Preparation of Porous Ellipsoidal Bismuth Oxyhalide Microspheres and Their Photocatalytic Performances
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ellipsoidal-Shaped BiOX
2.3. Characterization
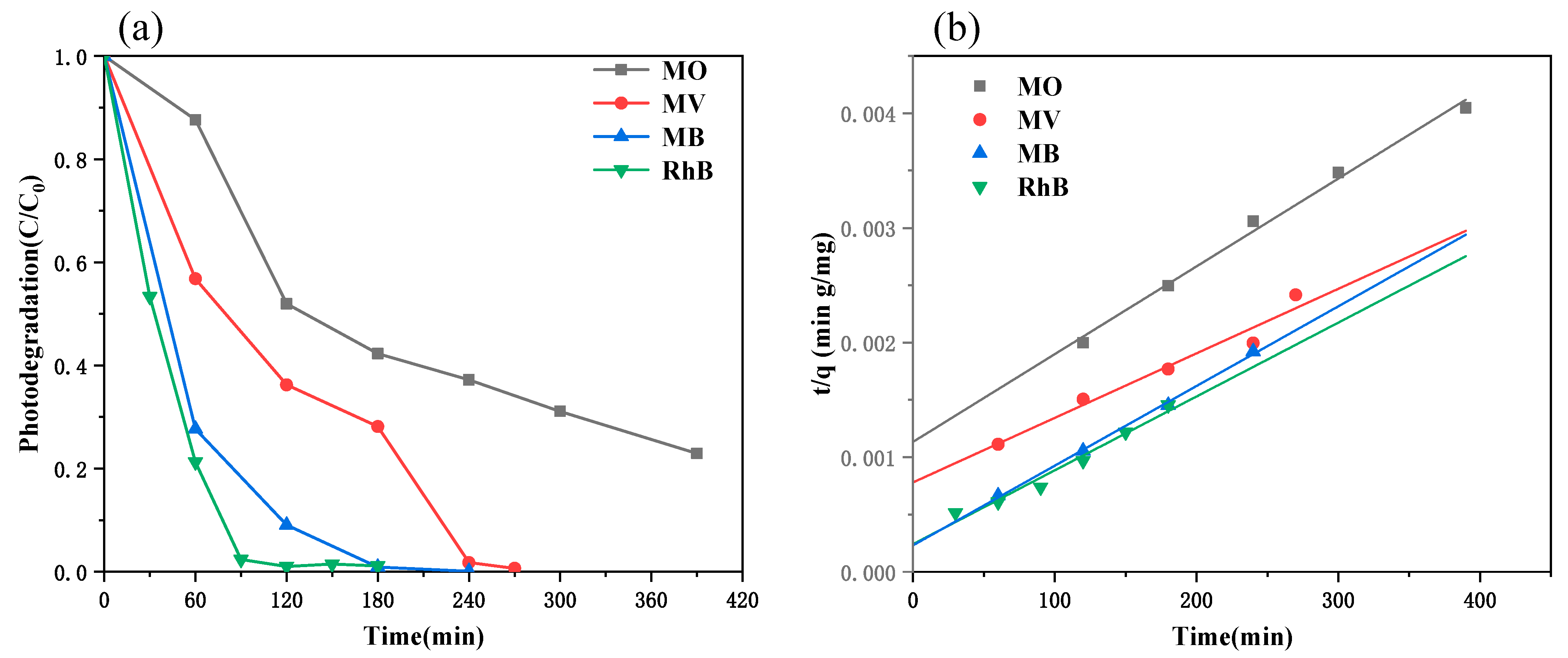
3. Results and Discussions
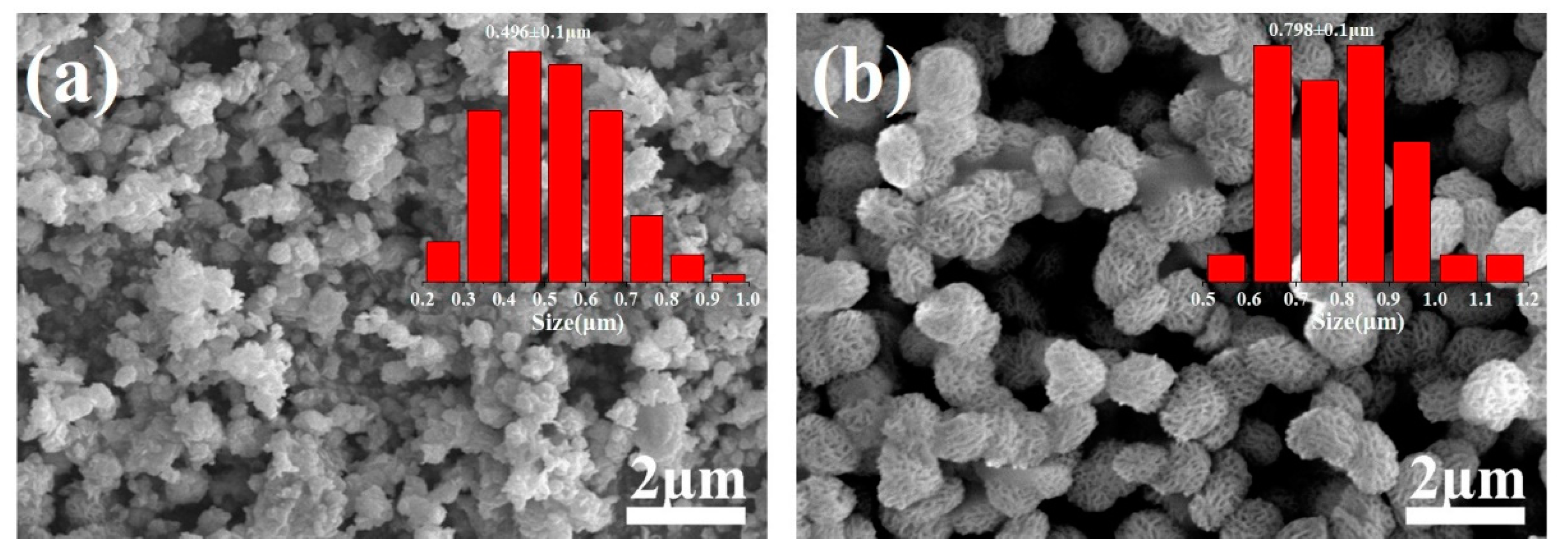
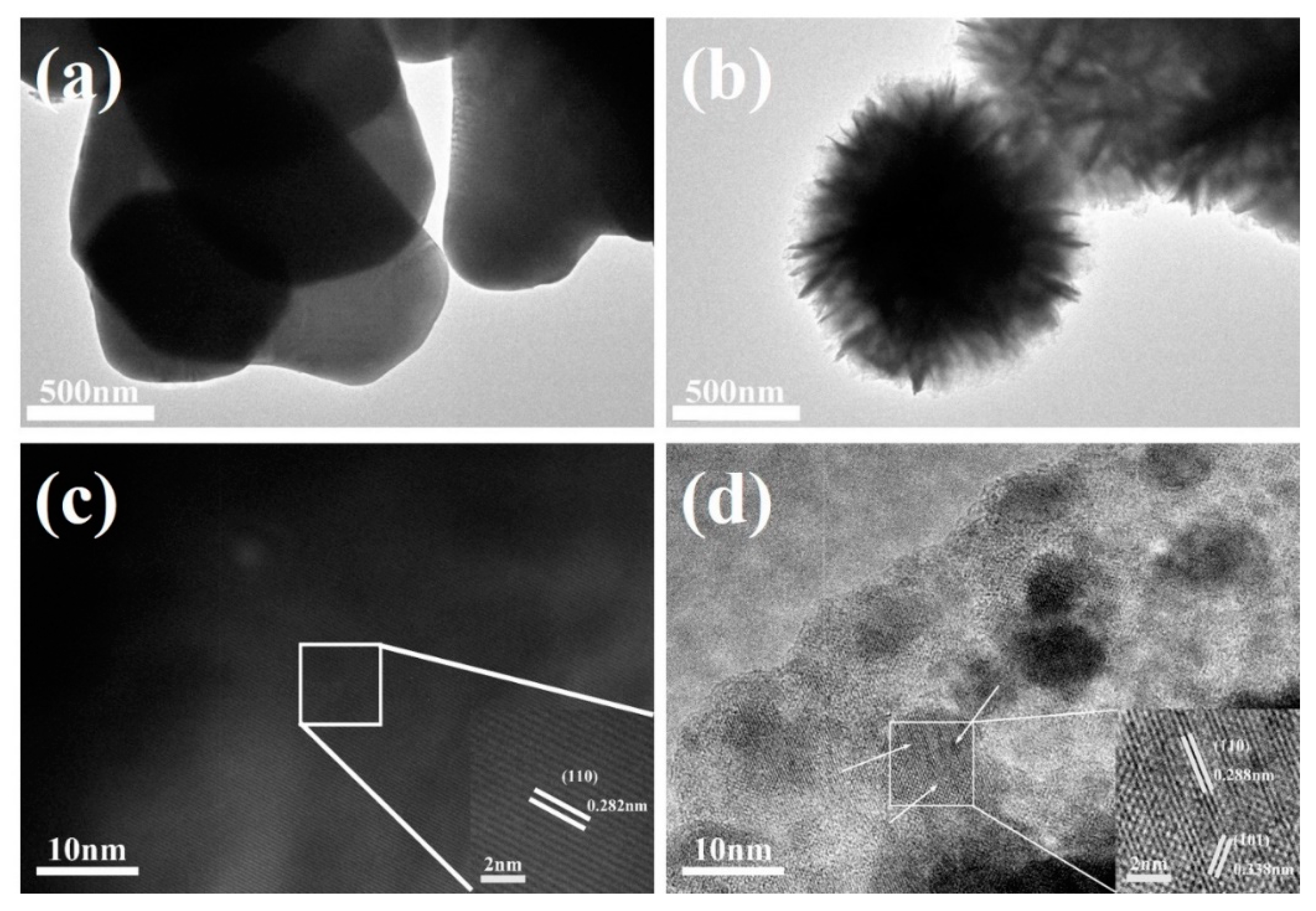
3.1. SEM and TEM Analysis
3.2. XRD Analysis
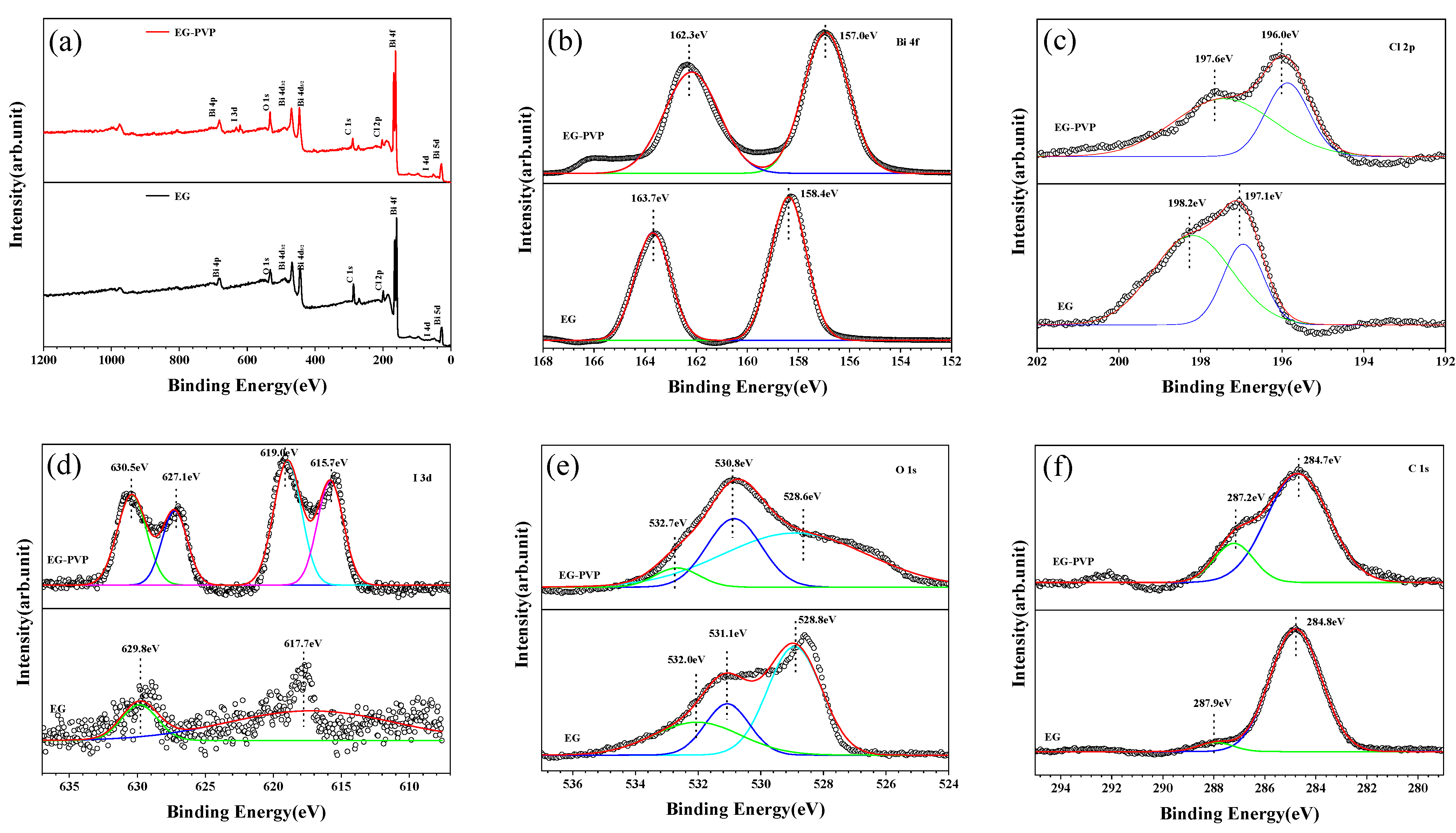
3.3. XPS Analysis
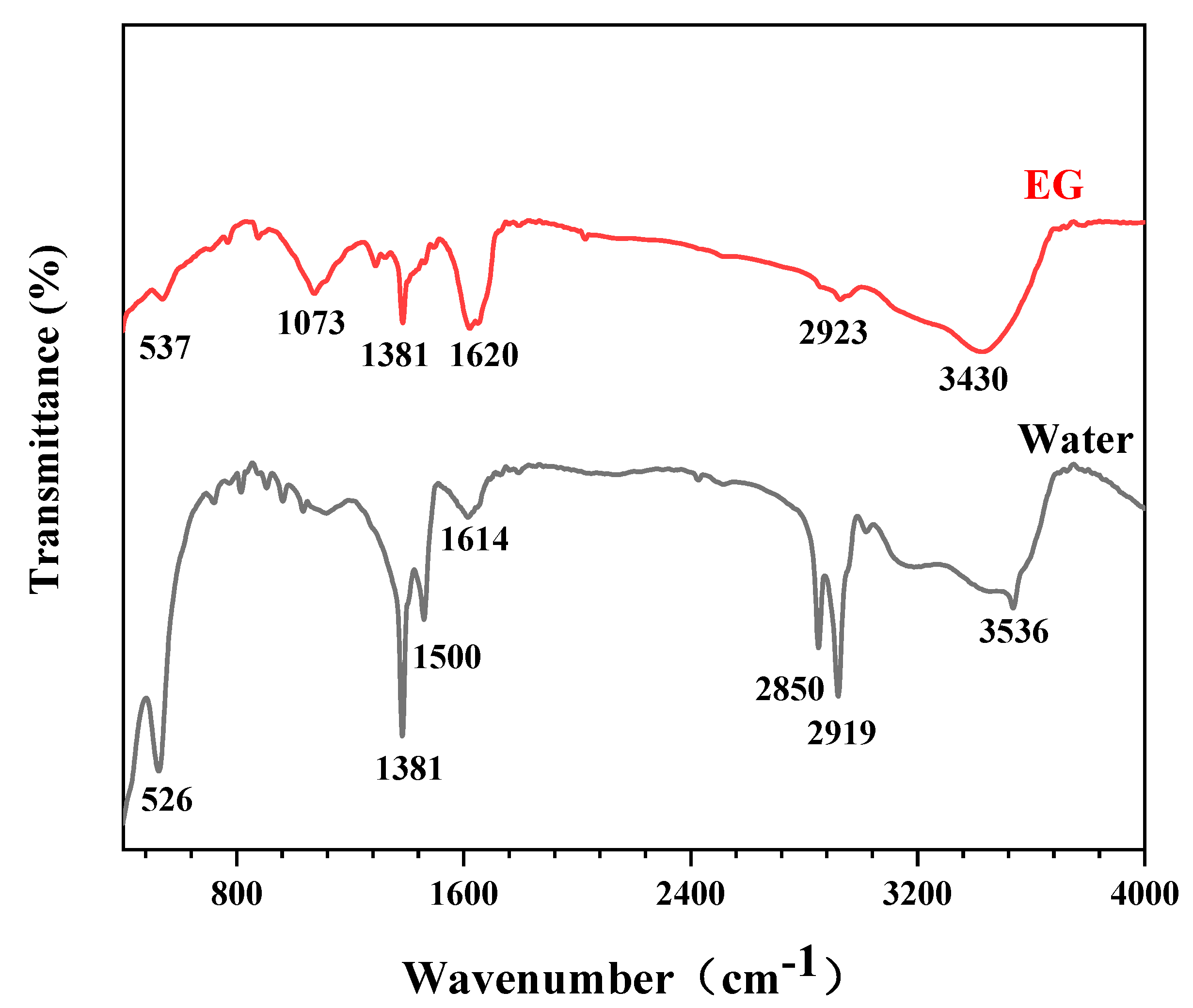
3.4. FTIR Analysis
3.5. UV-Vis and PL Analysis
3.6. Photoelectric Performance Analysis
3.7. Photocatalytic Activity
3.8. Photodegradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Q.; Tan, X.; Wang, Y.; Jin, R.; Gao, S. Enhanced photocatalytic performance of TiO2 NTs decorated with chrysanthemum-like BiOI nanoflowers. Sep. Purif. Technol. 2019, 215, 565–572. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Ren, X.; Gao, M.; Zhang, Y.; Zhang, Z.; Cao, X.; Wang, B.; Wang, X. Photocatalytic reduction of CO2 on BiOX: Effect of halogen element type and surface oxygen vacancy mediated mechanism. Appl. Catal. B Environ. 2020, 274, 119063. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Li, X.; Lin, X.; Du, B.; Luo, J.; Zhang, X. Mussel-Inspired Immobilization of Photocatalysts with Synergistic Photocatalytic-Photothermal Performance for Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 31066–31076. [Google Scholar] [CrossRef]
- Mahdavi, K.; Zinatloo-Ajabshir, S.; Yousif, Q.A.; Salavati-Niasari, M. Enhanced photocatalytic degradation of toxic contaminants using Dy2O3-SiO2 ceramic nanostructured materials fabricated by a new, simple and rapid sonochemical approach. Ultrason. Sonochem. 2022, 82, 105892. [Google Scholar] [CrossRef]
- Govindasamy, P.; Kandasamy, B.; Thangavelu, P.; Barathi, S.; Thandavarayan, M.; Shkir, M.; Lee, J. Biowaste derived hydroxyapatite embedded on two-dimensional g-C3N4 nanosheets for degradation of hazardous dye and pharmacological drug via Z-scheme charge transfer. Sci. Rep. 2022, 12, 11572. