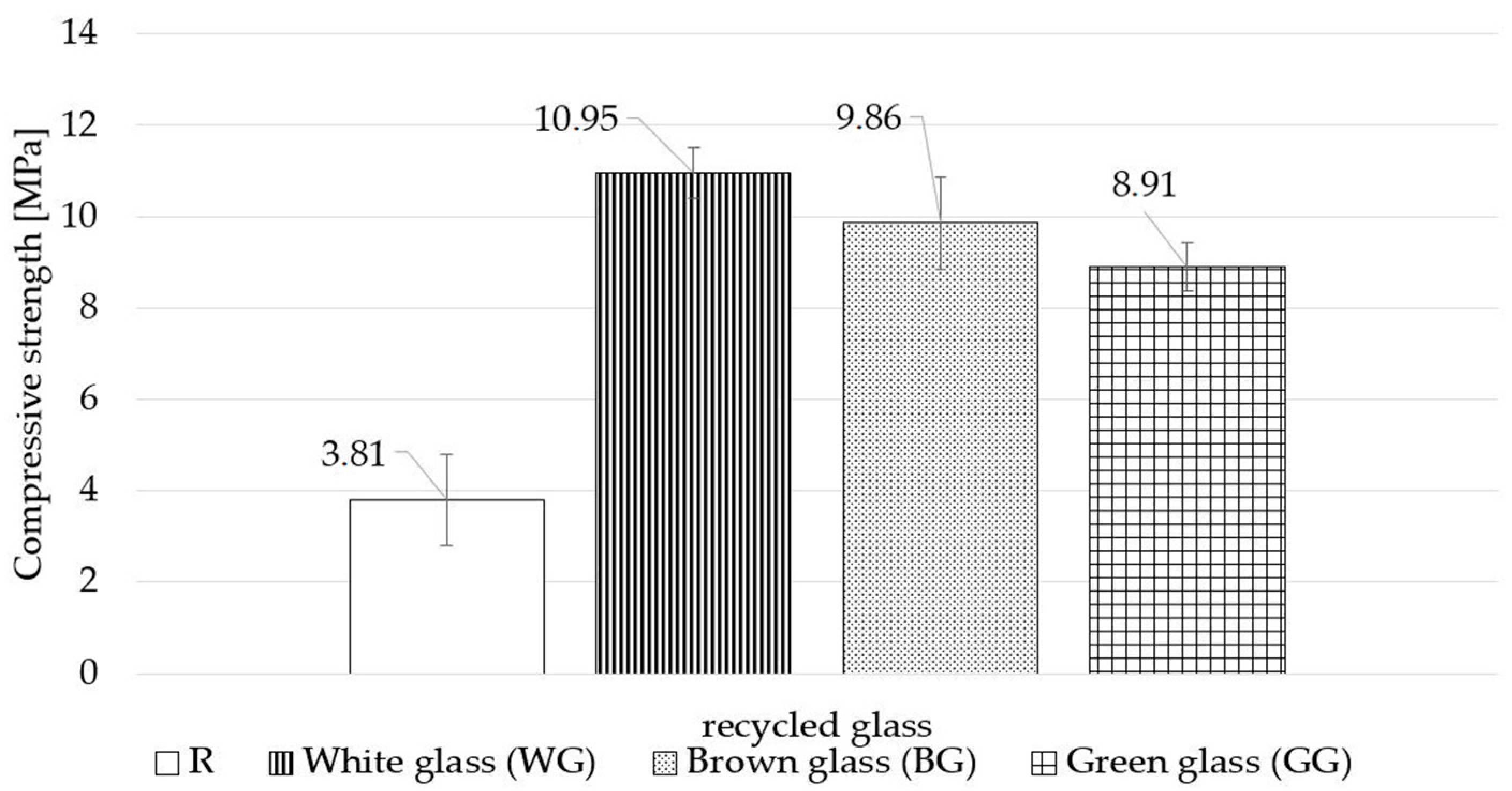
- regardless of the glass colour used, the tested samples demonstrate a significant increase in the compressive strength of finished products; the use of white waste glass (WG) results in a 184% increase in strength with a 159% increase for brown glass (BG) and 134% increase for green glass, compared to the samples with the standard composition;
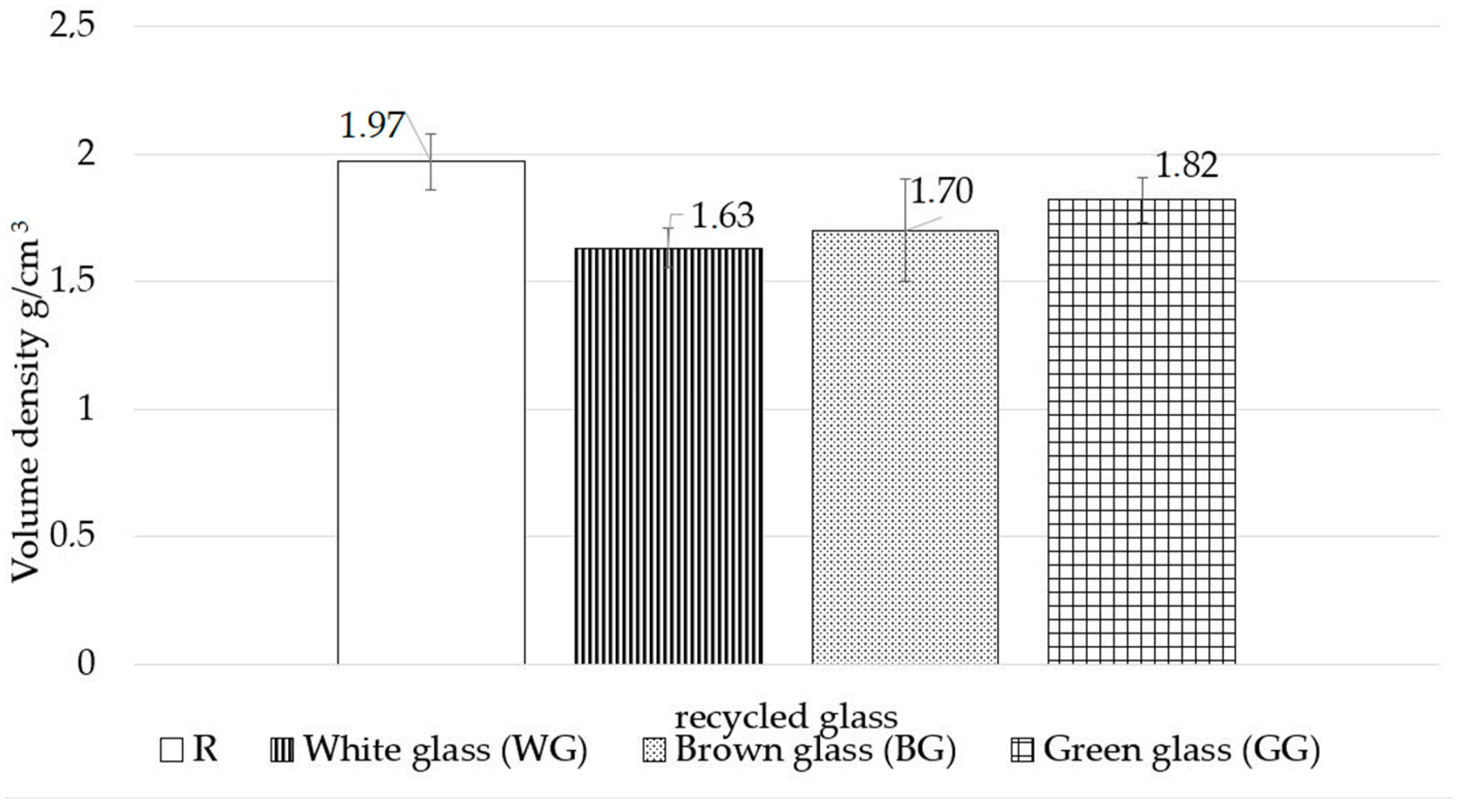
