Controlling the Surface Morphology of ZnO Nano-Thin Film Using the Spin Coating Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Precursor Solution
2.2. Characterization of the Film
3. Results and Discussion
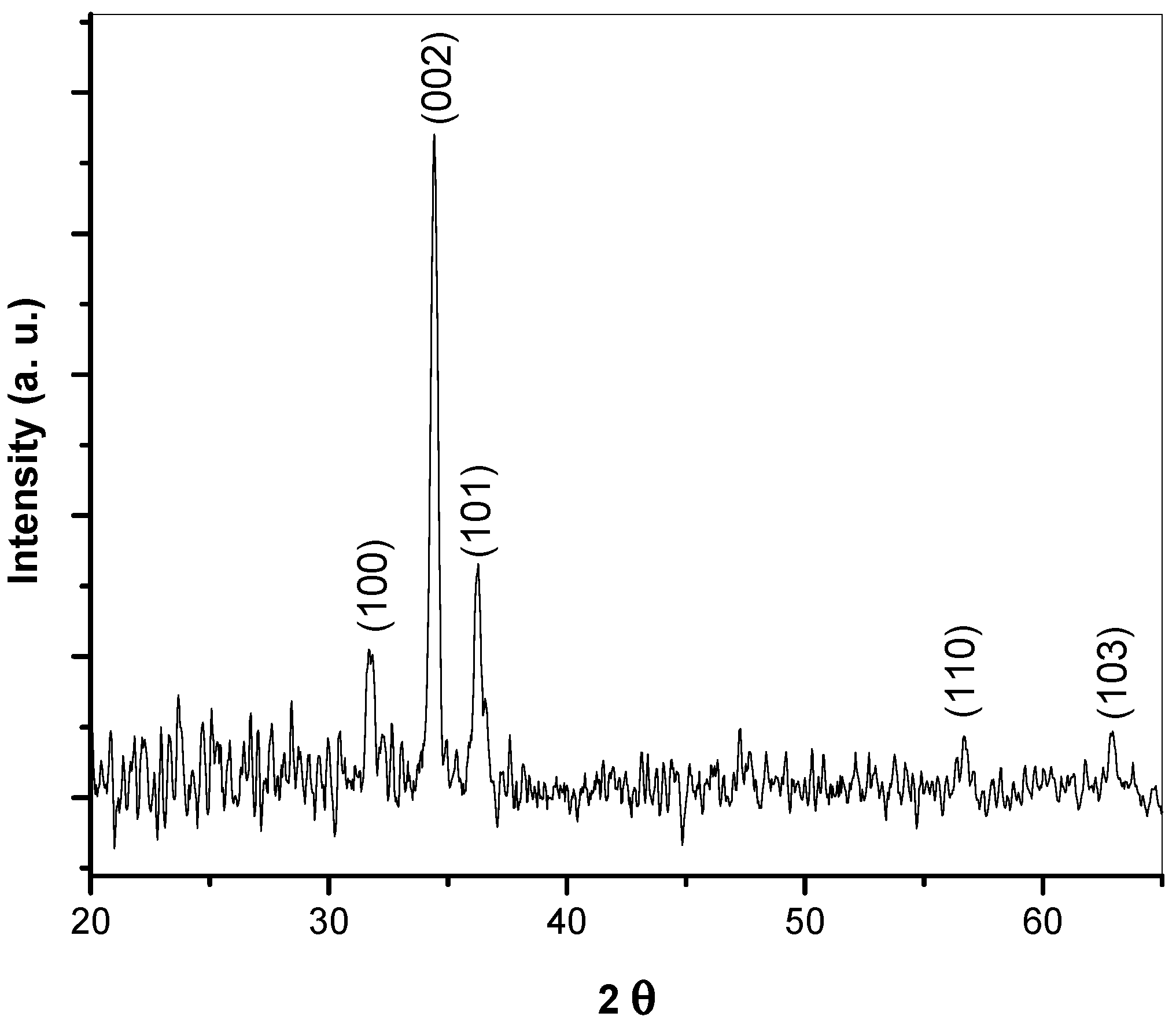
3.1. XRD Analysis
3.2. XPS Measurements
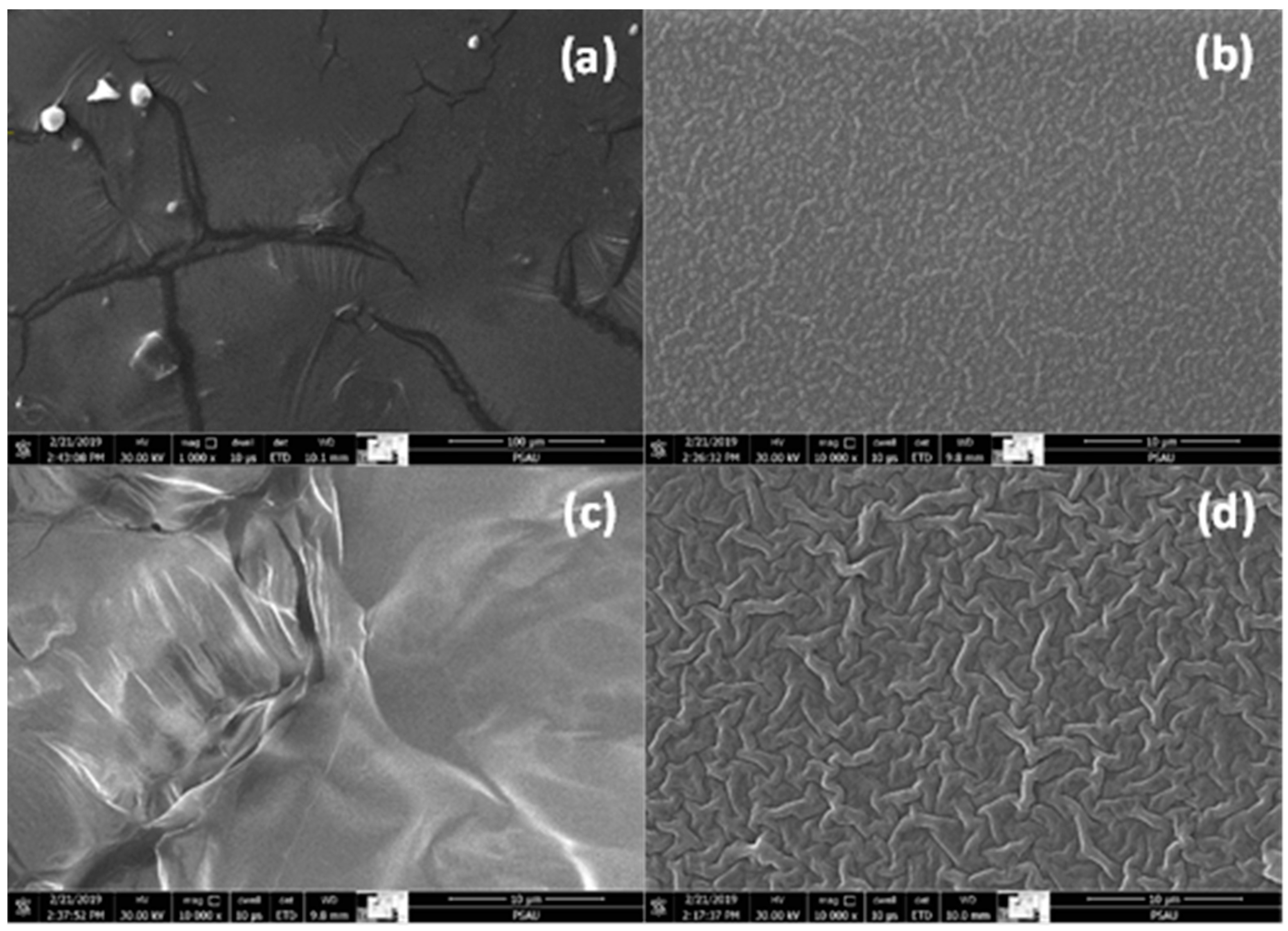
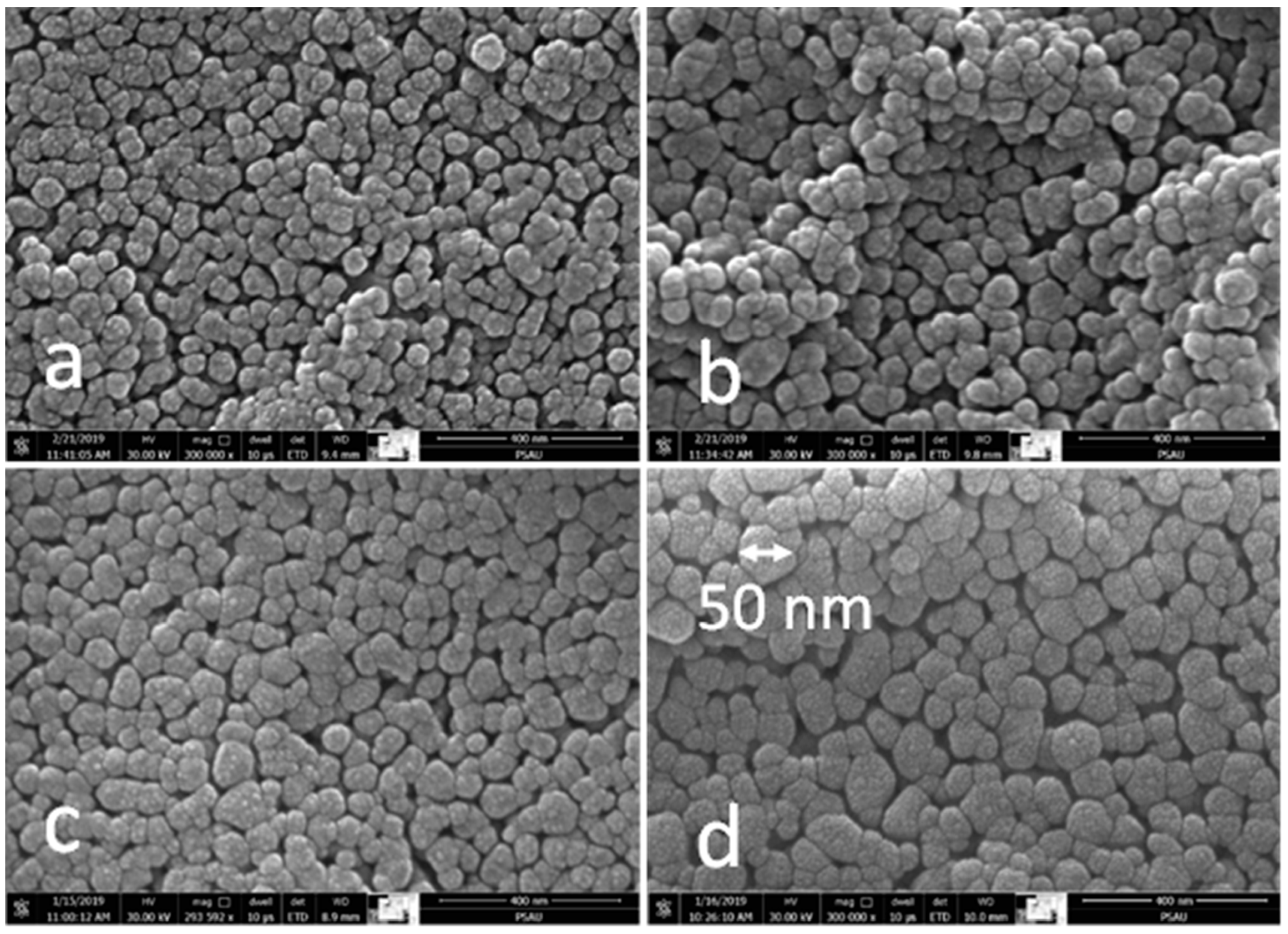
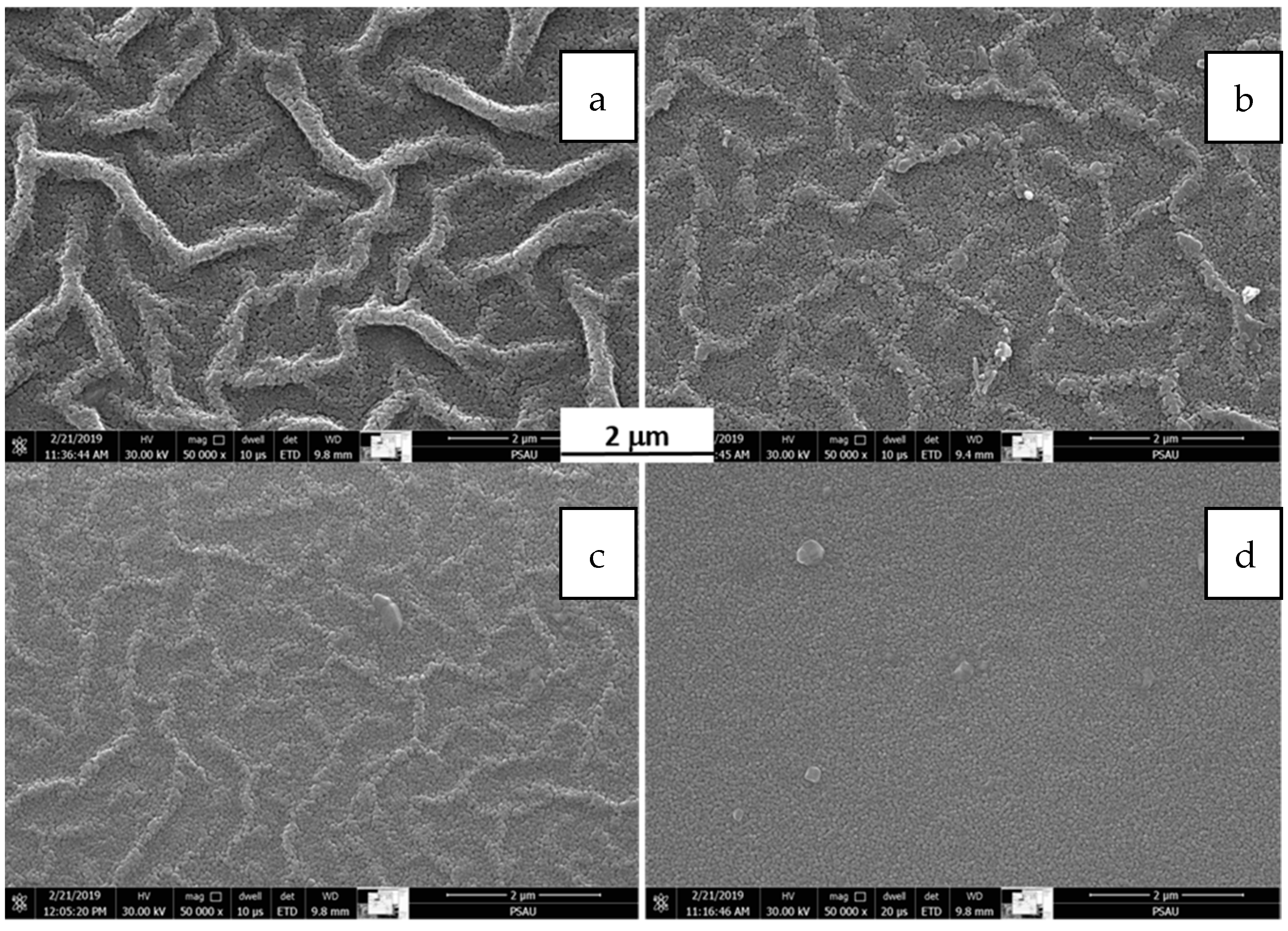
3.3. SEM Analysis
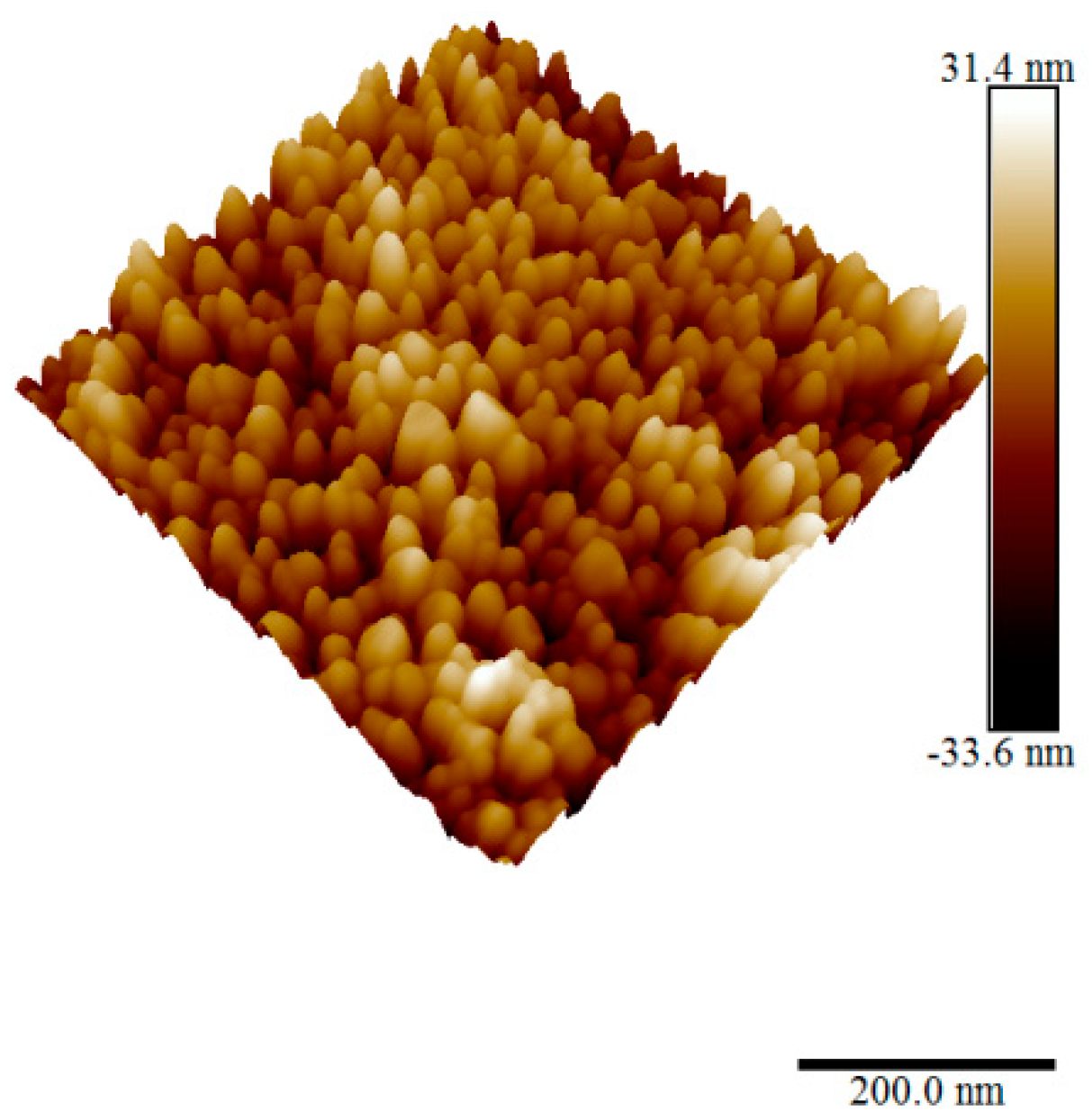
3.4. AFM Measurements
3.5. Ellipsometery Measurements
4. Conclusions
- For the 1.0 molar solution, the film surface had a wrinkled structure regardless of the spin coating speed.
- For the 0.5 molar solution, it was possible to tune the film surface morphology between wrinkle-free and wrinkled by varying the spin coating speed.
- The film had a critical thickness after which it wrinkled regardless of the spin coating speed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.-M.; Hong, Z.; Li, G.; Yang, Y. Recent Progress in Polymer Solar Cells: Manipulation of Polymer:Fullerene Morphology and the Formation of Efficient Inverted Polymer Solar Cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Jiang, L.; Cao, G. ZnO cathode buffer layers for inverted polymer solar cells. Energy Environ. Sci. 2015, 8, 3442–3476. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.