Effect of NaCl Solution and Simulated Concrete Pore Solution Environment on the Efficiency of Steel Bar Energized Corrosion
Abstract
:1. Introduction
2. Experimental
2.1. Test Sample and Solution
2.2. Corrosion Scheme
3. Experimental Phenomenon and Result Analysis
3.1. Test Phenomenon
3.2. Analysis of Test Results
3.2.1. Corrosion Efficiency of Concrete in Pore Solutions and NaCl Solution
3.2.2. Influence of Corrosion Degree of Reinforcement
3.2.3. Influence of Chloride ion Concentration
3.2.4. Influence of Current Density
4. Conclusions
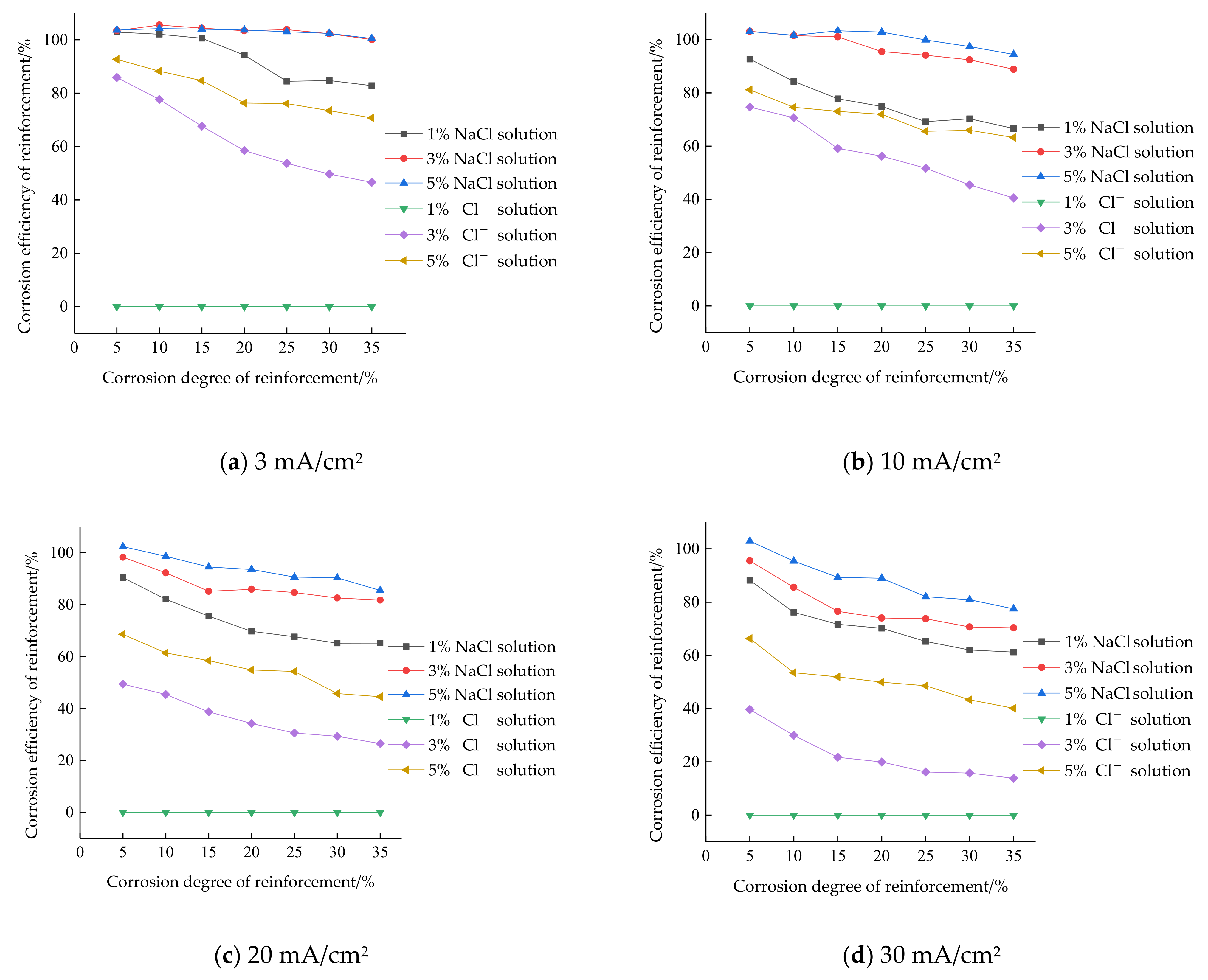
- The corrosion efficiency of reinforcement in a NaCl solution is higher than that of a concrete simulated pore fluid, and the actual corrosion depth is closer to the theoretical corrosion depth. In the NaCl solution, the corrosion efficiency of the reinforcing steel reached almost 100% on average for the chloride ion concentration of 3%, 3 mA/cm2 current density of conditions and the chloride ion concentration of 5%, 10 mA/cm2 current density of conditions; in the concrete simulated pore solution, the highest corrosion efficiency of the reinforcing steel is less than 80%.