- regardless of the glass colour used, the samples demonstrate a decrease in volumetric density and an increase in water absorption;
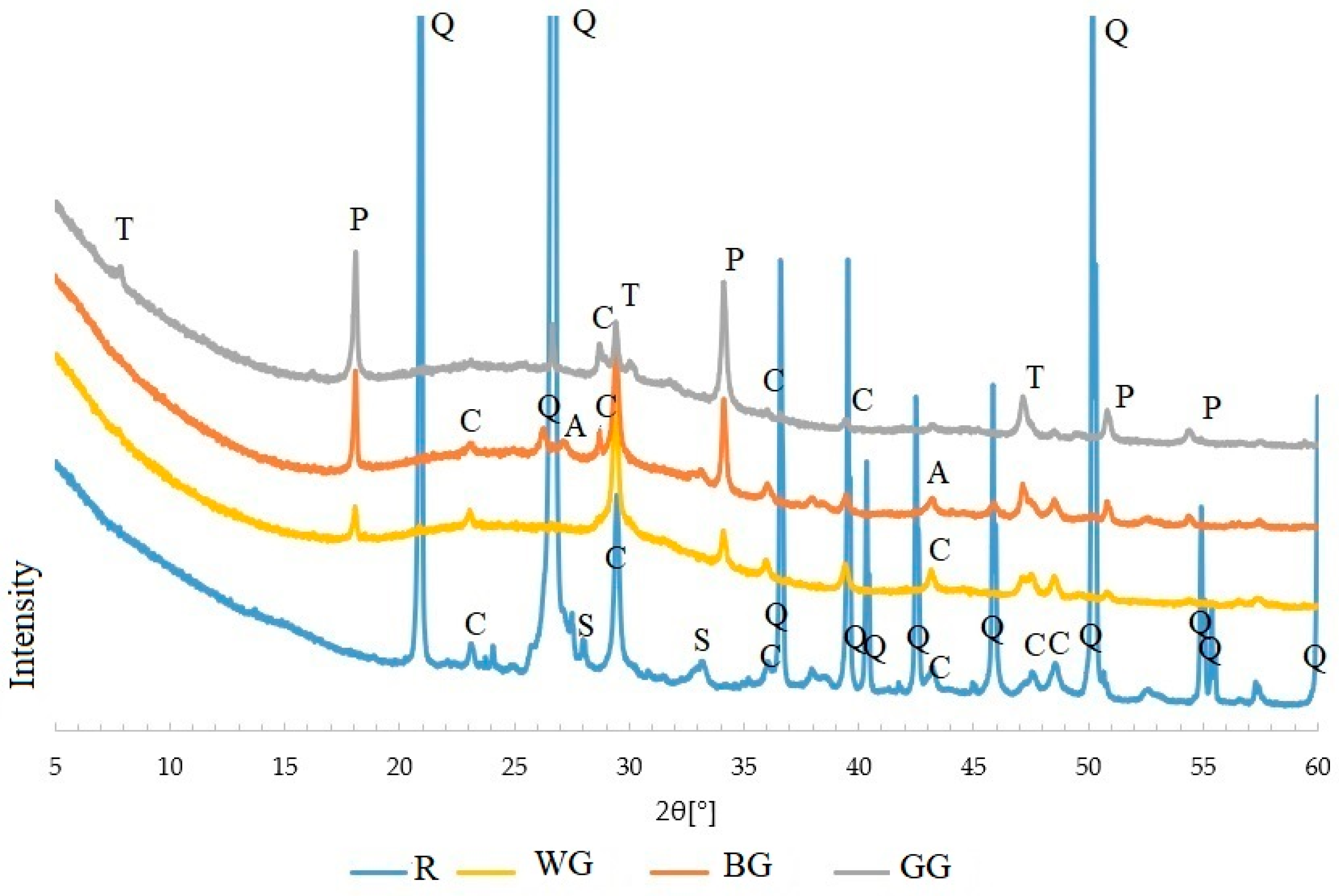
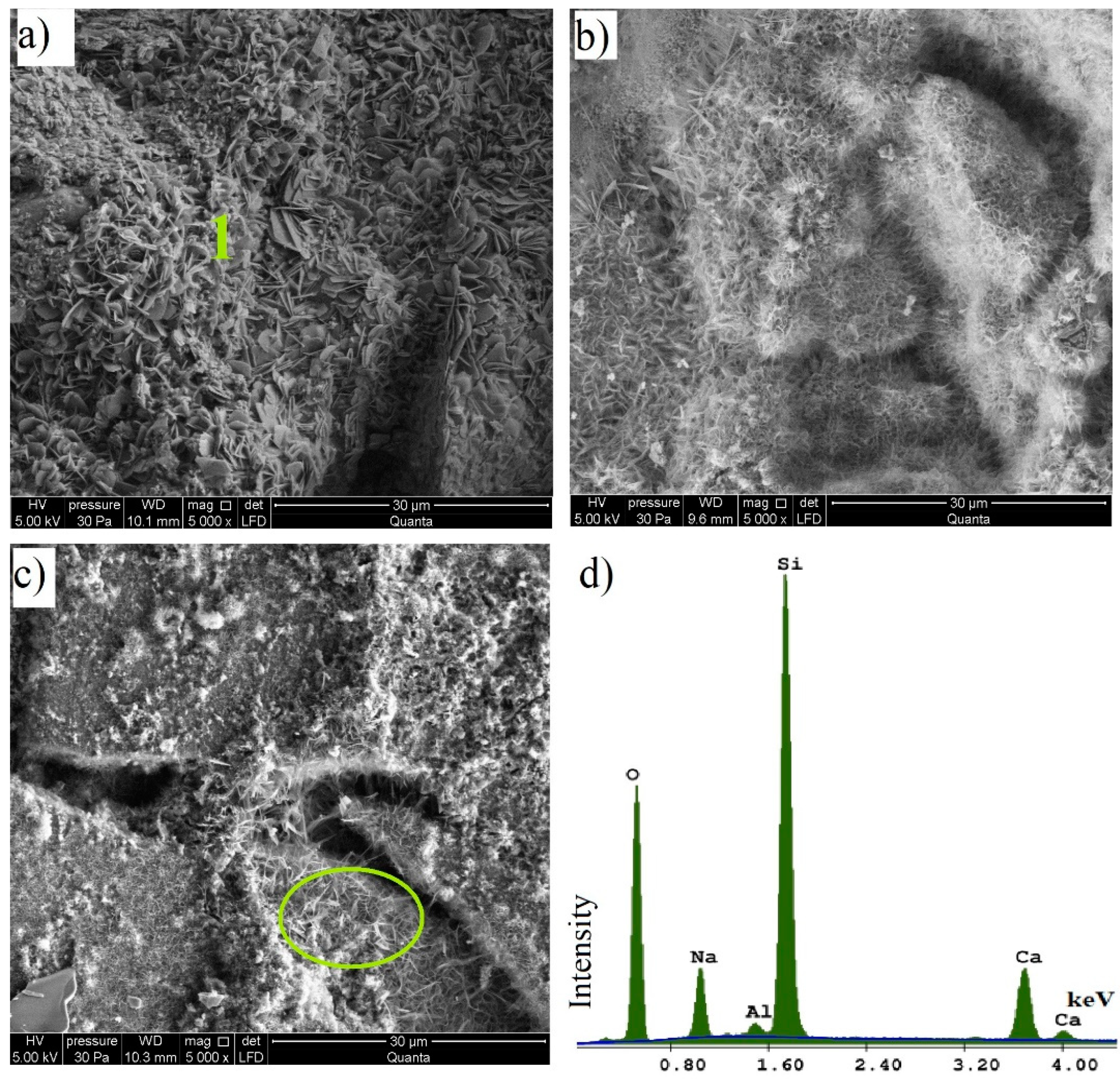
- in samples containing waste glass, the study reveals the formation of portlandite and calcite is the main product, regardless of the colour of the glass, additionally, based on the SEM/EDS analysis, a tobermorite phase was found;
- the microstructure image shows the formation of phases typical of autoclaved sand–lime products, the colour of the glass affects to a varying extent the crystallisation degree of hydrated calcium silicates;
- white glass aggregate is the most reactive and has the strongest effect on the properties of silica–lime products. Green glass proved to be the least reactive.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurostat. Waste Statistics, Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 29 April 2021).
- Eurostat. Packaging Waste Statistics, Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Packaging_waste_statistics (accessed on 18 October 2021).
- Glass Packing Institute. Glass Packaging European Production Continues to Steadily Grow. Available online: https://feve.org/about-glass/statistics/ (accessed on 2 July 2019).