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Srinivasan, M.; Vignesh, S.; Elavarasan, N.; Venkatesh, G.; Pazhanivel, T.; Ramasamy, P. Two-dimensional g-C3N4 nanosheets supporting Co3O4-V2O5 nanocomposite for remarkable photodegradation of mixed organic dyes based on a dual Z-scheme photocatalytic system. Diam. Relat. Mater. 2021, 118, 108540. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B-Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Z.; Li, F.B. Study of Au/Au(3+)-TiO2 photocatalysts toward visible photooxidation for water and wastewater treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, S.; Palanisamy, G.; Srinivasan, M.; Elavarasan, N.; Bhuvaneswari, K.; Venkatesh, G.; Pazhanivel, T.; Ramasamy, P.; Manthrammel, M.A.; Shkir, M. Fabricating SnO2 and Cu2O anchored on g-C3N4 nanocomposites for superior photocatalytic various organic pollutants degradation under simulated sunlight exposure. Diam. Relat. Mater. 2021, 120, 108606. [Google Scholar] [CrossRef]
- Xia, J.; Di, J.; Li, H.; Xu, H.; Li, H.; Guo, S. Ionic liquid-induced strategy for carbon quantum dots/BiOX (X = Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl. Catal. B Environ. 2016, 181, 260–269. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Yu, S.; Tian, N.; Dong, F.; Du, X.; Zhang, Y. Simultaneously promoting charge separation and photoabsorption of BiOX (X = Cl, Br) for efficient visible-light photocatalysis and photosensitization by compositing low-cost biochar. Appl. Surf. Sci. 2016, 386, 285–295. [Google Scholar] [CrossRef]
- Qi, L.; Yang, Y.; Zhang, P.; Le, Y.; Wang, C.; Wu, T. Hierarchical flower-like BiOIxBr(1−x) solid solution spheres with enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2019, 467–468, 792–801. [Google Scholar] [CrossRef]
- Su, X.; Yang, J.; Yu, X.; Zhu, Y.; Zhang, Y. In situ grown hierarchical 50%BiOCl/BiOI hollow flowerlike microspheres on reduced graphene oxide nanosheets for enhanced visible-light photocatalytic degradation of rhodamine B. Appl. Surf. Sci. 2018, 433, 502–512. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A-Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Garg, S.; Yadav, M.; Chandra, A.; Hernadi, K. A Review on BiOX (X= Cl, Br and I) Nano-/Microstructures for Their Photocatalytic Applications. J. Nanosci. Nanotechnol. 2019, 19, 280–294. [Google Scholar] [CrossRef]
