Chemically Synthesized Iron-Oxide-Based Pure Negative Electrode for Solid-State Asymmetric Supercapacitor Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Negative α-Fe2O3-NF Electrode
2.2. Synthesis of the Positive Co3O4-NF Electrode
3. Results and Discussion
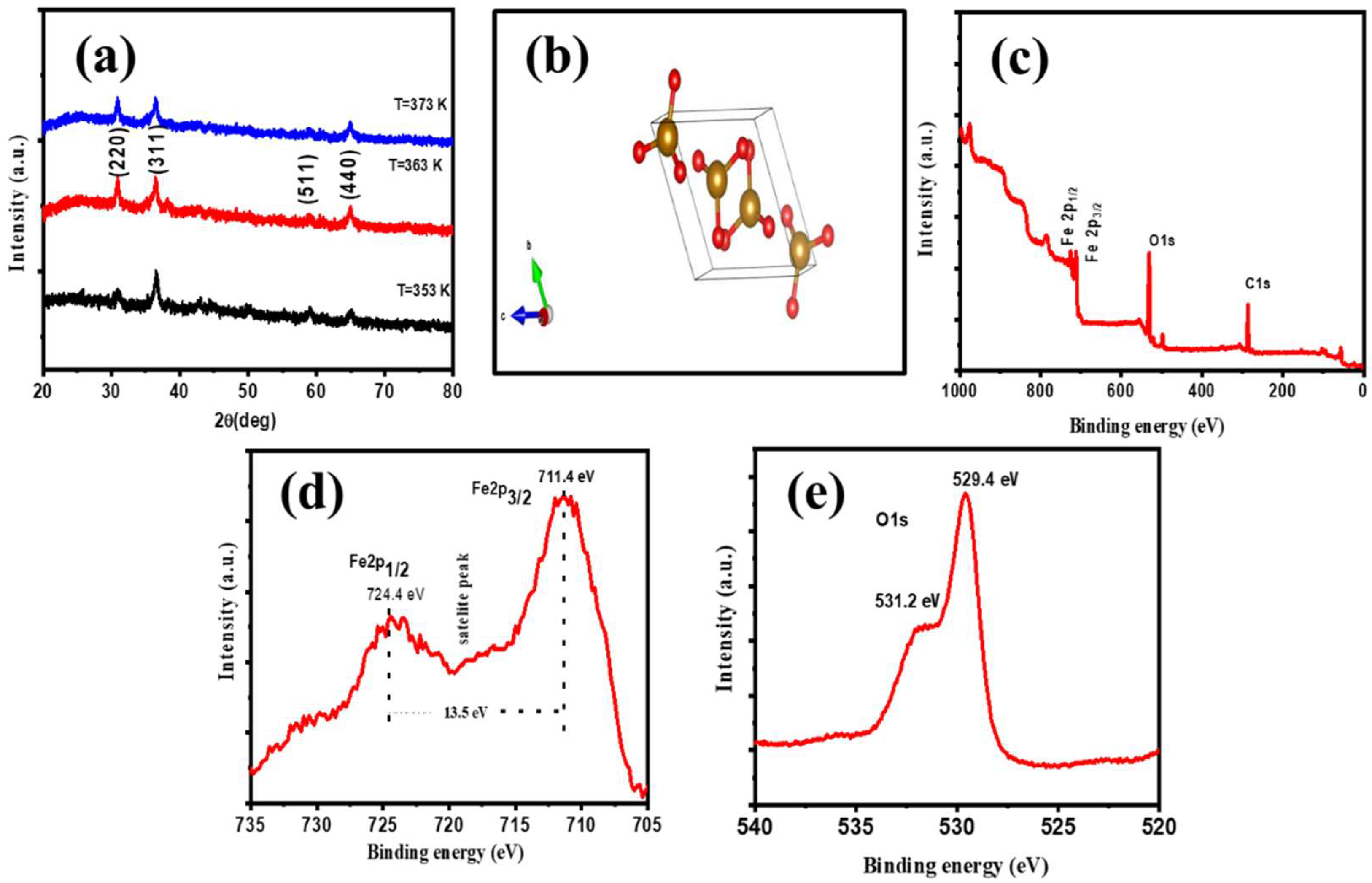
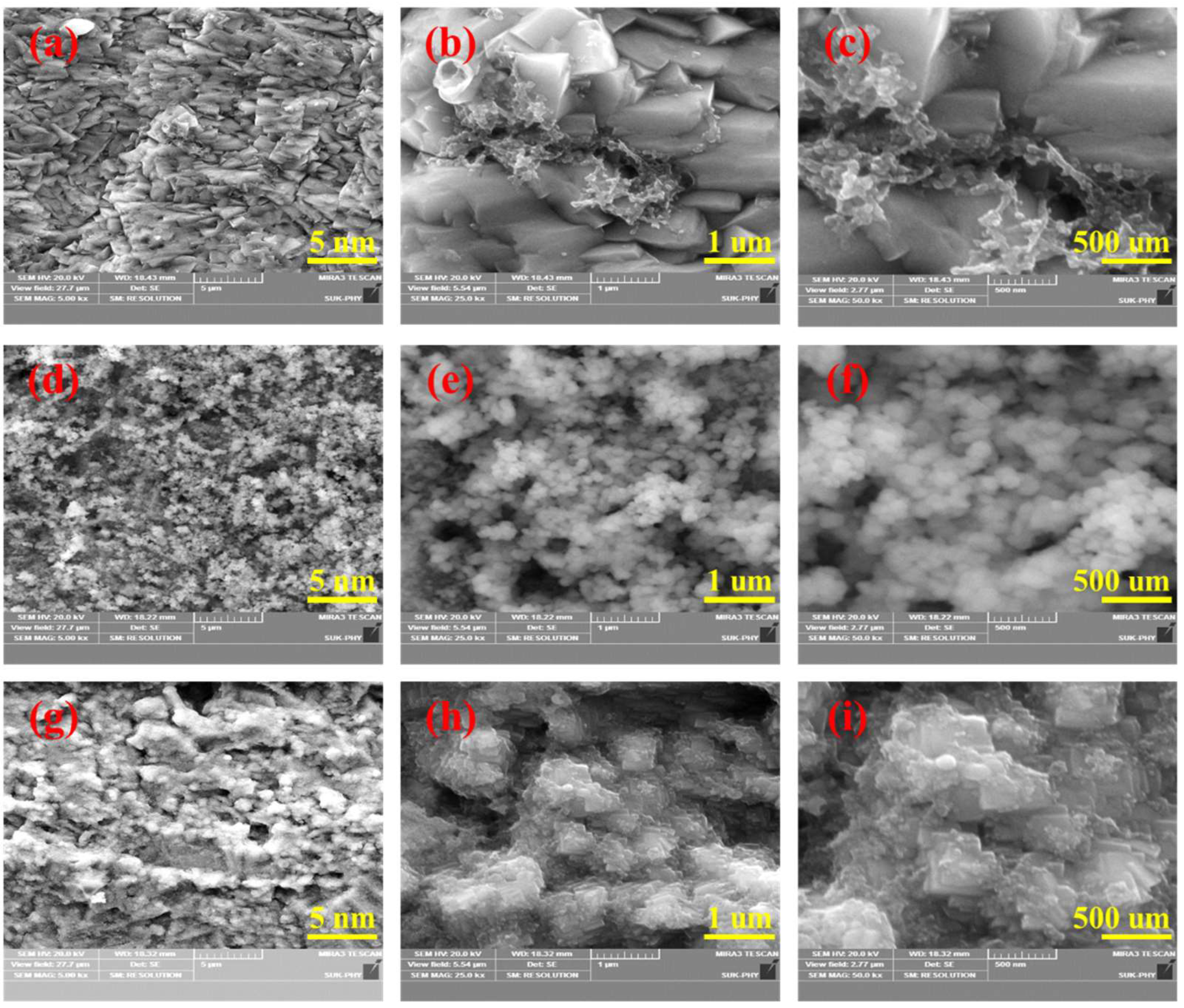
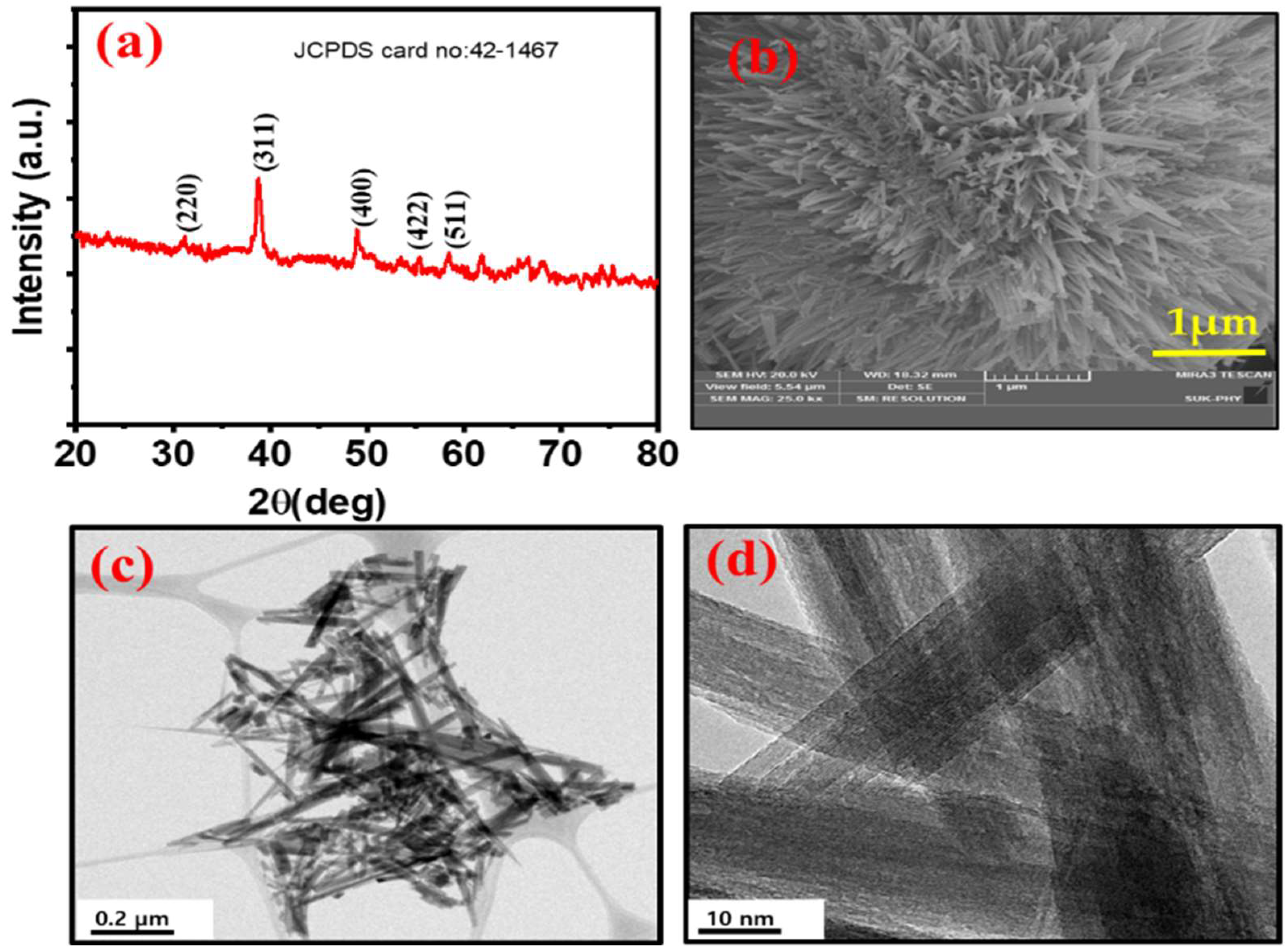
3.1. Structural and Morphological Characterization of the Negative α-Fe2O3-NF Electrode
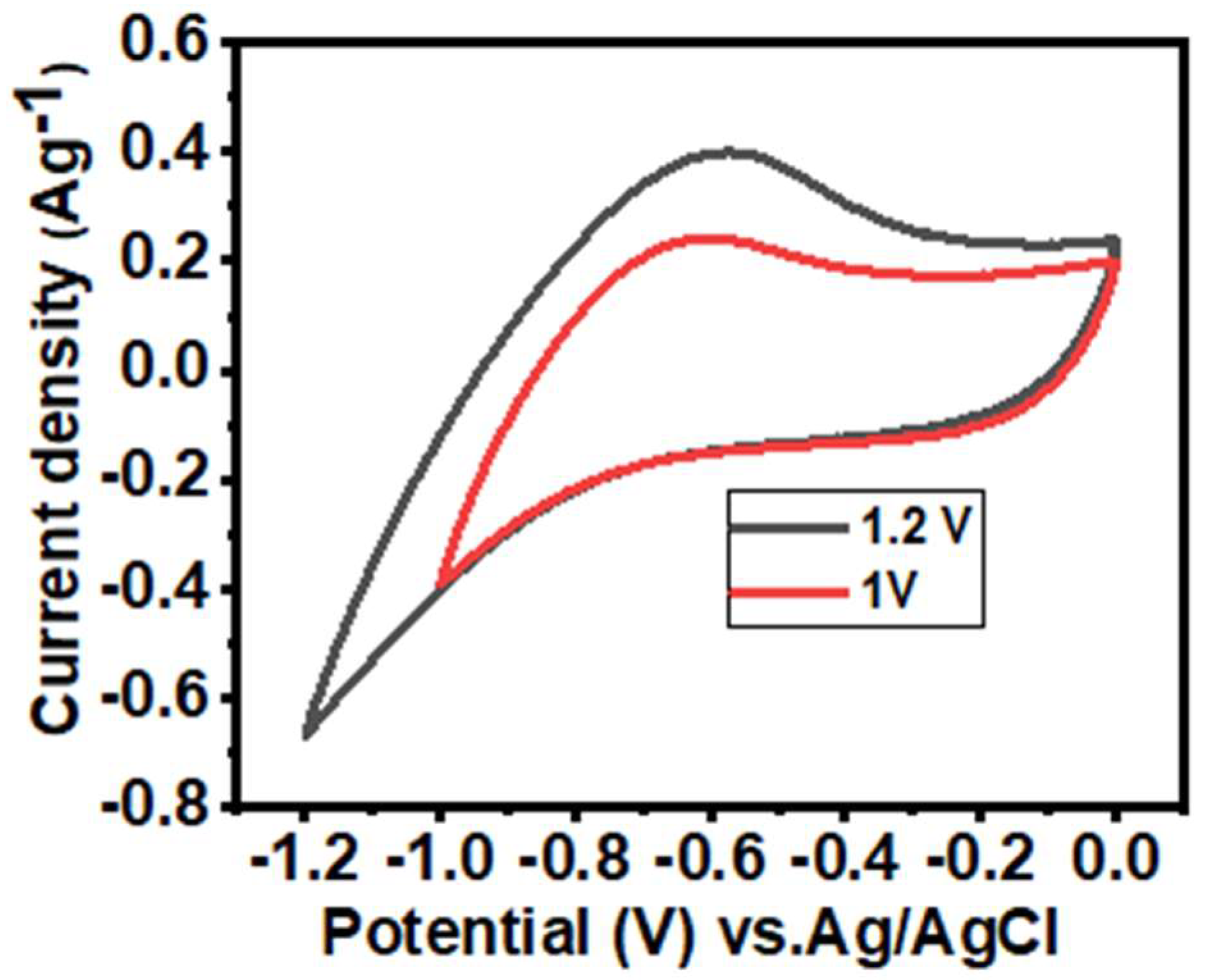
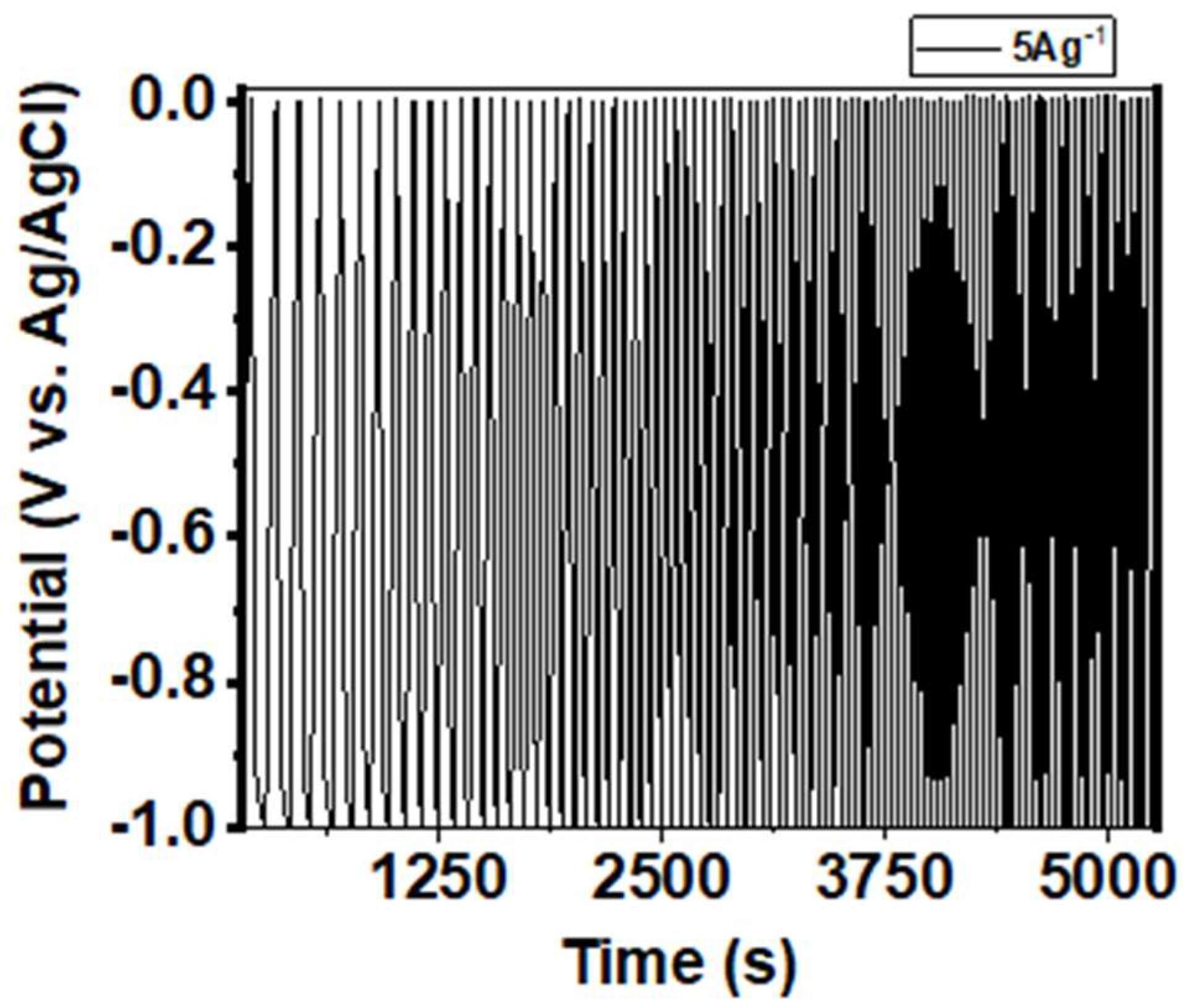
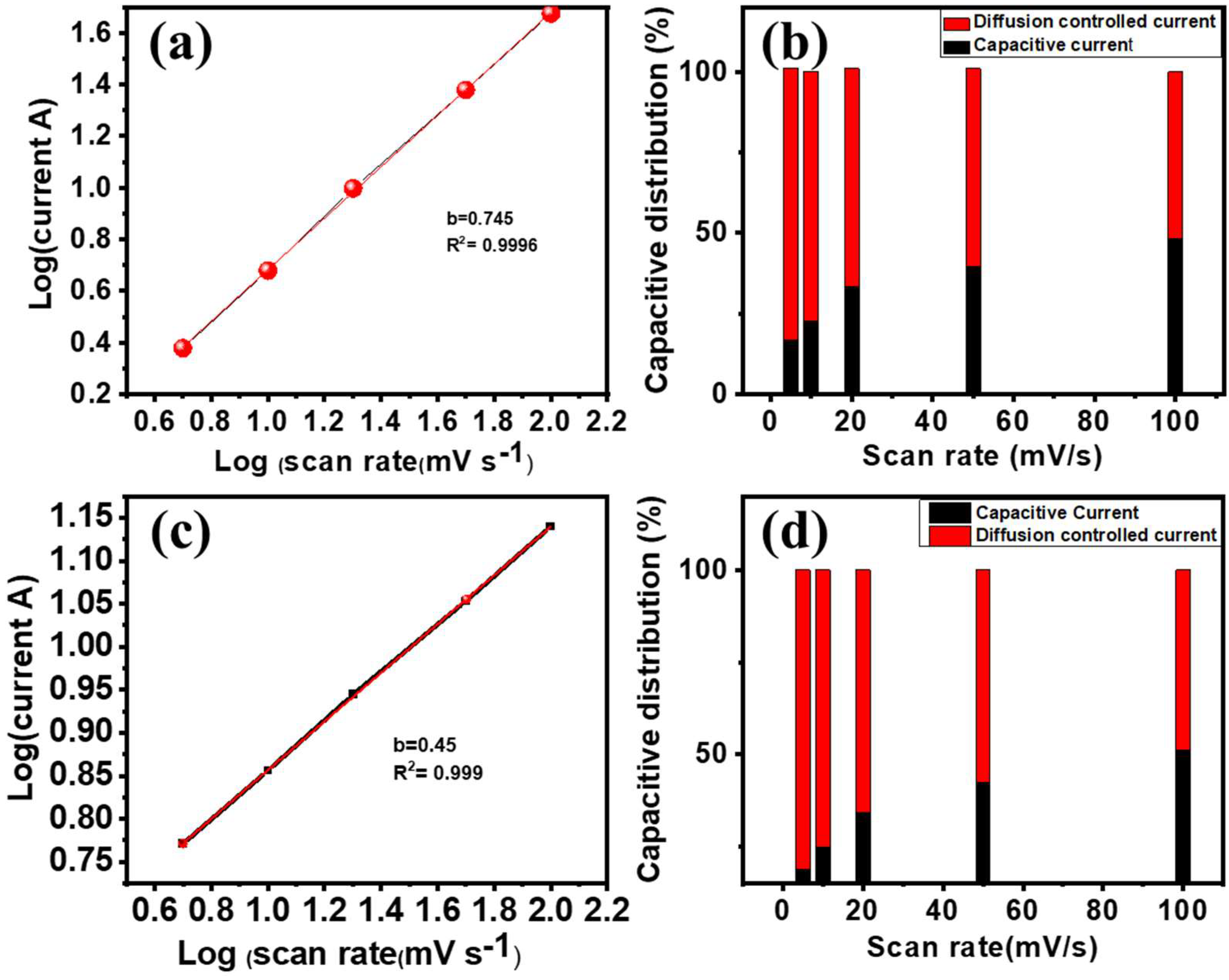
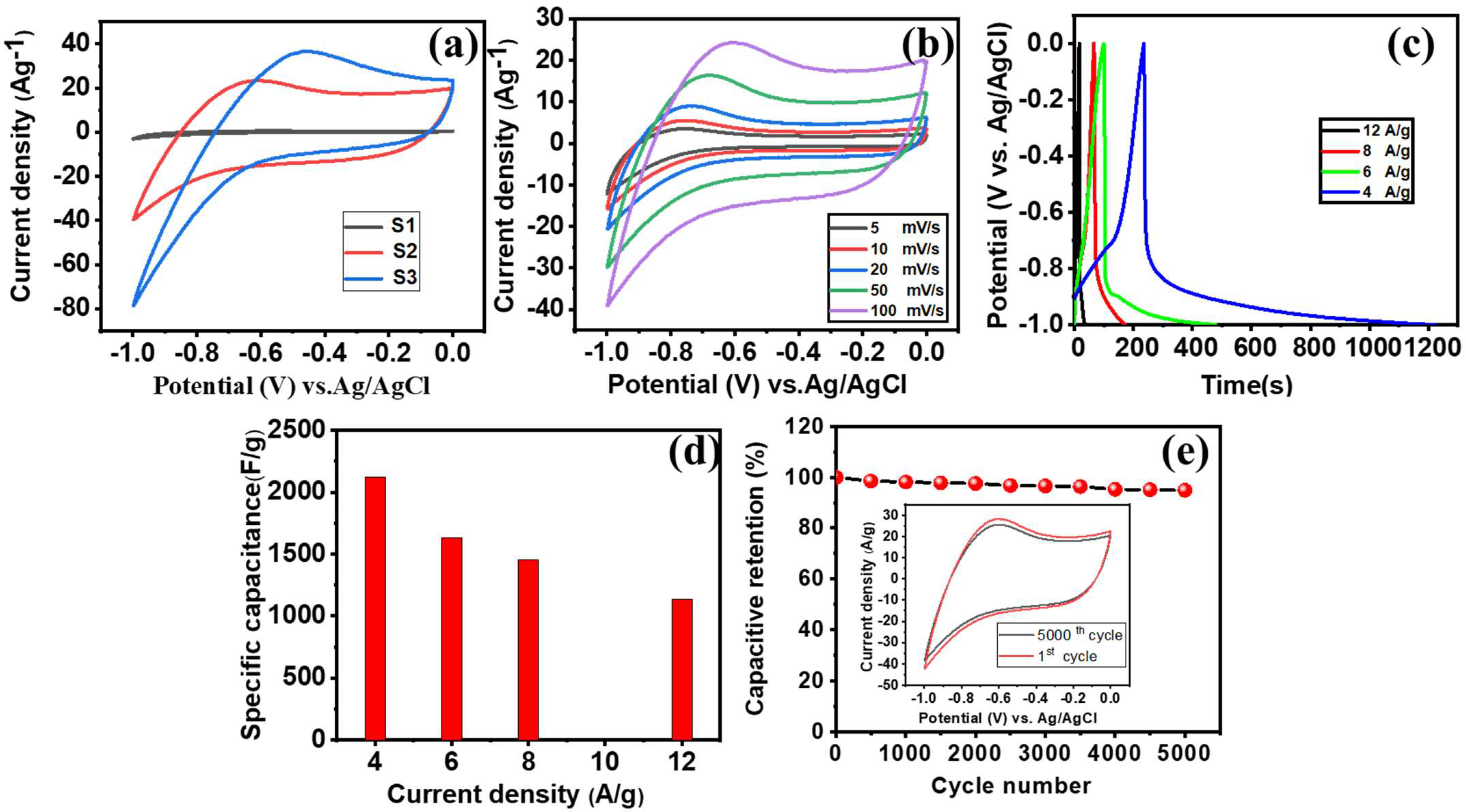
3.2. Electrochemical Performance of the α-Fe2O3-NF Electrode
3.3. Structural and Morphological Characterization of the Positive Co3O4-NF Electrode
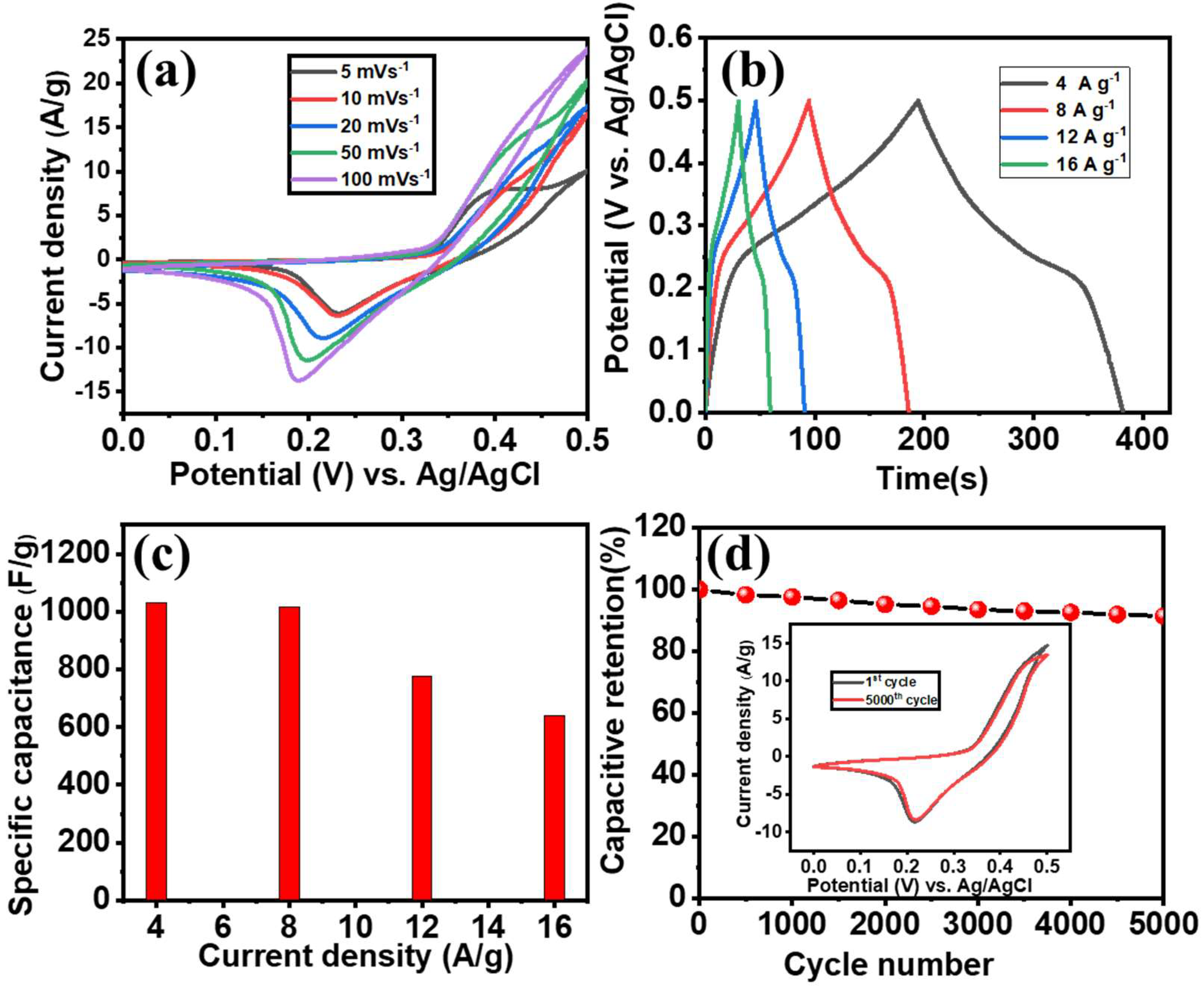
3.4. Electrochemical Performance of the Positive Co3O4-NF Electrode
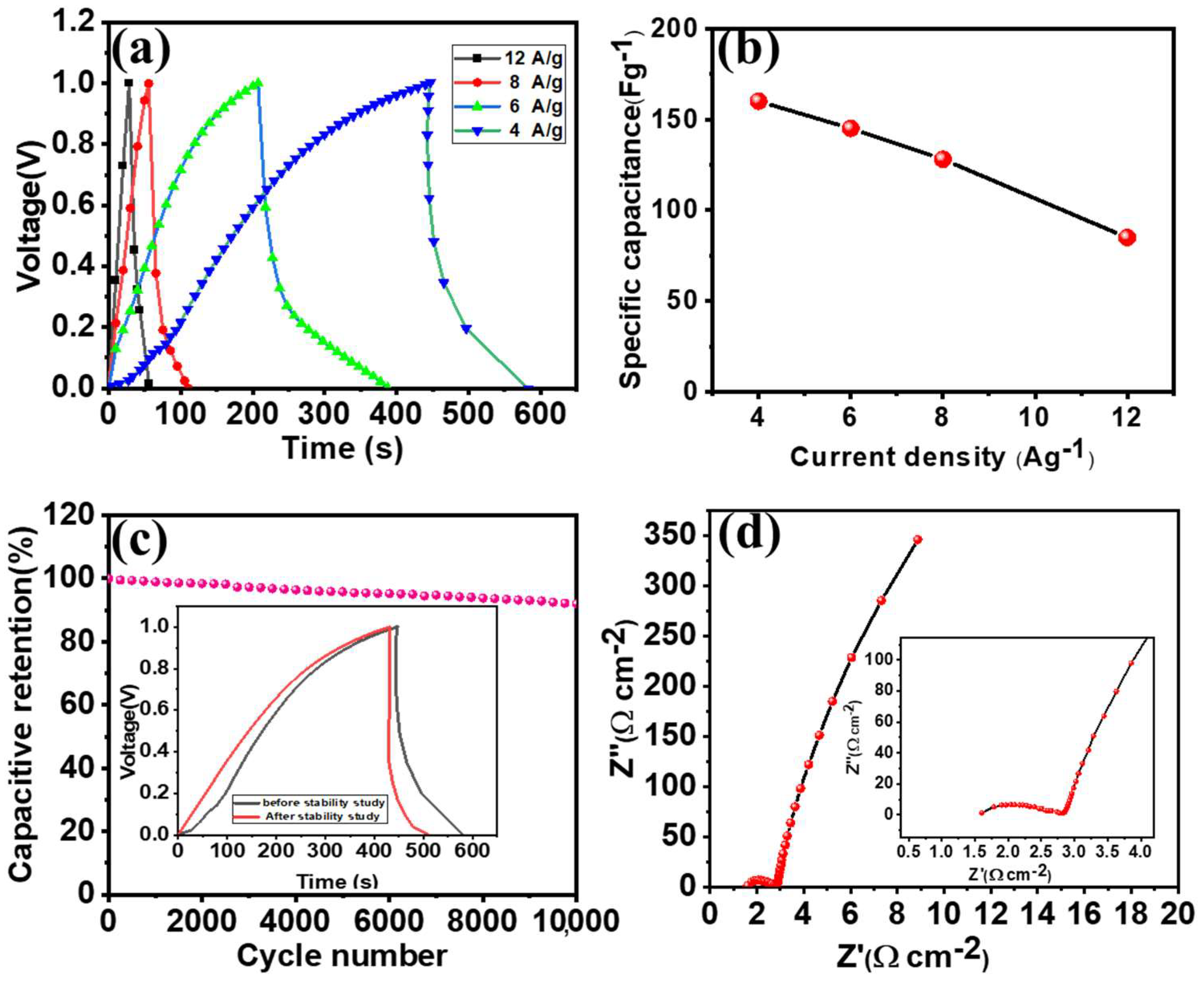
3.5. Supercapacitive Performance of the Solid-State Co3O4-NF//α-Fe2O3-NFASC Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Materials Characterization
Appendix A.2. Electrochemical Characterization
Appendix A.3. Asymmetric Supercapacitor Device Based on α-Fe2O3-NF//Co3O4-NF

| Material | Electrolyte | Capacitance | Stability | Ref. |
|---|---|---|---|---|
| Fe2O3/CF | 5 M LiCl | 180.4 mF/cm2 | - | [44] |
| Fe2O3-C | 3 M KOH | 247.5 mAh/g (2 mV/s) | 64% (5000) | [42] |
| Fe2O3 NF | 5 M LiCl | 145.9 mF/cm2 10 mA/cm2 | 87.2% (5000) | [45] |
| Fe2O3@C | 6 M KOH | 304.9 at 1 A/g | 90.7% (2000) | [46] |
| CF-Fe2O3 | 2 M KOH | 1.56 F/cm2 at 10 mA/cm2 | 102% (5000) | [47] |
| Fe2O3/CF | 0.5 M LiClO4 | 261 F/g at 1 A/g | 82.7% (10,000) | [48] |
