Hydrating Capabilities of the Biopolymers Produced by the Marine Thermophilic Bacillus horneckiae SBP3 as Evaluated by ATR-FTIR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Production and Characterization of the Biopolymers from SBP3
2.3. CFS Surface Tension and Emulsifying Properties
2.4. Wetting Property
2.5. Moisture Uptake
2.6. Hydrating States of BS-SBP3 and EPS-SBP3 Using ATR-FTIR Spectroscopy
2.7. Spectral Distance
2.8. Cross-Correlation Wavelet
3. Results
3.1. CFS Properties of B. horneckiae SBP3
3.2. The FTIR Analysis of the Biopolymers
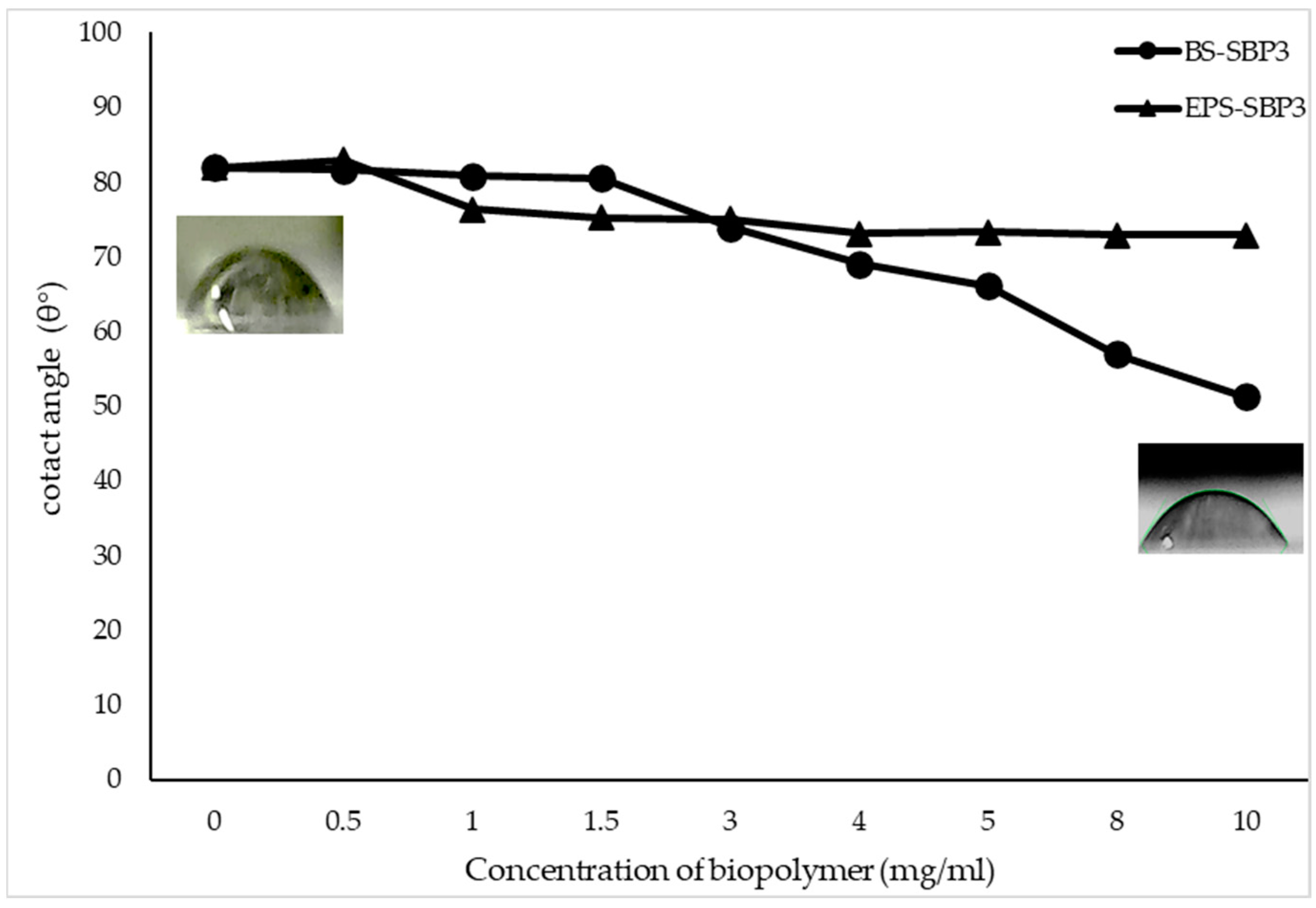
3.3. Wettability Assay
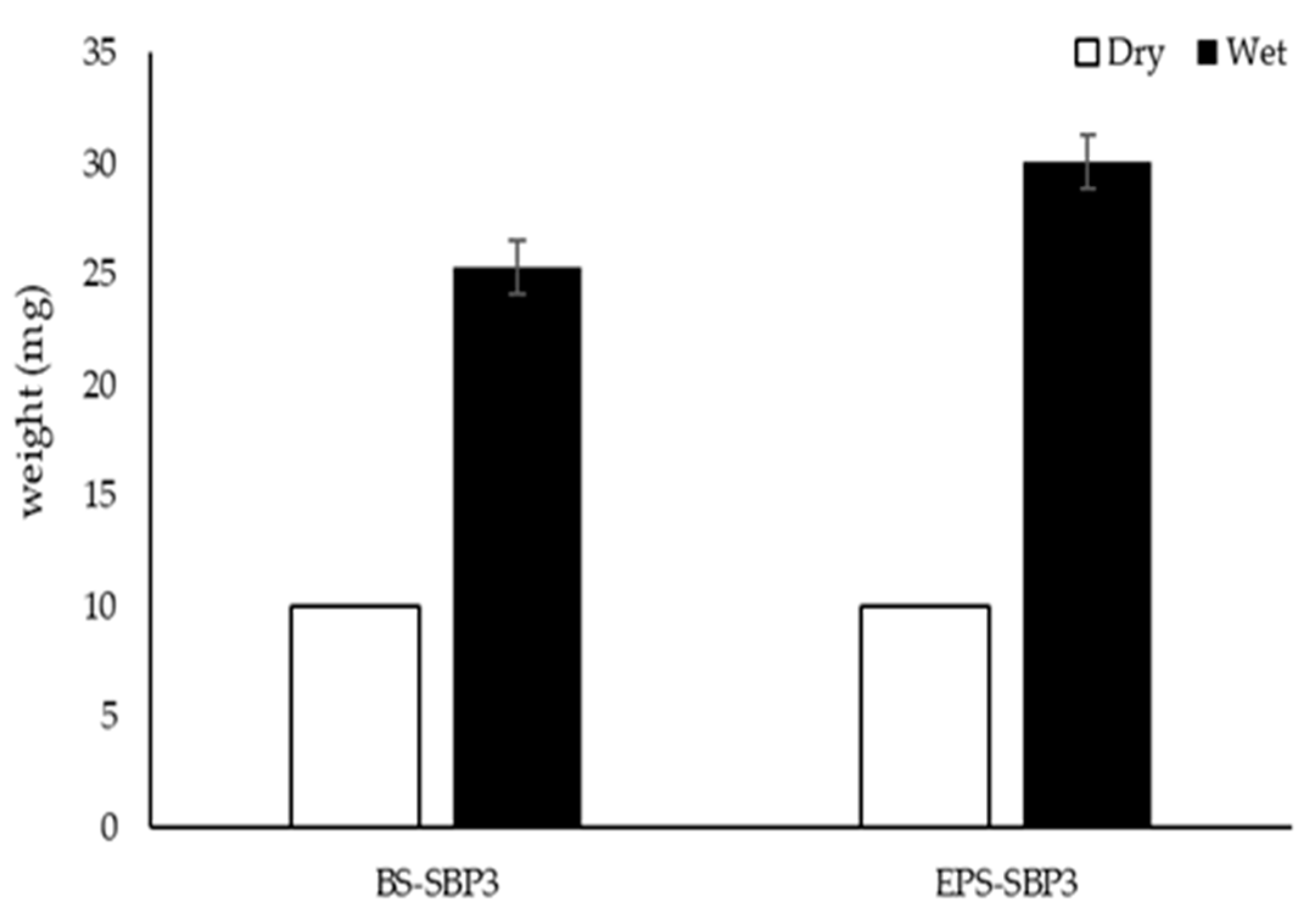