- Lu, J.; Meng, Q.; Lv, H.; Shui, L.; Jin, M.; Zhang, Z.; Chen, Z.; Yuan, M.; Wang, X.; Liu, J.-M.; et al. Synthesis of visible-light-driven BiOBrxI1-x solid solution nanoplates by ultrasound-assisted hydrolysis method with tunable bandgap and superior photocatalytic activity. J. Alloys Compd. 2018, 732, 167–177. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Li, H.; Mailhot, G.; Dong, W. Preparation and formation mechanism of BiOCl0.75I0.25 nanospheres by precipitation method in alcohol-water mixed solvents. J. Colloid Interface Sci. 2016, 478, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Li, X.; Zhang, D.; Dong, F.; Chen, S. Efficient visible light photocatalytic oxidation of NO with hierarchical nanostructured 3D flower-like BiOClxBr1−x solid solutions. J. Alloys Compd. 2016, 671, 318–327. [Google Scholar] [CrossRef]
- Zhao, R.; Jia, Z.; Li, T.; Liu, J.; Li, R.; Wang, Y.; Wang, Y.; Zhang, X.; Fan, C. Concise fabrication of 3D rose-like BiOBrxI1−x with exceptional wide spectrum visible-light photocatalytic activity. Inorg. Chem. Commun. 2019, 101, 150–159. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Pradhan, D.; Sohn, Y. AgX (X = Cl, Br, I)/BiOX nanoplates and microspheres for pure and mixed (methyl orange, rhodamine B and methylene blue) dyes. J. Ind. Eng. Chem. 2016, 35, 231–252. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Li, R.; Tao, L.; Li, R.; Wang, X.; Ning, P. Solvents mediated-synthesis of 3D-BiOX (X = Cl, Br, I) microspheres for photocatalytic removal of gaseous Hg0 from the zinc smelting flue gas. Fuel 2020, 268, 117211. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zheng, Y.F.; Song, X.C. Biomass Assisted Synthesis of 3D Hierarchical Structure BiOX(X Cl, Br)-(CMC) with Enhanced Photocatalytic Activity. J. Nanosci. Nanotechnol. 2019, 19, 5287–5294. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Pradhan, D.; Min, B.