-Q.; Dante, M.; Heeger, A.J. Efficient tandem polymer solar cells fabricated by all-solution processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Van Ijzendoorn, L.; De Voigt, M. Stability of the interface between indium-tin-oxide and poly (3,4-ethylenedioxythiophene)/poly (styrenesulfonate) in polymer light-emitting diodes. Appl. Phys. Lett. 2000, 77, 2255–2257. [Google Scholar] [CrossRef]
- Kawano, K.; Pacios, R.; Poplavskyy, D.; Nelson, J.; Bradley, D.D.; Durrant, J.R. Degradation of organic solar cells due to air exposure. Sol. Energy Mater. Sol. Cells 2006, 90, 3520–3530. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591. [Google Scholar] [CrossRef]
- Li, G.; Chu, C.-W.; Shrotriya, V.; Huang, J.; Yang, Y. Efficient inverted polymer solar cells. Appl. Phys. Lett. 2006, 88, 253503. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Wiranwetchayan, O.; Xi, J.; Yang, Z.; Park, K.; Li, C.; Cao, G. Effects of the morphology of a ZnO buffer layer on the photovoltaic performance of inverted polymer solar cells. Adv. Funct. Mater. 2012, 22, 2194–2201. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.-W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef]
- Yang, T.; Cai, W.; Qin, D.; Wang, E.; Lan, L.; Gong, X.; Peng, J.; Cao, Y. Solution-processed zinc oxide thin film as a buffer layer for polymer solar cells with an inverted device structure. J. Phys. Chem. C 2010, 114, 6849–6853. [Google Scholar] [CrossRef]