3.4. Moisture Uptake Assay
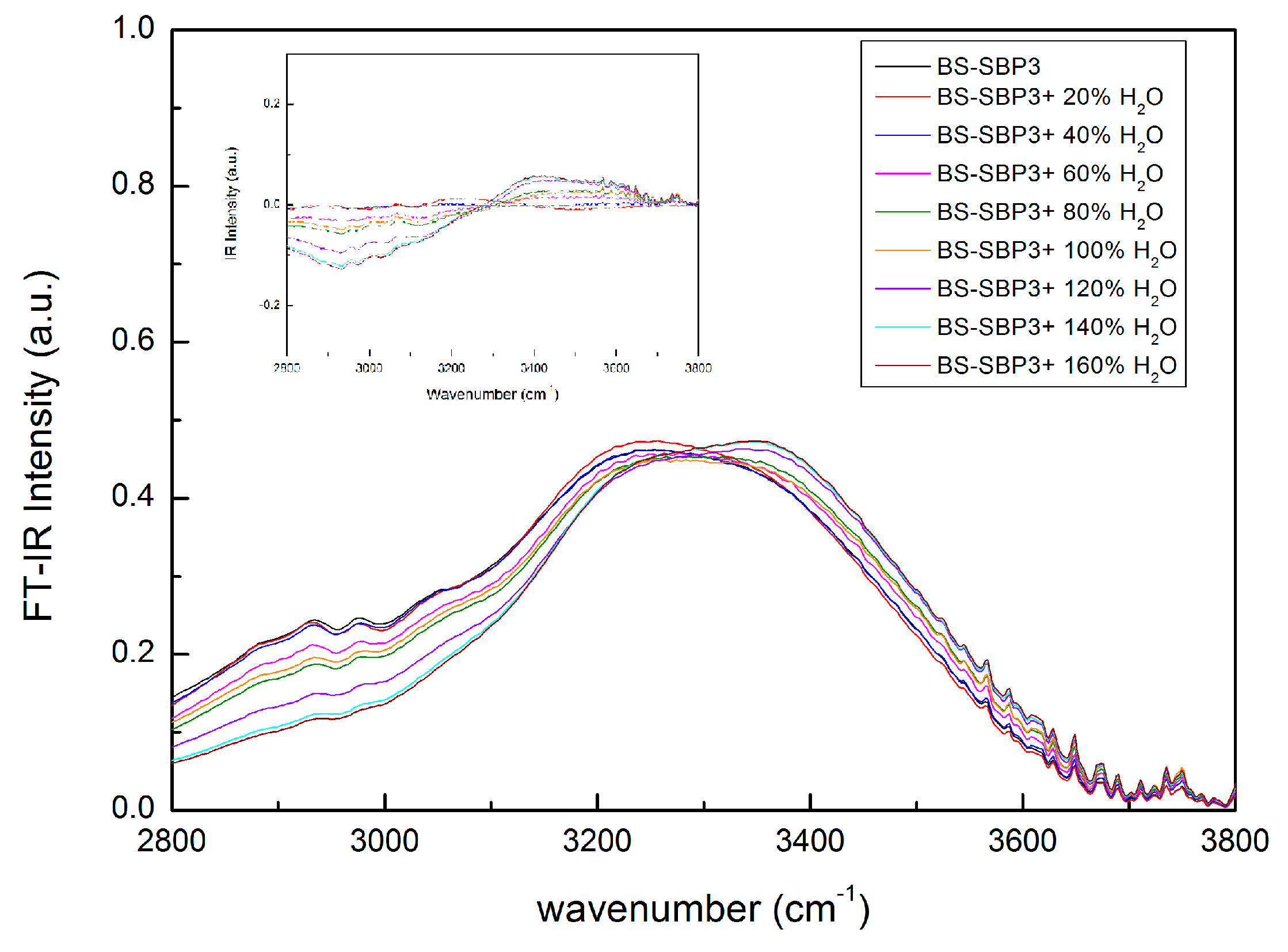
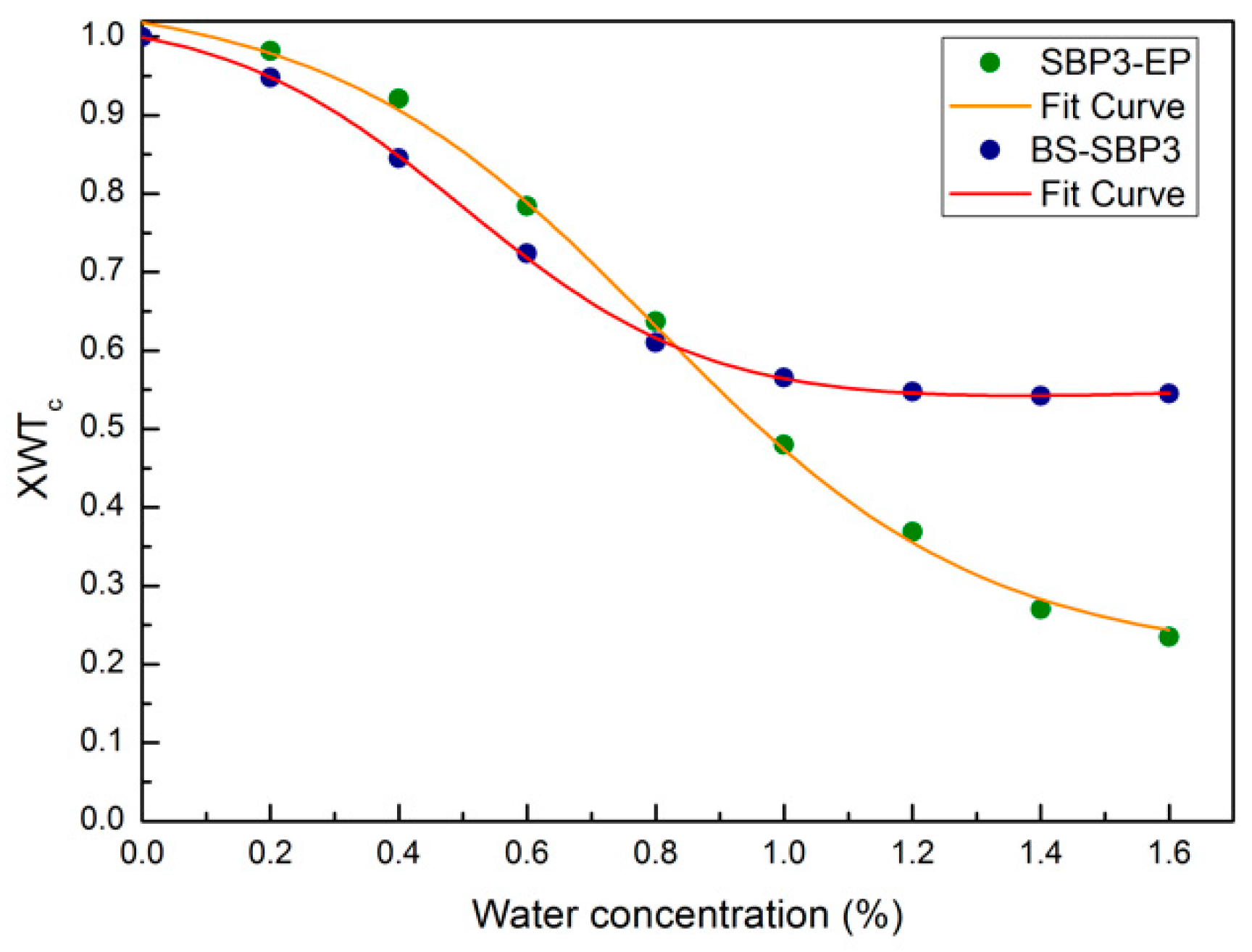
3.5. The Hydration State of Biopolymers by ATR-FTIR Analysis
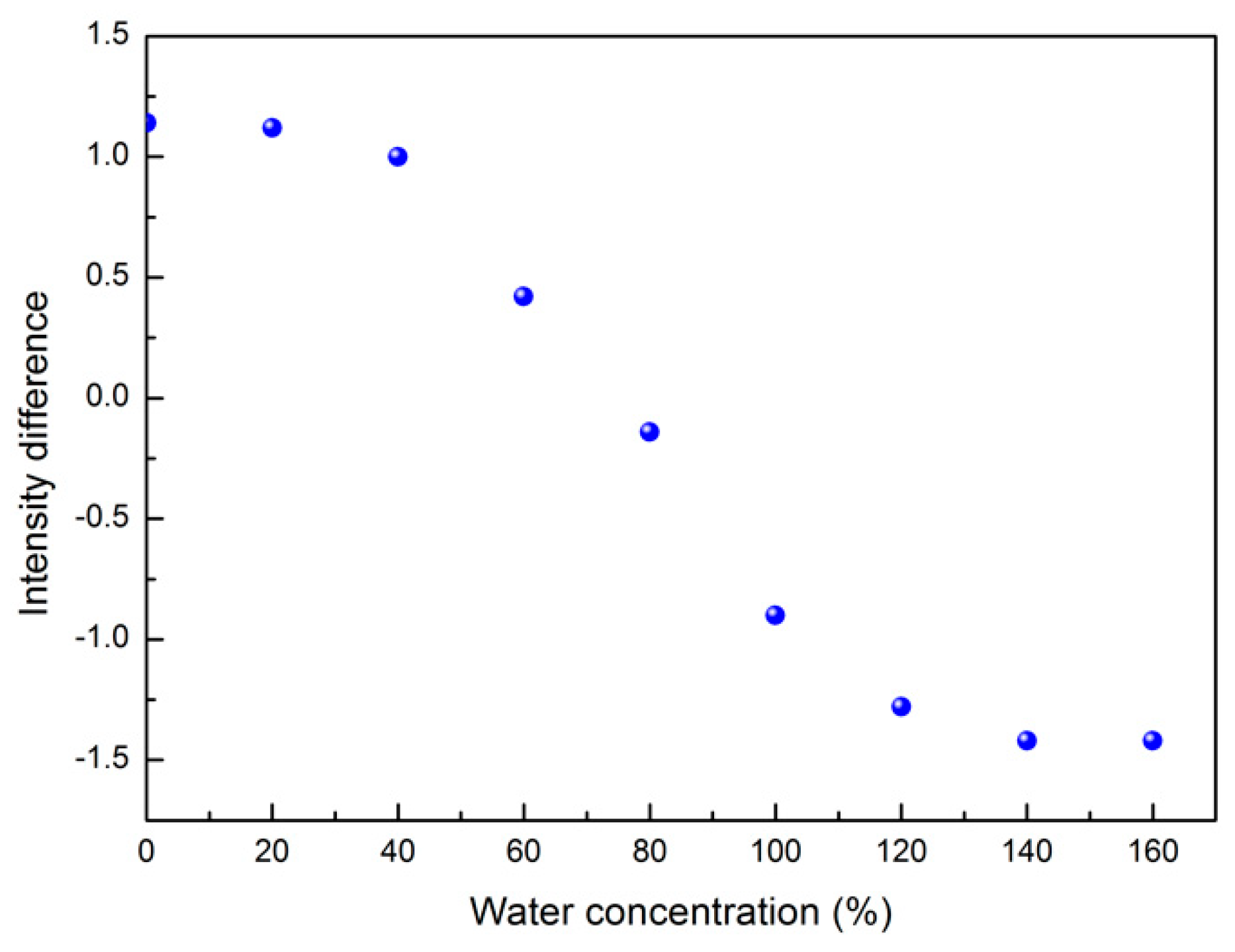
3.6. Spectral Distance Analysis
3.7. Cross-Correlation Wavelet Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Foster, S.; Chilton, P.J. Groundwater. The processes and global significance of aquifer degradation. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 1957–1972. [Google Scholar] [CrossRef] [PubMed]
- Bestelmeyer, B.T.; Okin, G.S.; Duniway, M.C.; Archer, S.R.; Sayre, N.F.; Williamson, J.C.; Herrick, J.E. Desertification, land use, and the transformation of global drylands. Front. Ecol. Environ. 