-K.; Sohn, Y. Adsorption/photocatalytic activity and fundamental natures of BiOCl and BiOClxI1−x prepared in water and ethylene glycol environments, and Ag and Au-doping effects. Appl. Catal. B Environ. 2014, 147, 711–725. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L. Generalized One-Pot Synthesis, Characterization, and Photocatalytic Activity of Hierarchical BiOX (X = Cl, Br, I) Nanoplate Microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, J.; Wang, S.; Liu, J.; Ren, K.; Zheng, X.; Luo, H.; Peng, Y.; Zou, X.; Bo, X.; et al. BiOCl Sub-Microcrystals Induced by Citric Acid and Their High Photocatalytic Activities. Cryst. Growth Des. 2012, 12, 793–803. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard Mater. 2012, 219–220, 26–34. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.-Z.; Ji, M.; Yan, C.; Gu, K.; Xia, J.; Li, H.; Li, S.; Liu, Z. Defect engineering in atomically-thin bismuth oxychloride towards photocatalytic oxygen evolution. J. Mater. Chem. A 2017, 5, 14144–14151. [Google Scholar] [CrossRef]
- Shkir, M.; AlFaify, S. Tailoring the structural, morphological, optical and dielectric properties of lead iodide through Nd(3+) doping. Sci. Rep. 2017, 7, 16091. [Google Scholar] [CrossRef] [PubMed]
- Ayeshamariam, A.; Sankaracharyulu, G.V.; Kashif, M.; Hussain, S.; Bououdina, M.; Jayachandran, M. Antibacterial Activity Studies of Ni and SnO2 Loaded Chitosan Beads. Mater. Sci. Forum 2015, 832, 110–122. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, J.; Jia, T.; Li, T.; Wang, Z.; Qi, Y.; Liu, Q.; Qi, X.; He, P. Fabrication of BiOI nanosheets with exposed (001) and (110) facets with different methods for photocatalytic oxidation elemental mercury. Colloid Interface Sci. Commun. 2021, 40, 100357. [Google Scholar] [CrossRef]
- Cheng, G.; Xiong, J.; Stadler, F.J. Facile template-free and fast refluxing synthesis of 3D desertrose-like BiOCl nanoarchitectures with superior photocatalytic activity. New J. Chem. 2013, 37, 3207–3213. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, K.; Zhang, L. Visible Light Photocatalysis of BiOI and Its Photocatalytic Activity Enhancement by in Situ Ionic Liquid Modification. J. Phys. Chem. C 2011, 115, 14300–14308. [Google Scholar] [CrossRef]