- Wong, K.; Yip, H.L.; Luo, Y.; Wong, K.Y.; Lau, W.M. Blocking reactions between indium-tin oxide and poly (3, 4-ethylene dioxythiophene): Poly (styrene sulphonate) with a self-assembly monolayer. Appl. Phys. Lett. 2002, 80, 2788–2790. [Google Scholar] [CrossRef]
- White, M.S.; Olson, D.C.; Shaheen, S.E.; Kopidakis, N.; Ginley, D.S. Inverted bulk-heterojunction organic photovoltaic device using a solution-derived ZnO underlayer. Appl. Phys. Lett. 2006, 89, 143517. [Google Scholar] [CrossRef]
- Ozgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 11. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Hassan, M.; Afify, A.S.; Ataalla, M.; Milanese, D.; Tulliani, J.-M. New ZnO-based glass ceramic sensor for H2 and NO2 detection. Sensors 2017, 17, 2538. [Google Scholar] [CrossRef]
- Chung, K.; Lee, C.-H.; Yi, G.-C. Transferable GaN layers grown on ZnO-coated graphene layers for optoelectronic devices. Science 2010, 330, 655–657. [Google Scholar] [CrossRef]
- Chen, Y.; Bagnall, D.M.; Koh, H.-J.; Park, K.-T.; Hiraga, K.; Zhu, Z.; Yao, T. Plasma assisted molecular beam epitaxy of ZnO on c-plane sapphire: Growth and characterization. J. Appl. Phys. 1998, 84, 3912–3918. [Google Scholar] [CrossRef]