| Fe2O3/CF | 2 M KOH | 908 F/g at 10 A/g | 90% (5000) | [36] |
| Fe2O3-NF | 2 M KOH | 2125 F/g at 4 A/g | 95.2% (5000) | Present work |
| ASC Devices | Electrolyte | Specific Capacitance | Energy Density | Power Density | Ref. |
|---|---|---|---|---|---|
| CuO//Fe2O3 | CMC-Na2SO4 | 79 F/g | 23 Wh/kg | 19 kW/kg | [49] |
| MnO2@CuO//Fe2O3@C | PVA-LiCl | 2.46 F/cm3 (0.13 A/cm2) | 0.85 mWh/cm3 | 0.1 W/cm3 | [50] |
| CF-Co3O4//CF-Fe2O3 | PVA-KOH | 17.5 F/cm3 (6 mA/cm2) | 6.75 mWh/cm3 | 104 mW/cm3 | [51] |
| MnO2///Fe2O3 | PVA-LiClO4 | 74 F/g (5 mV/s) | 33.1 Wh/kg | 1.32 kW/kg | [44] |
| MnO2//Fe2O3 | PVA-LiCl | 1.21 F/cm3 (0.5 mA/cm2) | 0.41 mWh/cm3 | - | [51] |
| MnO2/CF//Fe2O3/CF | PVA-LiCl | 1.5 F/cm3 (0.5 mA/cm2) | 0.55 mWh/cm3 | - | [44] |
| NiO//Fe2O3 | PVA KOH | 57.2 F/g | 12.4 Wh/kg | 951 W/kg | [32] |
| Co3O4-NF//Fe2O3-NF | PVA KOH | 155 F/g | 21.5 Wh/kg | 158 kW/kg | Present work |
References
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mat. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xing, J.; Gao, Y.; Lv, X.; He, Y.; Guo, Z.; Li, Y. Ordered assembly of NiCo2O4 multiple hierarchical structures for high-performance pseudocapacitors. ACS Appl. Mater. Interfaces 2014, 6, 11394–11402. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhou, G.; Zhang, H.; Cheng, C.; Ma, J.; Wei, W.; Chen, L.; Wang, T. Ultrathin porous NiCo2O4 nanosheet arrays on flexible carbon fabric for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 7405–7409. [Google Scholar] [CrossRef]
- Zhou, W.; Kong, D.; Jia, X.; Ding, C.; Cheng, C.; Wen, G. NiCo2O4 nanosheet supported hierarchical core–shell arrays for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 6310–6315. [Google Scholar] [CrossRef]