- Wu, J.; Xu, K.; Liu, Q.; Ji, Z.; Qu, C.; Qi, X.; Zhang, H.; Guan, Y.; He, P.; Zhu, L. Controlling dominantly reactive (010) facets and impurity level by in-situ reduction of BiOIO3 for enhancing photocatalytic activity. Appl. Catal. B Environ. 2018, 232, 135–145. [Google Scholar] [CrossRef]
- Sun, X.; Lu, J.; Wu, J.; Guan, D.; Liu, Q.; Yan, N. Enhancing photocatalytic activity on gas-phase heavy metal oxidation with self-assembled BiOI/BiOCl microflowers. J. Colloid Interface Sci. 2019, 546, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Bielicka–Giełdoń, A.; Wilczewska, P.; Malankowska, A.; Szczodrowski, K.; Ryl, J.; Zielińska-Jurek, A.; Siedlecka, E.M. Morphology, surface properties and photocatalytic activity of the bismuth oxyhalides semiconductors prepared by ionic liquid assisted solvothermal method. Sep. Purif. Technol. 2019, 217, 164–173. [Google Scholar] [CrossRef]
- Maensiri, S.; Laokul, P.; Promarak, V. Synthesis and optical properties of nanocrystalline ZnO powders by a simple method using zinc acetate dihydrate and poly(vinyl pyrrolidone). J. Cryst. Growth 2006, 289, 102–106. [Google Scholar] [CrossRef]
- Phoka, S.; Laokul, P.; Swatsitang, E.; Promarak, V.; Seraphin, S.; Maensiri, S. Synthesis, structural and optical properties of CeO=2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater. Chem. Phys. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Garg, S.; Yadav, M.; Chandra, A.; Gahlawat, S.; Ingole, P.P.; Pap, Z.; Hernadi, K. Plant leaf extracts as photocatalytic activity tailoring agents for BiOCl towards environmental remediation. Ecotoxicol. Environ Saf. 2018, 165, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Tang, F.; Muscat, A.J. Strong blue photoluminescence from single-crystalline bismuth oxychloride nanoplates. Nanotechnology 2008, 19, 295705. [Google Scholar] [CrossRef] [PubMed]
- Bárdos, E.; Márta, V.; Baia, L.; Todea, M.; Kovács, G.; Baán, K.; Garg, S.; Pap, Z.; Hernadi, K. Hydrothermal crystallization of bismuth oxybromide (BiOBr) in the presence of different shape controlling agents. Appl. Surf. Sci. 2020, 518, 146184. [Google Scholar] [CrossRef]