- Faÿ, S.; Kroll, U.; Bucher, C.; Vallat-Sauvain, E.; Shah, A. Low pressure chemical vapour deposition of ZnO layers for thin-film solar cells: Temperature-induced morphological changes. Sol. Energy Mater. Sol. Cells 2005, 86, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Carcia, P.F.; McLean, R.S.; Reilly, M.H.; Nunes, G. Transparent ZnO thin-film transistor fabricated by rf magnetron sputtering. Appl. Phys. Lett. 2003, 82, 1117–1119. [Google Scholar] [CrossRef]
- Matsubara, K.; Fons, P.; Iwata, K.; Yamada, A.; Sakurai, K.; Tampo, H.; Niki, S. ZnO transparent conducting films deposited by pulsed laser deposition for solar cell applications. Thin Solid Film 2003, 431, 369–372. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Elsayed, I.; Fahmy, T. Substrate temperature and laser fluence effects on properties of ZnO thin films deposited by pulsed laser deposition. J. Mater. Sci. Mater. Electron. 2018, 29, 19942–19950. [Google Scholar] [CrossRef]
- Ong, B.S.; Li, C.; Li, Y.; Wu, A.Y.; Loutfy, R. Stable, solution-processed, high-mobility ZnO thin-film transistors. J. Am. Chem. Soc. 2007, 129, 2750–2751. [Google Scholar] [CrossRef] [PubMed]
- Ilican, S.; Caglar, Y.; Caglar, M. Preparation and characterization of ZnO thin films deposited by sol-gel spin coating method. J. Optoelectron. Adv. Mater. 2008, 10, 2578–2583. [Google Scholar]