- Kim, K.; Kim, K.; Hwang, J. Thin Film Transistor-Liquid Crystal Display Cullet: A Raw Material for Production of Commercial Soda Lime Silicate Glasses. J. Clean. Prod. 2014, 79, 276–282. [Google Scholar] [CrossRef]
- Olofinnade, O.M.; Ndambuki, J.M.; Ede, A.N.; Booth, C. Application of Waste Glass Powder as a Partial Cement Substitute towards more Sustainable Concrete Production. Int. J. Eng. Res. 2017, 31, 77–93. [Google Scholar] [CrossRef]
- Flood, M.; Fennessy, L.; Lockrey, S.; Avendano, A.; Glover, J.; Kandare, E.; Bhat, T. Glass Fines: A review of cleaning and up-cycling possibilities. J. Clean. Prod. 2020, 267, 121875. [Google Scholar] [CrossRef]
- Ali, B.; Qureshi, L.A. Influence of glass fibers on mechanical and durability performance of concrete with recycled aggregates. Constr. Build. Mater. 2019, 228, 116783. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Value-Added utilisation of waste glass in concrete. Cem. Concr. Res. 2004, 34, 81–89. [Google Scholar] [CrossRef]
- Topçu, I.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2004, 34, 267–274. [Google Scholar] [CrossRef]
- Ismail, Z.Z.; Al-Hashmi, E.A. Recycling of waste glass as a partial replacement for fine aggregate in concrete. Waste Manag. 2009, 29, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Czapik, P. Microstructure and Degradation of Mortar Containing Waste Glass Aggregate as Evaluated by Various Microscopic Techniques. Materials 2020, 13, 2186. [Google Scholar] [CrossRef]
- Alducin-Ochoa, J.; Martín-Del-Río, J.; Torres-González, M.; Flores-Alés, V.; Hernández-Cruz, D. Performance of mortars based on recycled glass as aggregate by accelerated decay tests (ADT). Constr. Build. Mater. 2021, 300, 124057. [Google Scholar] [CrossRef]
- Hamzah, H.K.; Huseien, G.F.; Asaad, M.A.; Georgescu, D.P.; Ghoshal, S.; Alrshoudi, F. Effect of waste glass bottles-derived nanopowder as slag replacement on mortars with alkali activation: Durability characteristics. Case Stud. Constr. Mater. 2021, 15, e00775. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Tu, Z.; Poon, C.S.; Shi, C. Improvement of properties of architectural mortars prepared with 100% recycled glass by CO2 curing. Constr. Build. Mater. 2018, 179, 138–150. [Google Scholar] [CrossRef]
- Bohn, B.P.; Von Mühlen, C.; Pedrotti, M.F.; Zimmer, A. A novel method to produce a ceramic paver recycling waste glass. Clean. Eng. Technol. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Matteucci, F.; Dondi, M.; Guarini, G. Effect of soda-lime glass on sintering and technological properties of porcelain stoneware tiles. Ceram. Int. 2002, 28, 873–880. [Google Scholar] [CrossRef] [Green Version]
- Walczak, P.; Małolepszy, J.; Reben, M.; Szymański, P.; Rzepa, K. Utilization of Waste Glass in Autoclaved Aerated Concrete. Procedia Eng. 2015, 122, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Borek, K.; Czapik, P.; Dachowski, R. Recycled Glass as a Substitute for Quartz Sand in Silicate Products. Materials 2020, 13, 1030. [Google Scholar] [CrossRef] [Green Version]