- Chen, L.; Huang, R.; Xiong, M.; Yuan, Q.; He, J.; Jia, J.; Yao, M.Y.; Luo, S.L.; Au, C.T.; Yin, S.F. Room-temperature synthesis of flower-like BiOX (X horizontal lineCl, Br, I) hierarchical structures and their visible-light photocatalytic activity. Inorg. Chem. 2013, 52, 11118–11125. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Dai, K.; Liang, C.; Liu, Q. One-step growth of nanosheet-assembled BiOCl/BiOBr microspheres for highly efficient visible photocatalytic performance. Appl. Surf. Sci. 2018, 430, 639–646. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Li, G.; Qin, F.; Chen, R. Well-crystallized square-like 2D BiOCl nanoplates: Mannitol-assisted hydrothermal synthesis and improved visible-light-driven photocatalytic performance. RSC Adv. 2011, 1, 1542–1553. [Google Scholar] [CrossRef]
- Cinelli, G.; Cuomo, F.; Ambrosone, L.; Colella, M.; Ceglie, A.; Venditti, F.; Lopez, F. Photocatalytic degradation of a model textile dye using Carbon-doped titanium dioxide and visible light. J. Water Process Eng. 2017, 20, 71–77. [Google Scholar] [CrossRef]
- Venditti, F.; Cuomo, F.; Ceglie, A.; Avino, P.; Russo, M.V.; Lopez, F. Visible light caffeic acid degradation by carbon-doped titanium dioxide. Langmuir 2015, 31, 3627–3634. [Google Scholar] [CrossRef]
- Wang, R.; Shi, K.; Huang, D.; Zhang, J.; An, S. Synthesis and degradation kinetics of TiO2/GO composites with highly efficient activity for adsorption and photocatalytic degradation of MB. Sci. Rep. 2019, 9, 18744. [Google Scholar] [CrossRef]
- Ben Abdelaziz, M.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Ezzaouia, H.; Schneider, R. One pot synthesis of bismuth oxide/graphitic carbon nitride composites with high photocatalytic activity. Mol. Catal. 2019, 463, 110–118. [Google Scholar] [CrossRef]
- Ren, J.; Wu, Y.Z.; Pan, J.M.; Yan, X.H.; Chen, M.; Wang, J.J.; Wang, D.F.; Zhou, C.; Wang, Q.; Cheng, X.N. Novel ternary Ag/CeVO4/g-C3N4 nanocomposite as a highly efficient visible-light-driven photocatalyst. J. Adv. Ceram. 2017, 7, 50–57. [Google Scholar] [CrossRef]