- Kamalasanan, M.; Chandra, S. Sol-gel synthesis of ZnO thin films. Thin Solid Film 1996, 288, 112–115. [Google Scholar] [CrossRef]
- Andrade, E.; Miki-Yoshida, M. Growth, structure and optical characterization of high quality ZnO thin films obtained by spray pyrolysis. Thin Solid Film 1999, 350, 192–202. [Google Scholar]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Joo, J.; Kim, D.; Yun, D.-J.; Jun, H.; Rhee, S.-W.; Lee, J.S.; Yong, K.; Kim, S.; Jeon, S. The fabrication of highly uniform ZnO/CdS core/shell structures using a spin-coating-based successive ion layer adsorption and reaction method. Nanotechnology 2010, 21, 325604. [Google Scholar] [CrossRef]
- Jagadamma, L.K.; Abdelsamie, M.; El Labban, A.; Aresu, E.; Ndjawa, G.O.N.; Anjum, D.H.; Cha, D.; Beaujuge, P.M.; Amassian, A. Efficient inverted bulk-heterojunction solar cells from low-temperature processing of amorphous ZnO buffer layers. J. Mater. Chem. A 2014, 2, 13321–13331. [Google Scholar] [CrossRef]
- Park, H.-Y.; Lim, D.; Kim, K.-D.; Jang, S.-Y. Performance optimization of low-temperature-annealed solution-processable ZnO buffer layers for inverted polymer solar cells. J. Mater. Chem. A 2013, 1, 6327–6334. [Google Scholar] [CrossRef]