- The corrosion efficiency in NaCl solution changes in two stages: in the first stage, the corrosion efficiency does not change with the increase of the corrosion degree of the reinforcement; in the second stage, the corrosion efficiency less than 100% decreases with the increase of the corrosion degree of the reinforcement; the corrosion efficiency of simulated pore fluid of concrete decreases with the increase of the corrosion degree of reinforcement, which is more significant than that in NaCl solution.
- Under the same conditions, the chloride ion concentration of the 5% NaCl solution of steel corrosion efficiency is significantly higher than the chloride ion concentration of the 3% NaCl solution. The corrosion efficiency of reinforcement is higher in the chloride ion solution with a high concentration. The maximum difference in corrosion efficiency between 3% and 5% NaCl solution under four current densities is 1.31%, 7.33%, 7.78% and 13.94%, respectively, while in concrete simulated pore solution, it is 24.13%, 22.70%, 23.66% and 32.36%, respectively. The change of the chloride ion concentration in the simulated pore solution of concrete has a greater impact on the corrosion efficiency than in the NaCl solution.
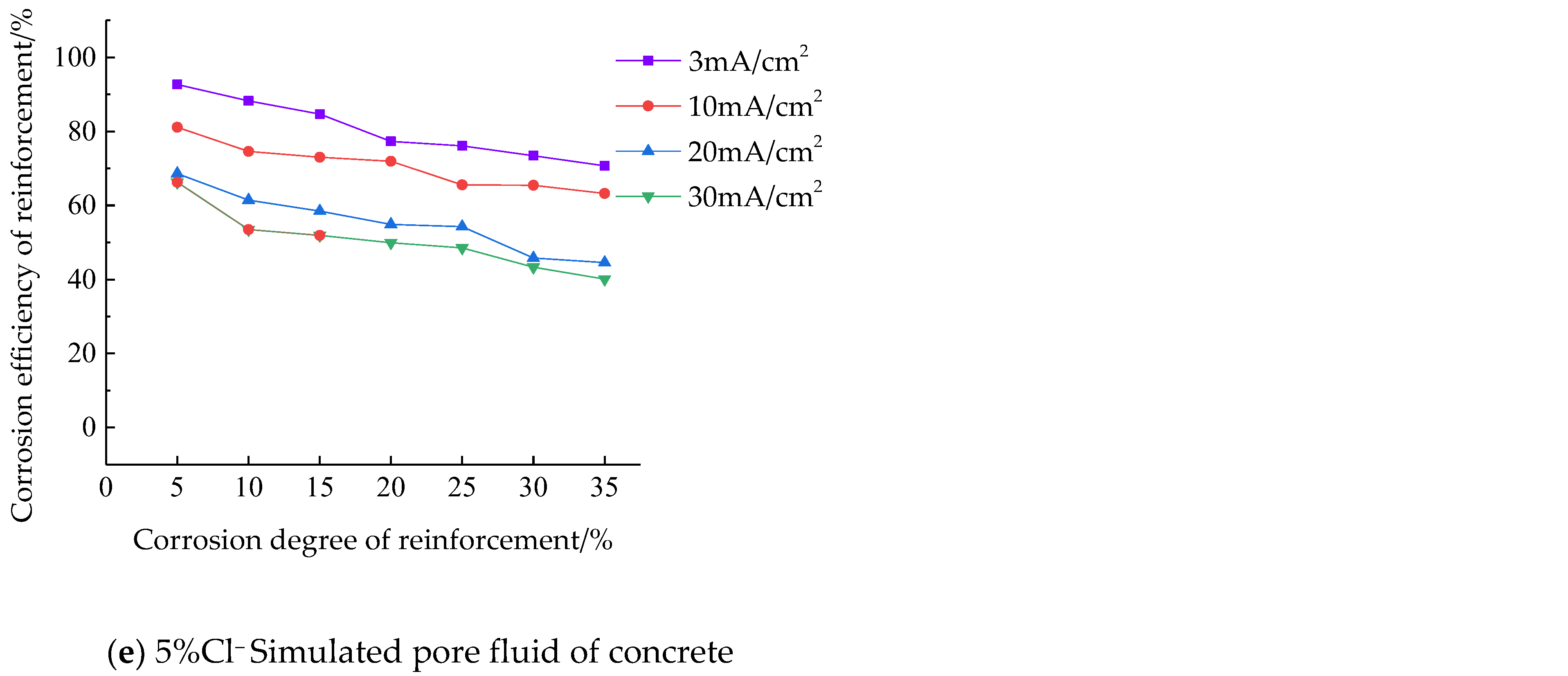
- Under the same conditions, the corrosion efficiency is the highest when the current density is 3 mA/cm2, the lowest when the current density is 30 mA/cm2, and the corrosion efficiency of 10 mA/cm2 current density and 20 mA/cm2 current density is between 3 mA/cm2 current density and 30 mA/cm2 current density. The corrosion efficiency of reinforcement under a low current density is higher than that under a high current density.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Wang, M.; Zhou, X.; Yu, W.; Gao, Y.; Zhang, Y. Exploring the Emerging Evolution Trends of Probabilistic Service Life Prediction of Reinforced Concrete Structures in the Chloride Environment by Scientometric Analysis. Adv. Civ. Eng. 2021, 2021, 8883142. [Google Scholar] [CrossRef]
- Kenny, A.; Katz, A. Steel-concrete interface influence on chloride threshold for corrosion–Empirical reinforcement to theory. Conster. Build. Mater. 2020, 244, 118376. [Google Scholar] [CrossRef]