- Sumathi, S.; Kavipriya, A. Structural, optical and photocatalytic activity of cerium doped zinc aluminate. Solid State Sci. 2017, 65, 52–60. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Zhang, C.; Fu, L.; Wang, Y.; Yang, D. Preparation, characterization and photocatalytic application of TiO2–graphene photocatalyst under visible light irradiation. Ceram. Int. 2015, 41, 2502–2511. [Google Scholar] [CrossRef]
- Abhilash, M.R.; Akshatha, G.; Srikantaswamy, S. Photocatalytic dye degradation and biological activities of the Fe2O3/Cu2O nanocomposite. RSC Adv. 2019, 9, 8557–8568. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Ehtisham-ul-Haque, S.; Bilal, M.; Ahmad, N.; Ahmad, A.; Abbas, M.; Nisar, J.; Khan, M.I.; Nazir, A.; Ghaffar, A.; et al. Synthesis and characterization of Zn doped WO3 nanoparticles: Photocatalytic, antifungal and antibacterial activities evaluation. Mater. Res. Express 2020, 7, 015407. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, Y.; Yu, X.; Guo, J.; Zhu, Y.; Zhang, Y. A facile two-step method to synthesize immobilized CdS/BiOCl film photocatalysts with enhanced photocatalytic activities. J. Alloy. Compd. 2019, 771, 309–316. [Google Scholar] [CrossRef]
- Tran Huu, H.; Thi, M.D.N.; Nguyen, V.P.; Thi, L.N.; Phan, T.T.T.; Hoang, Q.D.; Luc, H.H.; Kim, S.J.; Vo, V. One-pot synthesis of S-scheme MoS2/g-C3N4 heterojunction as effective visible light photocatalyst. Sci. Rep. 2021, 11, 14787. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Devi, T.B.; Nath, J. Photodegradation of methyl violet 6B and methylene blue using tin-oxide nanoparticles (synthesized via a green route). J. Photochem. Photobiol. A Chem. 2016, 325, 116–124. [Google Scholar] [CrossRef]
- Li, T.B.; Chen, G.; Zhou, C.; Shen, Z.Y.; Jin, R.C.; Sun, J.X. New photocatalyst BiOCl/BiOI composites with highly enhanced visible light photocatalytic performances. Dalton Trans. 2011, 40, 6751–6758. [Google Scholar] [CrossRef]
- Ge, M.; Liu, L.; Chen, W.; Zhou, Z. Sunlight-driven degradation of Rhodamine B by peanut-shaped porous BiVO4nanostructures in the H2O2-containing system. CrystEngComm 2012, 14, 1038–1044. [Google Scholar] [CrossRef]