- Yu, X.; Yu, X.; Hu, Z.; Zhang, J.; Zhao, G.; Zhao, Y. Effect of sol–gel derived ZnO annealing rate on light-trapping in inverted polymer solar cells. Mater. Lett. 2013, 108, 50–53. [Google Scholar] [CrossRef]
- Ma, Z.; Tang, Z.; Wang, E.; Andersson, M.R.; Inganäs, O.; Zhang, F. Influences of surface roughness of ZnO electron transport layer on the photovoltaic performance of organic inverted solar cells. J. Phys. Chem. C 2012, 116, 24462–24468. [Google Scholar] [CrossRef]
- Ambade, R.B.; Ambade, S.B.; Mane, R.S.; Lee, S.-H. Interfacial engineering importance of bilayered ZnO cathode buffer on the photovoltaic performance of inverted organic solar cells. ACS Appl. Mater. Interfaces 2015, 7, 7951–7960. [Google Scholar] [CrossRef]
- Burrell, A.K.; Mark McCleskey, T.; Jia, Q.X. Polymer assisted deposition. Chem. Commun. 2008, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Lin, Y.; Minogue, E.M.; Burrell, A.K.; McCleskey, T.M.; Jia, Q.; Lu, P. Polymer Assisted Deposition (PAD) of thin metal films: A new technique to the preparation of metal oxides and reduced metal films. MRS Proc. 2011, 893, 0893-JJ05-13. [Google Scholar] [CrossRef]
- Vila-Fungueiriño, J.M.; Rivas-Murias, B.; Rubio-Zuazo, J.; Carretero-Genevrier, A.; Lazzari, M.; Rivadulla, F. Polymer assisted deposition of epitaxial oxide thin films. J. Mater. Chem. C 2018, 6, 3834–3844. [Google Scholar] [CrossRef]