- Liu, Q.; Dave, E.; Yang, J.; Li, L. A three-phase, multi-component ionic transport model for simulation of chloride penetration in concrete. Eng. Struct. 2015, 86, 122–133. [Google Scholar] [CrossRef]
- McPolin, D.; Basheer, P.A.M.; Long, A.E.; Grattan, K.T.V.; Sun, T. Obtaining progressive chloride profiles in cementitious materials. Constr. Build. Mater. 2005, 19, 666–673. [Google Scholar] [CrossRef]
- Van den Heede, P.; De Keersmaecker, M.; Elia, A.; Adriaens, A.; De Belie, N. Service life and global warming potential of chloride exposed concrete with high volumes of fly ash. Cem. Concr. Compos. 2017, 80, 210–223. [Google Scholar] [CrossRef]
- Cairns, J.; Plizzari, G.A.; Du, Y.; Law, D.W.; Franzoni, C. Mechanical properties of corrosion-damaged reinforcement. ACI Mater. J. 2005, 102, 256. [Google Scholar]
- Chen, J.; Fu, C.; Ye, H.; Jin, X. Corrosion of steel embedded in mortar and concrete under different electrolytic accelerated corrosion methods. Constr. Build. Mater. 2020, 241, 117971. [Google Scholar] [CrossRef]
- Taha, N.A.; Morsy, M. Study of the behavior of corroded steel bar and convenient method of repairing. HBRC J. 2016, 12, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Bazán, A.M.; Gálvez, J.C.; Reyes, E.; Galé-Lamuela, D. Study of the rust penetration and circumferential stresses in reinforced concrete at early stages of an accelerated corrosion test by means of combined SEM, EDS and strain gauges. Constr. Build. Mater. 2018, 184, 655–667. [Google Scholar] [CrossRef]
- Li, Q.; Jin, X.; Yan, D.; Fu, C.; Xu, J. Study of wiring method on accelerated corrosion of steel bars in concrete. Constr. Build. Mater. 2021, 269, 121286. [Google Scholar] [CrossRef]
- Al-alaily, H.S.; Hassan, A.A.A. A study on the effect of curing temperature and duration on rebar corrosion. Mag. Concr. Res. 2018, 70, 260–270. [Google Scholar] [CrossRef]
- Apostolopoulos, C.A.; Demis, S.; Papadakis, V.G. Chloride-induced corrosion of steel reinforcement–Mechanical performance and pit depth analysis. Conster. Build. Mater. 2013, 38, 139–146. [Google Scholar] [CrossRef]
- Ahmad, S. Techniques for inducing accelerated corrosion of steel in concrete. Arab. J. Sci. Eng. 2009, 34, 95. [Google Scholar]
- Bicer, K.; Yalciner, H.; Balkıs, A.P.; Kumbasaroglu, A. Effect of corrosion on flexural strength of reinforced concrete beams with polypropylene fibers. Conster. Build. Mater. 2018, 185, 574–588. [Google Scholar] [CrossRef]