- Xiao, X.; Hao, R.; Liang, M.; Zuo, X.; Nan, J.; Li, L.; Zhang, W. One-pot solvothermal synthesis of three-dimensional (3D) BiOI/BiOCl composites with enhanced visible-light photocatalytic activities for the degradation of bisphenol-A. J. Hazard Mater. 2012, 233–234, 122–130. [Google Scholar] [CrossRef] [PubMed]






| Dyes | K2 (g/mg min) | qe (mg/g) | T0.5 (min) |
|---|---|---|---|
| MO | 1.51 × 1013 ± 5.38 × 1011 | 1.31 × 105 ± 6.44 × 104 | 148.1 ± 19.87 |
| MV | 4.05 × 1013 ± 4.27 × 1012 | 1.78 × 105 ± 1.99 × 105 | 138.6 ± 35.9 |
| MB | 8.96 × 1013 ± 8.99 × 1012 | 1.44 × 105 ± 4.49 × 104 | 33.2 ± 6.01 |
| RhB | 9.99 × 1013 ± 1.35 × 1013 | 1.55 × 105 ± 1.33 × 105 | 37.5 ± 12.9 |
| S. No. | Sample | Dyes | Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| 1 | Bi2O3/g-CN | RhB | 180 | ~83 | [50] |
| 2 | 10 wt% Ag/CeVO4/g-C3N4 | MB | 120 | ~94 | [51] |
| 3 | ZnAl1.98Ce0.02O4 | MB | 240 | 72.5 | [52] |
| 4 | TiO2–graphene | MB | 100 | 98.8 | [53] |
| 5 | Fe2O3/Cu2O nanomaterial | RhB | 120 | 79.15 | [54] |
| 6 | Zn doped WO3 | MB | 120 | 78 | [55] |
| 7 | 6 wt% CdS/BiOCl | RhB | ** | 78 | [56] |
| 8 | MoS2/g-C3N4 | RhB | 420 | ~90 | [57] |
| 9 | SnO2 nanoparticles | MB | 240 | ~96 | [58] |
| 10 | 80%BiOCl/20%BiOI | RhB | 90 | 98 | This |
| MB | 120 | 90.4 | work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Wu, C.; Zhang, F.; Wang, T.; Yao, Y. Preparation of Porous Ellipsoidal Bismuth Oxyhalide Microspheres and Their Photocatalytic Performances. Materials 2022, 15, 6035. https://doi.org/10.3390/ma15176035
Luo B, Wu C, Zhang F, Wang T, Yao Y. Preparation of Porous Ellipsoidal Bismuth Oxyhalide Microspheres and Their Photocatalytic Performances. Materials. 2022; 15(17):6035. https://doi.org/10.3390/ma15176035
Chicago/Turabian StyleLuo, Bing, Canfeng Wu, Fuzeng Zhang, Tingting Wang, and Yingbang Yao. 2022. "Preparation of Porous Ellipsoidal Bismuth Oxyhalide Microspheres and Their Photocatalytic Performances" Materials 15, no. 17: 6035. https://doi.org/10.3390/ma15176035