- Dachowski, R.; Stepien, A. Impact of modification of sand-lime mass with organic compounds on the microstructure and mechanical features of silicate bricks. In Proceedings of the 9th International Conference Environmental Engineering, Vilnius, Lithuania, 22–23 May 2014. [Google Scholar] [CrossRef] [Green Version]
- Jasińska, I. Effect of foam glass granules fillers modification of lime-sand products on their microstructure. Open Eng. 2019, 9, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Główny Urząd Statystyczny. Rynek Producentów Wyrobów Budowlanych w Latach 2015–2018; Główny Urząd Statystyczny: Warsaw, Poland, 2020.
- Stepien, A.; Kostrzewa, P.; Dachowski, R. Influence of barium and lithium compounds on silica autoclaved materials properties and on the microstructure. J. Clean. Prod. 2019, 236, 117507. [Google Scholar] [CrossRef]
- Pytel, Z. Modyfikowanie Składu Fazowego i Mikrostruktury Autoklawizowanych Tworzyw Wapienno-Piaskowych; Ceramika: Krakow, Poland, 2014. [Google Scholar]
- Komisarczyk, K.; Dachowski, R.; Czapik, P. Silicate composites modified with the use of diabase and barite aggregate as an alternative for low-energy construction. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 12072. [Google Scholar] [CrossRef]
- Komisarczyk, K.; Czapik, P.; Komisarczyk, K. Quartz bentonite sandmix in sand-lime products. Open Eng. 2019, 9, 367–373. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Jiao, X.; Sun, P.; Li, J.; Wuri, L.; Zhang, T.C. Electrolytic manganese residue based autoclaved bricks with Ca(OH)2 and thermal-mechanical activated K-feldspar additions. Constr. Build. Mater. 2020, 230, 116848. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Chen, T.; Chen, Y.; Bao, S. Preparation of high strength autoclaved bricks from hematite tailings. Constr. Build. Mater. 2012, 28, 450–455. [Google Scholar] [CrossRef]
- Fang, Y.; Gu, Y.; Kang, Q.; Wen, Q.; Dai, P. Utilization of copper tailing for autoclaved sand–lime brick. Constr. Build. Mater. 2011, 25, 867–872. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liang, S.; Zhang, Y.; Ye, Q.; Zhang, L.; Pan, C.; Lv, R.; Li, Q.; Xiao, K.; et al. Preparation of water-permeable bricks derived from fly ash by autoclaving. Constr. Build. Mater. 2021, 271, 121556. [Google Scholar] [CrossRef]
- Yatsenko, E.; Smoliy, V.; Klimova, L.; Yatsenko, L.A. Use of TPP Ash and Slag Waste in Silicate Technologies. In Proceedings of the IEEE International Conference Management of Municipal Waste as an Important Factor of Sustainable Urban Development, St. Petersburg, Russia, 4–6 October 2018. [Google Scholar] [CrossRef]
- Wójcicki, J.; Ciągło, A.; Czerwiński, Z.; Galewicz, M.; Hasslinger, K.; Hilgertner, A.; Skirgajłło, J.; Sztandera, W.; Świketlik, W.; Tomaszewski, J.; et al. Technologia Szkła, 3rd ed.; Arkady: Warsaw, Poland, 1987. [Google Scholar]
- Jin, W.; Meyer, C.; Baxter, S. Glascrete—Concrete with glass aggregate. ACI Struct. J. 2000, 97, 208–213. [Google Scholar]