- Lu, C.; Jin, W.; Liu, R. Reinforcement corrosion-induced cover cracking and its time prediction for reinforced concrete structures. Corros. Sci. 2011, 53, 1337–1347. [Google Scholar] [CrossRef]
- Austin, S.A.; Lyons, R.; Ing, M.J. Electrochemical behavior of steel-reinforced concrete during accelerated corrosion testing. Corrosion 2004, 60, 203–212. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Rodriguez, J.; Diez, J.M. Factors controlling cracking of concrete affected by reinforcement corrosion. Mater. Struct. 1998, 31, 435–441. [Google Scholar] [CrossRef]
- Nossoni, G.; Harichandran, R. Current Efficiency in Accelerated Corrosion Testing of Concrete. Corrosion 2012, 68, 801–809. [Google Scholar] [CrossRef]
- Maaddawy, T.; Soudki, K.A. Effectiveness of Impressed Current Technique to Simulate Corrosion of Steel Reinforcement in Concrete. J. Mater. Civil Eng. 2003, 15, 41–47. [Google Scholar] [CrossRef]
- Wang, X.G.; Du, X.B.; Yan, J.Y.; Fang, Z.X. Experimental comparative of galvanostatic methods for accelerated corrosion of steel bars in RC members. J. Build. Struct. 2015, 36, 104–110. [Google Scholar]
- Yang, X.M.; Wu, T.Y.; Chen, Y.L. Relationship of corrosion of concrete reinforcement to accelerated corrosion current. Proc. Inst. Civ. Eng. Constr. Mater. 2019, 172, 263–268. [Google Scholar] [CrossRef]
- Feng, W.P.; Dong, Z.J.; Liu, W.; Cui, H.Z.; Tang, W.; Xing, F. An experimental study on the influence of applied voltage on current efficiency of rebars with a modified accelerated corrosion test. Cem. Concr. Comp. 2021, 122, 104120. [Google Scholar] [CrossRef]
- Auyeung, Y.B.; Balaguru, P.; Chung, L. Bond behavior of corroded reinforcement bars. Aci Mater. J. 2000, 97, 214–220. [Google Scholar]
- Xu, G.; Xu, L.L.; Wang, Q.; Wen, T.; Liu, L. Study on corrosion efficiency of reinforcing steel bar in electrifying corrosion after concrete cover cracking. Concrete 2016, 1, 38–42. [Google Scholar]
- Chen, S.; Ren, J.; Li, Q.; Yang, G. Stirrup accelerate corrosion test method and applicability. Concrete 2017, 39, 40–43. [Google Scholar]
- Saremi, M.; Mahallati, E. A study on chloride-induced depassivation of mild steel in simulated concrete pore solution. Cem. Concr. Comp. 2002, 32, 1915–1921. [Google Scholar] [CrossRef]
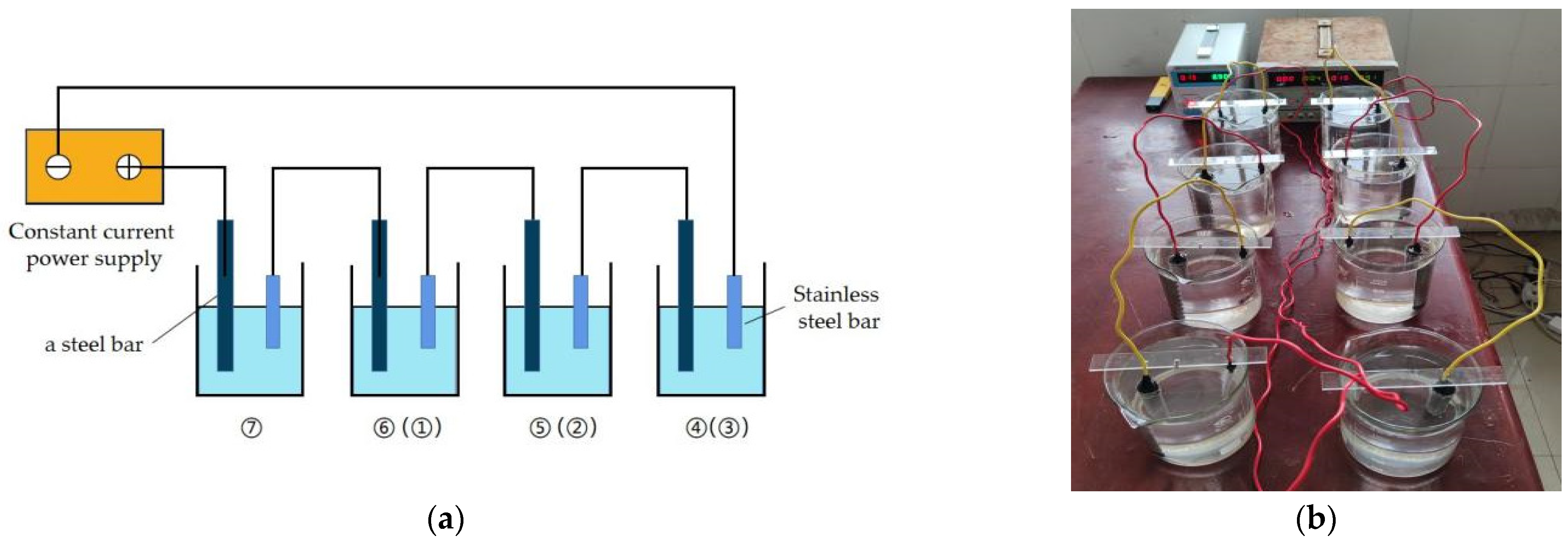


