The Observation of Cellular Precipitation in an Ni36Co18Cr20Fe19Al7 High-Entropy Alloy after Quenching and Annealing
Abstract
:1. Introduction
2. Experimental Procedures
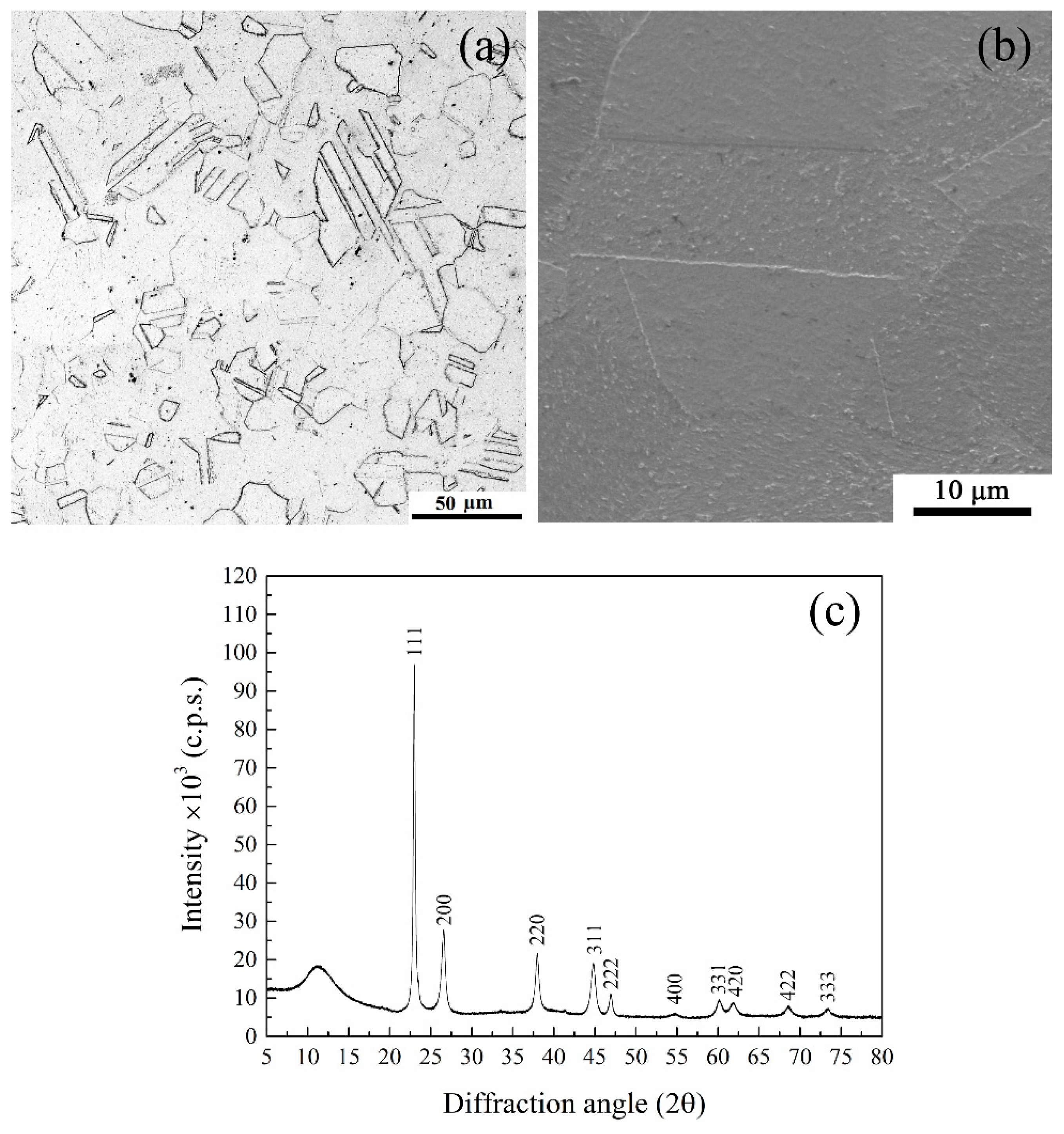
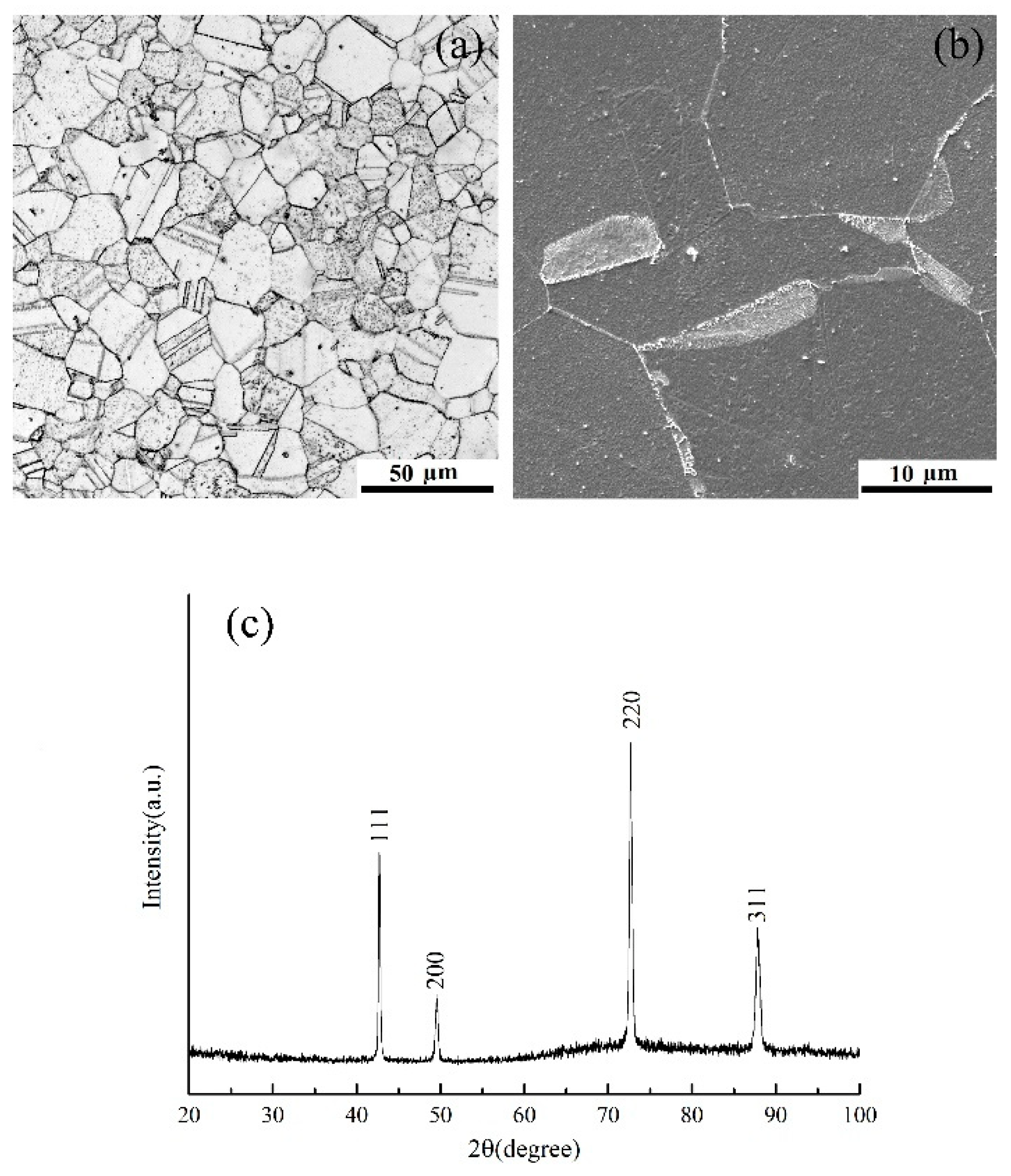
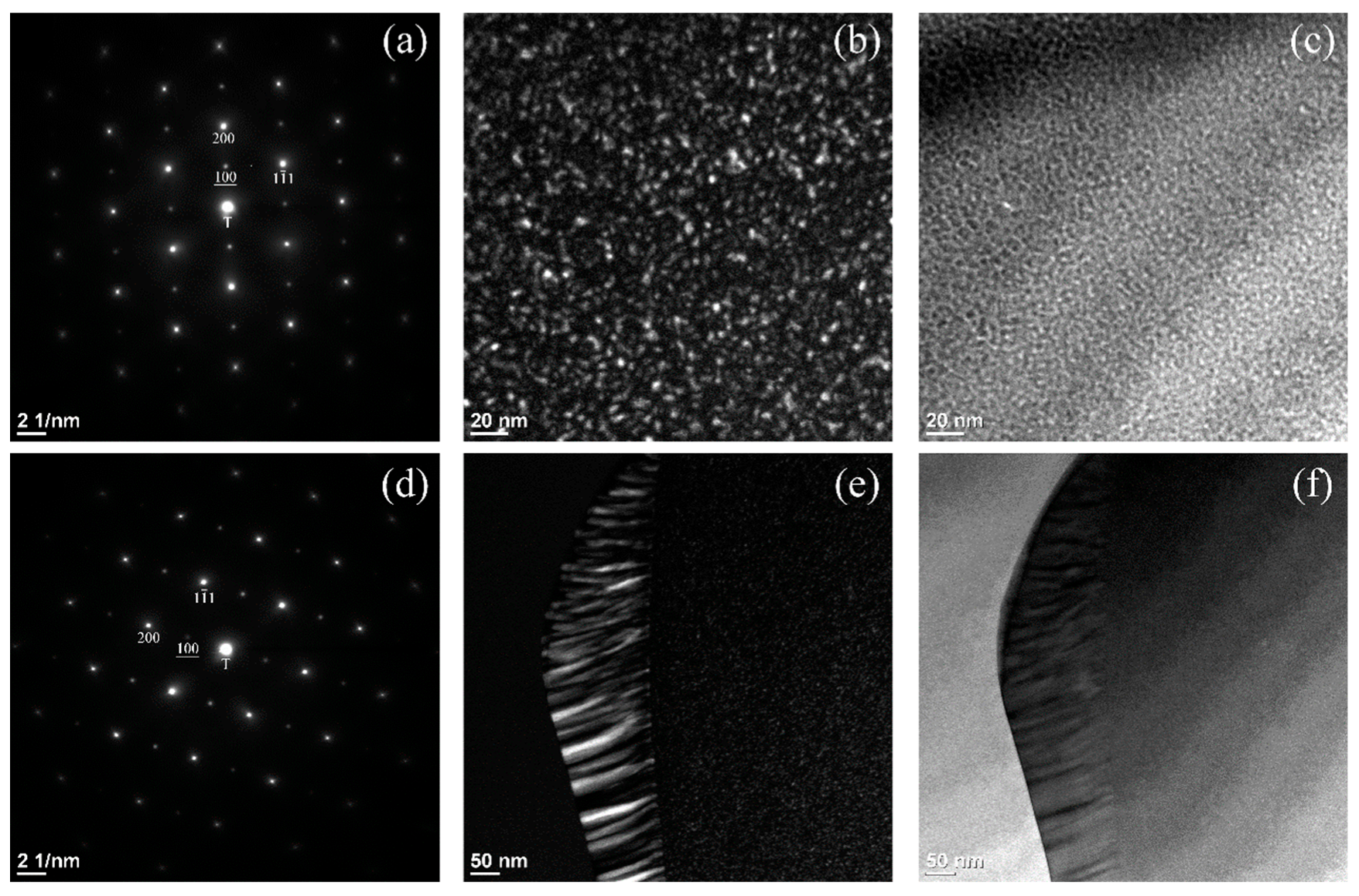
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Qiao, J.W.; Ma, S.G.; Huang, E.W.; Chuang, C.P.; Liaw, P.K.; Zhang, Y. Microstructural Characteristics and Mechanical Behaviors of AlCoCrFeNi High-Entropy Alloys at Ambient and Cryogenic Temperatures. Mater. Sci. Forum 2011, 688, 419–425. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructure and Compressive Properties of Multiprincipal Component AlCoCrFeNiCX Alloys. J. Alloys Compd. 2011, 509, 3476–3480. [Google Scholar] [CrossRef]
- Shun, T.T.; Du, Y.C. Microstructure and Tensile Behaviors of FCC Al0.3CoCrFeNi High Entropy Alloy. J. Alloys Compd. 2009, 479, 157–160. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; Choudhuri, D.; Lee, M.; Hwang, J.Y.; Nam, S.J.; Ryu, H.; Hong, S.H.; Banerjee, R. Stability of Ordered L12 and B2 Precipitates in Face Centered Cubic Based High Entropy Alloys-Al0.3CoFeCrNi and Al0.3CuFeCrNi2. Scr. Mater. 2016, 123, 130–134. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Investigation of the Phase Stabilities in AlNiCoCrFe High Entropy Alloys. J. Alloys Compd. 2017, 691, 119–129. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase Separation in Equiatomic AlCoCrFeNi High-Entropy Alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L. Evolution of Microstructure, Hardness, and Corrosion Properties of High-Entropy Al0.5CoCrFeNi Alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.-W. Microstructure and Mechanical Property of As-Cast, -Homogenized, and -Deformed AlXCoCrFeNi (0 ≤ x ≤ 2) High-Entropy Alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of Vanadium Addition on the Microstructure and Properties of AlCoCrFeNi High Entropy Alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Chen, J.; Niu, P.; Liu, Y.; Lu, Y.; Wang, X.; Peng, Y.; Liu, J. Effect of Zr Content on Microstructure and Mechanical Properties of AlCoCrFeNi High Entropy Alloy. Mater. Des. 2016, 94, 39–44. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation Behavior of Arc Melted AlCoCrFeNi Multi-Component High-Entropy Alloys. J. Alloys Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Mohanty, S.; Maity, T.N.; Mukhopadhyay, S.; Sarkar, S.; Gurao, N.P.; Bhowmick, S.; Biswas, K. Powder Metallurgical Processing of Equiatomic AlCoCrFeNi High Entropy Alloy: Microstructure and Mechanical Properties. Mater. Sci. Eng. A 2017, 679, 299–313. [Google Scholar] [CrossRef]
- Xu, X.D.; Liu, P.; Guo, S.; Hirata, A.; Fujita, T.; Nieh, T.G.; Liu, C.T.; Chen, M.W. Nanoscale Phase Separation in a Fcc-Based CoCrCuFeNiAl0.5 High-Entropy Alloy. Acta Mater. 2015, 84, 145–152. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring Nanostructures and Mechanical Properties of AlCoCrFeNi2.1 Eutectic High Entropy Alloy Using Thermo-Mechanical Processing. Mater. Sci. Eng. A 2016, 675, 99–109. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al Addition on the Microstructure and Mechanical Property of AlXCoCrFeNi High-Entropy Alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Yeh, J.W. Phases, Microstructure and Mechanical Properties of AlXCoCrFeNi High-Entropy Alloys at Elevated Temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Lv, Y.; Hu, R.; Yao, Z.; Chen, J.; Xu, D.; Liu, Y.; Fan, X. Cooling Rate Effect on Microstructure and Mechanical Properties of AlXCoCrFeNi High Entropy Alloys. Mater. Des. 2017, 132, 392–399. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat Treatment Impacts the Micro-Structure and Mechanical Properties of AlCoCrFeNi High Entropy Alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Lee, K.S.; Bae, B.; Kang, J.H.; Lim, K.R.; Na, Y.S. Multi-Phase Refining of an AlCoCrFeNi High Entropy Alloy by Hot Compression. Mater. Lett. 2017, 198, 81–84. [Google Scholar] [CrossRef]
- Tang, Z.; Senkov, O.N.; Parish, C.M.; Zhang, C.; Zhang, F.; Santodonato, L.J.; Wang, G.; Zhao, G.; Yang, F.; Liaw, P.K. Tensile Ductility of an AlCoCrFeNi Multi-Phase High-Entropy Alloy through Hot Isostatic Pressing (HIP) and Homogenization. Mater. Sci. Eng. A 2015, 647, 229–240. [Google Scholar] [CrossRef]