- Cai, D.; Xiao, S.; Wang, D.; Liu, B.; Wang, L.; Liu, Y.; Li, H.; Wang, Y.; Li, Q.; Wang, T. Morphology controlled synthesis of NiCo2O4 nanosheet array nanostructures on nickel foam and their application for pseudocapacitors. Electrochim. Acta 2014, 142, 118–124. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X. Controlled growth of NiCo2O4 nanorods and ultrathin nanosheets on carbon nanofibers for high-performance supercapacitors. Sci. Rep. 2013, 3, 1470. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Y.; Cao, X.; Ouyang, B.; Sun, W.; Tan, C.; Zhang, Y.; Ma, Q.; Liang, S.; Yan, Q.; et al. Two-dimensional NiCo2O4 nanosheet-coated three-dimensional graphene networks for high-rate, long-cycle-life supercapacitors. Nanoscale 2015, 7, 7035–7039. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Cao, L.; Cui, X.; Yang, Y.; Xiao, P.; Zhang, Y. The impact of morphologies and electrolyte solutions on the supercapacitive behavior for Fe2O3 and the charge storage mechanism. Electrochim. Acta 2015, 178, 171–178. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Progress and development of Fe3O4 electrodes for supercapacitors. J. Mater. Chem. A 2016, 4, 10767–10778. [Google Scholar] [CrossRef]
- Khatavkar, S.N.; Sartale, S.D. α-Fe2O3 thin films by liquid phase deposition: Low-cost option for supercapacitor. J. Solid State Electrochem. 2016, 21, 2555–2566. [Google Scholar] [CrossRef]
- He, Y.M.; Chen, W.J.; Li, X.D.; Zhang, Z.X.; Fu, J.C.; Zhao, C.H.; Xie, E.Q. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Wei, C.; Lei, Z.; Yang, H.; Zhu, M.; Yan, H.; Fu, Y.; Geng, F.; Jie, Y.; Chen, X. Porous Fe3O4/carbon composite electrode material prepared from metal-organic framework template and effect of temperature on its capacitance. Nano Energy 2014, 8, 133–140. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W. Morphology-dependent nanocatalysts: Rod-shaped oxides. Chem Soc. Rev. 2014, 43, 1543–1574. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. Alpha-Fe2O3 as a photocatalytic material: A review. Applied Catalysis A: General. Appl. Catal. A 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Nguyen, T.; Silva, T.M.; Carmezim, M.J.; Montemor, M.F. Electrodeposited MoOx films as negative electrode materials for redox supercapacitors. Electrochim. Acta 2017, 225, 19–28. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Zhai, T.; Wang, G.; Xie, S.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 2013, 13, 2628–2633. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Zan, G. A high-performance supercapacitor with well-dispersed Bi2O3 nanospheres and active-carbon electrodes. Eur. Inorg. Chem. 2015, 201, 5751–5756. [Google Scholar] [CrossRef]
- Bak, C.; Kim, K.; Jung, K.; Kim, J.B.; Jang, J.H. Efficient photoelectrochemical water splitting of nanostructured hematite on a three-dimensional nanoporous metal electrode. J. Mater. Chem. A 2014, 2, 17249–17252. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Hunge, Y.M. Photoelectrocatalytic degradation of 4-chlorophenol using nanostructured α-Fe2O3 thin films under sunlight illumination. J. Mar. Sci. 2017, 28, 11260–11267. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kulkarni, S.B. Chemical synthesis of Co3O4 nanowires for symmetric supercapacitor device. J. Mar. Sci. 2018, 29, 16401–16409. [Google Scholar] [CrossRef]