- Karamberi, A.; Moutsatsou, A. Participation of coloured glass cullet in cementitious materials. Cem. Concr. Compos. 2005, 27, 319–327. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Chemical Reactions of Glass Cullet Used as Cement Component. J. Mater. Civ. Eng. 2001, 13, 412–417. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of curing temperature and glass type on the pozzolanic reactivity of glass powder. Cem. Concr. Res. 2014, 58, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Bignozzi, M.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass waste as supplementary cementing materials: The effects of glass chemical composition. Cem. Concr. Compos. 2014, 55, 45–52. [Google Scholar] [CrossRef]
- Al-Zubaid, A.B.; Shabeeb, K.M.; Ali, A.I. Study The Effect of Recycled Glass on The Mechanical Properties of Green Concrete. Energy Procedia 2017, 119, 680–692. [Google Scholar] [CrossRef]
- Zimmer, A.; Bragança, S.R. A review of waste glass as a raw material for whitewares. J. Environ. Manag. 2019, 244, 161–171. [Google Scholar] [CrossRef]
- PN-EN 459-2:2010. Wapno Budowlane—Część 2: Metody Badań; Polish Committee for Standarization: Warsaw, Poland, 2010. [Google Scholar]
- PN-EN 933-1:2012. Badania Geometrycznych Właściwości Kruszyw—Część 1: Oznaczanie Składu Ziarnowego—Metoda Przesiewania; Polish Committee for Standarization: Warsaw, Poland, 2012. [Google Scholar]
- PN-EN 1008:2004. Woda Zarobowa do Betonu—Specyfikacja Pobierania Próbek, Badanie i Ocena Przydatności Wody Zarobowej do Betonu, w tym Wody Odzyskanej z Procesów Produkcji Betonu; Polish Committee for Standarization: Warsaw, Poland, 2004. [Google Scholar]
- PN-EN 772-1+A1:2015-10. Metody Badań Elementów Murowych- Część 1: Określenie Wytrzymałości na Ściskanie; Polish Committee for Standarization: Warsaw, Poland, 2015. [Google Scholar]
- PN-EN 772-21:2011. Metody Badań Elementów Murowych- Część 21: Określenie Absorpcji Wody Ceramicznych i Silikaotwych Elementów Murowych Przez Absorpcję Zimnej Wody; Polish Committee for Standarization: Warsaw, Poland, 2011. [Google Scholar]
- Stepien, A.; Potrzeszcz-Sut, B.; Prentice, D.P.; Oey, T.J.; Balonis, M. The Role of Glass Compounds in Autoclaved Bricks. Buildings 2020, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Sasui, S.; Kim, G.; Nam, J.; van Riessen, A.; Eu, H.; Chansomsak, S.; Alam, S.F.; Cho, C.H. Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material. Materials 2020, 13, 3906. [Google Scholar] [CrossRef] [PubMed]
- Drochytka, R.; Černý, V. Influence of fluidized bed combustion fly ash admixture on hydrothermal synthesis of tobermorite in the mixture with quartz sand, high temperature fly ash and lime. Constr. Build. Mater. 2020, 230, 117033. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z. Effects of Environmental Factors on Concrete Carbonation Depth and Compressive Strength. Materials 2018, 11, 2167. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.H.; Du, H. Use of waste glass as sand in mortar: Part I—Fresh, mechanical and durability properties. Cem. Concr. Compos. 2013, 35, 109–117. [Google Scholar] [CrossRef]
- Černý, V.; Drochytka, R.; Kulísek, K.; Húšt'Avová, J. Influence of secondary raw materials on synthesis of tobermorite in lime-silicate composite. In Proceedings of the ICAAC—6th International Conference on Autoclaved Aerated Concrete, Podstam, Germany, 4–6 September 2018. [Google Scholar] [CrossRef]