| No. | Cl− Concentration | Current Density (mA/cm2) | Corrosion Degree |
|---|---|---|---|
| X-1-3-Y | 1% | 3 | 5%, 10%, 15%,20%, 25%, 30%, 35% |
| X-1-10-Y | 10 | ||
| X-1-20-Y | 20 | ||
| X-1-30-Y | 30 | ||
| X-3-3-Y | 3% | 3 | 5%, 10%, 15%, 20%, 25%, 30%, 35% |
| X-3-10-Y | 10 | ||
| X-3-20-Y | 20 | ||
| X-3-30-Y | 30 | ||
| X-5-3-Y | 5% | 3 | 5%, 10%, 15%, 20%, 25%, 30%, 35% |
| X-5-10-Y | 10 | ||
| X-5-20-Y | 20 | ||
| X-5-30-Y | 30 |
| Current Density(mA/cm2) | No. | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 3 | 3000 | 6000 | 9000 | 12,000 | 15,000 | 18,000 | 21,000 |
| 10 | 900 | 1800 | 2700 | 3600 | 4500 | 5400 | 6300 |
| 20 | 450 | 900 | 1350 | 1800 | 2250 | 2700 | 3150 |
| 30 | 300 | 600 | 900 | 1200 | 1500 | 1800 | 2100 |
| Solution | Current Density | |||
|---|---|---|---|---|
| 3 mA/cm2 | 10 mA/cm2 | 20 mA/cm2 | 30 mA/cm2 | |
| 1%NaCl | 15% | - | - | - |
| 3%NaCl | 35% | 15% | - | - |
| 5%NaCl | 35% | 20% | 5% | 5% |
| Solution | Current Density | |||
|---|---|---|---|---|
| 3 mA/cm2 | 10 mA/cm2 | 20 mA/cm2 | 30 mA/cm2 | |
| 1%NaCl | 19.49% | 28.12% | 27.87% | 30.58% |
| 3%NaCl | 3.23% | 13.84% | 16.79% | 26.36% |
| 5%NaCl | 3.06% | 8.32% | 16.52% | 24.75% |
| 3%Cl− solution | 45.75% | 45.72% | 46.26% | 65.21% |
| 5%Cl− solution | 23.68% | 22.07% | 35.02% | 39.05% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianyu, Y.; Xin, Y.; Cong, J.; Weijun, Y. Effect of NaCl Solution and Simulated Concrete Pore Solution Environment on the Efficiency of Steel Bar Energized Corrosion. Materials 2022, 15, 7040. https://doi.org/10.3390/ma15197040
Jianyu Y, Xin Y, Cong J, Weijun Y. Effect of NaCl Solution and Simulated Concrete Pore Solution Environment on the Efficiency of Steel Bar Energized Corrosion. Materials. 2022; 15(19):7040. https://doi.org/10.3390/ma15197040
Chicago/Turabian StyleJianyu, Yang, Ye Xin, Jiang Cong, and Yang Weijun. 2022. "Effect of NaCl Solution and Simulated Concrete Pore Solution Environment on the Efficiency of Steel Bar Energized Corrosion" Materials 15, no. 19: 7040. https://doi.org/10.3390/ma15197040
APA StyleJianyu, Y., Xin, Y., Cong, J., & Weijun, Y. (2022). Effect of NaCl Solution and Simulated Concrete Pore Solution Environment on the Efficiency of Steel Bar Energized Corrosion. Materials, 15(19), 7040. https://doi.org/10.3390/ma15197040