- Hjiri, M. Highly sensitive NO2 gas sensor based on hematite nanoparticles synthesized by sol–gel technique. J. Mar. Sci. Eng. 2020, 31, 5025–5031. [Google Scholar] [CrossRef]
- Xiao, T.; Che, P.; Xiao, R.; Xiang, P.; Jiang, L.; Tao, F.; Tan, X.; Chen, X. 3D interconnected Fe-Co-S nanosheets network directly grown on graphene coated nickel foam with enhanced electrochemical performance for asymmetric supercapacitors. Appl. Surf. Sci. 2021, 543, 148747. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Skrifterutgittav det Norske Videnskaps-Akademi I Oslo 1: Matematisk—NaturvidenskpelingKlasse; New York Botanical Garden: Bronx, NY, USA, 1998; pp. 1–165. [Google Scholar]
- Wang, J.-C.; Ren, J.; Yao, H.-C.; Zhang, L.; Wang, J.-S.; Zang, S.-Q.; Han, L.-F.; Li, Z.-J. Synergistic photocatalysis of Cr (VI) reduction and 4-chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation. J. Hazard. Mater. 2016, 311, 11–19. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Zetaruk, D.G. X-ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977, 49, 1521–1529. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Lee, K.K.; Deng, S.; Fan, H.M.; Mhaisalkar, S.; Tan, H.R.; Tok, E.S.; Loh, K.P.; Chin, W.S.; Sow, C.H. α-Fe2O3 nanotubes-reduced graphene oxide composites as synergistic electrochemical capacitor materials. Nanoscale 2012, 4, 2958–2961. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Q.; Yang, K.; Tan, Y.; Tain, W.; Zhu, L.; Li, Z.; Yang, C. Solvothermally induced α-Fe2O3/graphene nanocomposites with ultrahigh capacitance and excellent rate capability for supercapacitors. J. Mater. Chem. A 2015, 3, 22005–22011. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, B.; Wang, Z.; Peter, F. Super long-life all solid-state asymmetric supercapacitor based on NiO nanosheets and α-Fe2O3 nanorods. Chem. Eng. J. 2016, 306, 193–203. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, C.; Ma, L.; Fu, L.; Li, G.; Hu, Q.; Liu, Q. MOF-derived Fe2O3 decorated with MnO2 nanosheet arrays as anode for high energy density hybrid supercapacitor. Chem. Eng. J. 2021, 417, 129243. [Google Scholar] [CrossRef]
- Tang, D.; Wang, W.; Wang, G. The perfect matching between the low-cost Fe2O3 nanowire anode and the NiO nanoflake cathode significantly enhance the energy density of asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 6662–6670. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Liu, S.; Kulkarni, S.B. Ultrasound assisted growth of NiCo2O4@carbon cloth for high energy storage device application. Ultrason. Sonochem. 2019, 56, 290–292. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, X.; Lou, X.W. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Xu, J.; Wang, T.; Liu, X.; Duan, X.; Ma, Y.; Zhou, Y.; Pei, C. Multi-channeled hierarchical porous carbon incorporated Co3O4 nanopillar arrays as 3D binder-free electrode for high performance supercapacitors. Nano Energy 2016, 20, 94–107. [Google Scholar] [CrossRef]
- Demarconnay, L.; Raymundo-Pinero, E.; Beguin, F. Adjustment of electrodes potential window in an asymmetric carbon/MnO2 supercapacitor. J. Power Sources 2011, 196, 580–586. [Google Scholar] [CrossRef]
- Sahoo, R.; Roy, A.; Ray, C.; Mondal, C.; Negishi, Y.; Yusuf, S.M.; Pal, A.; Pal, T. Decoration of Fe3O4 base material with Pd loaded CdS nanoparticle for superior photocatalytic efficiency. J. Phys. Chem. C 2014, 118, 11485–11494. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Ouyanga, J.; Jia, D.; Zhou, Y. Flexible and solid-state asymmetric supercapacitor based on ternary graphene/MnO2/carbon black hybrid film with high power performance. Electrochim. Acta. 2015, 182, 861–870. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kulkarni, S.B. Synthesis of multifunctional FeCo2O4 electrode using ultrasonic treatment for photocatalysis and energy storage. Ultrason. Sonochem. 2019, 58, 104663. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-stabilized high-capacity ferroferric oxide nanorod array for flexible solid-state alkaline battery–supercapacitor hybrid device with high environmental suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Pham, H.D.; Nanjundan, A.-K.; Fernando, J.F.S.; Jayaramulu, K.; Golberg, D.; Han, Y.-K.; Dubal, D.P. True meaning of psudocapacitors and their performance metrics: Asymmetric versus hybrid supercapacitors. Small 2020, 16, 2002806. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ding, Y.; Lin, Z.; Chen, Z.; Li, Y.; Qiang, P.; Ebrahimi, M.; Mai, W.; Wong, C.P.; Wang, Z.L. Low-Cost High-Performance Solid-State Asymmetric Supercapacitors Based on MnO2 Nanowires and Fe2O3 Nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high-performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, H.; Wu, F.; Wang, R.; Pan, M. One-step self-assembly synthesis α- Fe2O3 with carbon-coated nanoparticles for stabilized and enhanced supercapacitors electrode. Energies 2017, 10, 1296. [Google Scholar] [CrossRef]
- Li, T.; Yu, H.; Zhi, L.; Zhang, W.; Dang, L.; Liu, Z.; Lei, Z. Facile electrochemical fabrication of porous Fe2O3 nanosheets for flexible asymmetric supercapacitors. J. Phys. Chem. C 2017, 121, 18982–18991. [Google Scholar] [CrossRef]