- Wongkeo, W.; Chaipanich, A. Compressive strength, microstructure and thermal analysis of autoclaved and air cured structural lightweight concrete made with coal bottom ash and silica fume. Mater. Sci. Eng. A 2010, 527, 3676–3684. [Google Scholar] [CrossRef]
- Wongkeo, W.; Thongsanitgarn, P.; Pimraksa, K.; Chaipanich, A. Compressive strength, flexural strength and thermal conductivity of autoclaved concrete block made using bottom ash as cement replacement materials. Mater. Des. 2012, 35, 434–439. [Google Scholar] [CrossRef]



| White Glass | Brown Glass | Green Glass | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Jin, W., Meyer, C., Baxter, S. | Karamberi A., Moutsatsou A. | Poland/HS “Pollena Czechy” | Jin, W., Meyer, C., Baxter, S. | Karamberi A., Moutsatsou A. | Poland/HS “Ujście” | Jin, W., Meyer, C., Baxter, S. | Karamberi A., Moutsatsou A. | Poland/HS “Ujście” | |
| SiO2 | 73.20–73.50 | 70.65 | 71.20 | 71.90–72.40 | 71.20 | 71.30 | 71.30 | 70.5 | 71.00 |
| Na2O + K2O | 13.60–14.10 | 13.80 | 13.00 | 13.80–14.40 | 13.75 | 14.00 | 13.10 | 13.40 | 14.00 |
| CaO + MgO | 10.70–10.80 | 13.15 | 14.50 | 11.60 | 12.95 | 11.40 | 12.20 | 12.90 | 11.20 |
| Al2O3 | 1.70–1.90 | 1.75 | 1.55 | 1.70–1.80 | 1.90 | 2.50 | 2.20 | 1.80 | 2.50 |
| SO3 | 0.20–0.24 | 0.45 | - | 0.12–0.14 | 0.30 | - | 0.05 | 0.25 | - |
| Fe2O3 | 0.04–0.05 | 0.45 | 0.03 | 0.30 | 0.35 | 0.30 | 0.56 | 0.45 | 0.60 |
| Cr2O3 | - | - | - | 0.01 | 0.06 | - | 0.43 | 0.25 | 0.20 |
| Chemical Composition of Lime | Requirements for Lime | Lime Used in the Study |
|---|---|---|
| CaO + MgO | ≥90% | ≥91% |
| MgO | ≤5% | ≤2.0% |
| CO2 | ≤4% | ≤3.0% |
| SO3 | ≤2% | ≤0.50% |
| Individual Fractions | Requirements for Sand | Sand Used in the Study |
|---|---|---|
| 2.5–0.5 mm | <30% | 2.5–0.5 mm 19% |
| 0.5–0.05 mm | ≥65% | 0.5–0.05 mm 81% |
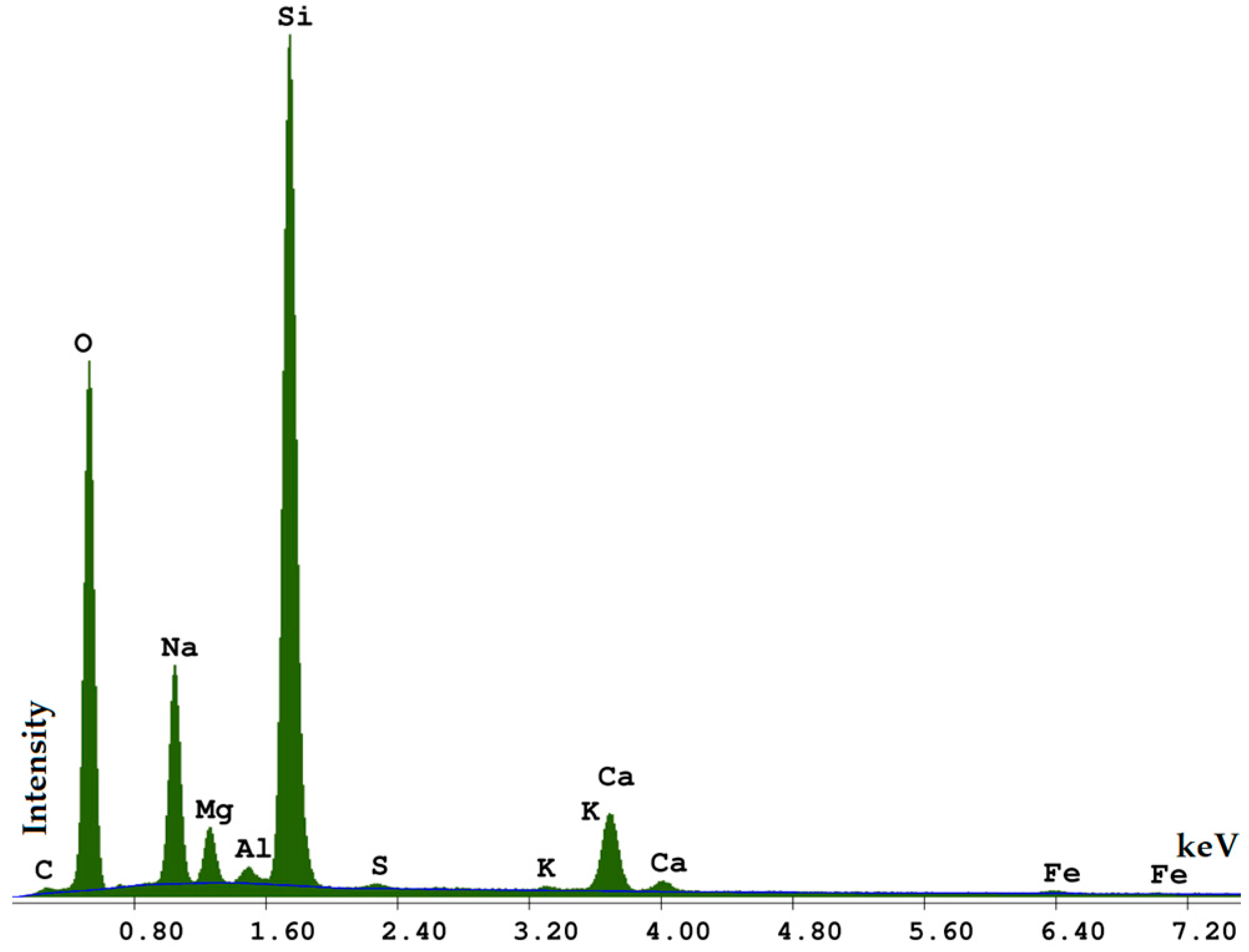
| Element | Weight (%) | Atomic (%) | Net Int. |
|---|---|---|---|
| C | 3.73 | 6.43 | 53.28 |
| O | 41.79 | 54.14 | 1976.71 |
| Mg | 0.44 | 0.38 | 34.33 |
| Al | 4.50 | 3.46 | 363.93 |
| Si | 46.60 | 34.39 | 3655.03 |
| K | 0.71 | 0.38 | 32.18 |
| Fe | 2.23 | 0.83 | 30.50 |
| Reference (R) | White Glass (WG) | Brown Glass (BG) | Green Glass (GG) | |
|---|---|---|---|---|
| Glass | 0 | 92 | 92 | 92 |
| Quartz sand | 92 | 0 | 0 | 0 |
| Lime | 8 | 8 | 8 | 8 |
| Compressive strength |
|
| Volumetric density |
|
| Water absorption |
|
| Microstructure |
|
| Phase composition |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borek, K.; Czapik, P. Utilization of Waste Glass in Autoclaved Silica–Lime Materials. Materials 2022, 15, 549. https://doi.org/10.3390/ma15020549
Borek K, Czapik P. Utilization of Waste Glass in Autoclaved Silica–Lime Materials. Materials. 2022; 15(2):549. https://doi.org/10.3390/ma15020549
Chicago/Turabian StyleBorek, Katarzyna, and Przemysław Czapik. 2022. "Utilization of Waste Glass in Autoclaved Silica–Lime Materials" Materials 15, no. 2: 549. https://doi.org/10.3390/ma15020549