- Kumar, A.S.; Nagaraja, K.K.; Nagaraja, H.S. Polymer assisted preparation and characterization of ZnO and Sn doped ZnO nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012077. [Google Scholar] [CrossRef]
- Jia, Q.X.; Mccleskey, T.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529–532. [Google Scholar] [CrossRef]
- Dai, T.-F.; Hsu, W.-C.; Hsu, H.-C. Improvement of photoluminescence and lasing properties in ZnO submicron spheres by elimination of surface-trapped state. Opt. Express 2014, 22, 27169–27174. [Google Scholar] [CrossRef]
- Farrag, A.A.; Balboul, M.R. Nano ZnO thin films synthesis by sol–gel spin coating method as a transparent layer for solar cell applications. J. Sol-Gel. Sci. Technol. 2017, 82, 269–279. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, I.A.; Afify, A.S. Controlling the Surface Morphology of ZnO Nano-Thin Film Using the Spin Coating Technique. Materials 2022, 15, 6178. https://doi.org/10.3390/ma15176178
Elsayed IA, Afify AS. Controlling the Surface Morphology of ZnO Nano-Thin Film Using the Spin Coating Technique. Materials. 2022; 15(17):6178. https://doi.org/10.3390/ma15176178
Chicago/Turabian StyleElsayed, I. A., and Ahmed S. Afify. 2022. "Controlling the Surface Morphology of ZnO Nano-Thin Film Using the Spin Coating Technique" Materials 15, no. 17: 6178. https://doi.org/10.3390/ma15176178