- Cho, S.; Patil, B.; Yu, S.; Ahn, S.; Hwang, J.; Park, C.; Do, K.; Ahn, H. Flexible swiss roll fiber-shaped asymmetric supercapacitor using MnO2 and Fe2O3 on carbon fibers. Electrochim. Acta 2018, 269, 499–508. [Google Scholar] [CrossRef]
- Shinde, A.V.; Chodankar, N.R.; Lokhande, A.C.; Ji, T.; Kim, J.H.; Lokhande, C.D. Highly energetic flexible all-solid-state asymmetric supercapacitor with Fe2O3 and CuO thin films. RSC Adv. 2016, 6, 58839–58843. [Google Scholar] [CrossRef]
- Yu, Z.; Moore, J.; Calderon, J.; Zhai, L.; Thomas, J. Coil-Type Asymmetric SupercapacitorElectrical Cables. Small 2015, 11, 5289–5295. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, Y.; Yu, M.; Zhai, T.; Liang, C.; Xie, S.; Balogun, M.S.; Tong, Y. Oxygen-deficient hematite nanorods as high-performance and novel negative electrodes for flexible asymmetric supercapacitors. Adv. Mater. 2014, 26, 3148–3155. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, A.A.; Hunge, Y.M.; Ko, S.; Kang, S.-W. Chemically Synthesized Iron-Oxide-Based Pure Negative Electrode for Solid-State Asymmetric Supercapacitor Devices. Materials 2022, 15, 6133. https://doi.org/10.3390/ma15176133
Yadav AA, Hunge YM, Ko S, Kang S-W. Chemically Synthesized Iron-Oxide-Based Pure Negative Electrode for Solid-State Asymmetric Supercapacitor Devices. Materials. 2022; 15(17):6133. https://doi.org/10.3390/ma15176133
Chicago/Turabian StyleYadav, A. A., Y. M. Hunge, Seongjun Ko, and Seok-Won Kang. 2022. "Chemically Synthesized Iron-Oxide-Based Pure Negative Electrode for Solid-State Asymmetric Supercapacitor Devices" Materials 15, no. 17: 6133. https://doi.org/10.3390/ma15176133