- Ecob, R.C.; Bee, J.V.; Ralph, B. The Cellular Reaction in Dilute Copper-Titanium Alloys. Metall. Trans. A 1980, 11, 1407–1414. [Google Scholar] [CrossRef]
- Miki, M.; Laughlin, D.E. Cellular Decomposition in a Cu-25Ni-15Co Side-Band Alloy. Metall. Trans. A 1985, 16, 1751–1757. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Pande, C.S. A Synthesis of Mechanisms for Initiation of the Cellular (or Discontinuous Precipitation) Reaction. Acta Mater. 1998, 47, 175–181. [Google Scholar] [CrossRef]
- Cheng, W.C.; Cheng, C.Y.; Hsu, C.W.; Laughlin, D.E. Phase Transformation of the L12 Phase to Kappa-Carbide after Spinodal Decomposition and Ordering in an Fe-C-Mn-Al Austenitic Steel. Mater. Sci. Eng. A 2015, 642, 128–135. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenedy, G.R.; Chemeli, K.R.; Cheng, W.-C. The Observation of Cellular Precipitation in an Ni36Co18Cr20Fe19Al7 High-Entropy Alloy after Quenching and Annealing. Materials 2022, 15, 6613. https://doi.org/10.3390/ma15196613
Kenedy GR, Chemeli KR, Cheng W-C. The Observation of Cellular Precipitation in an Ni36Co18Cr20Fe19Al7 High-Entropy Alloy after Quenching and Annealing. Materials. 2022; 15(19):6613. https://doi.org/10.3390/ma15196613
Chicago/Turabian StyleKenedy, Gurumayum Robert, Korir Rosemary Chemeli, and Wei-Chun Cheng. 2022. "The Observation of Cellular Precipitation in an Ni36Co18Cr20Fe19Al7 High-Entropy Alloy after Quenching and Annealing" Materials 15, no. 19: 6613. https://doi.org/10.3390/ma15196613