2015, 13, 28–36. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. 2 Tilman. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Dekker, L.W.; Ritsema, C.J. Preferential flow paths in a water repellent clay soil with grass cover. Water Resour. Res. 1996, 32, 1239–1249. [Google Scholar] [CrossRef]
- Roper, M.M. Managing soils to enhance the potential for bioremediation of water repellency. Aust. J. Soil Res. 2005, 43, 803–810. [Google Scholar] [CrossRef]
- Chen, J.; Ormes, J.D.; Higgins, J.D.; Taylor, L.S. Impact of surfactants on the crystallization of aqueous suspensions of celecoxib amorphous solid dispersion spray dried particles. Mol. Pharm. 2015, 12, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Tadayonnejad, M.; Mosaddeghi, M.R.; Dashtaki, S.G. Changing soil hydraulic properties and water repellency in a pomegranate orchard irrigated with saline water by applying polyacrylamide. Agric. Water Manag. 2017, 188, 12–20. [Google Scholar] [CrossRef]
- McDonald, G.I.; Richardson, B.A.; Zambino, P.J.; Klopfenstein, N.B.; Kim, M.S. Pedicularis and Castilleja are natural hosts of Cronartium ribicola in North America: A first report. For. Pathol. 2006, 36, 73–82. [Google Scholar] [CrossRef]
- Kostka, S.J. Amelioration of water repellency in highly managed soils and the enhancement of turfgrass performance through the systematic application of surfactants. J. Hydrol. 2000, 231–232, 359–368. [Google Scholar] [CrossRef]
- Bouazza, A.; Gates, W.P.; Ranhith, P.G. Hydraulic conductivity of biopolymer-treated silty sand. Geotechnique 2009, 59, 71–72. [Google Scholar] [CrossRef]
- Wiszniewski, M.; Cabalar, A.F. Hydraulic conductivity of a biopolymer treated sand. New Frontiers. In Geotechnical Engineering, Conference Paper in Geotechnical Special Publication, Proceedings of the 3rd Geotechnical International Conference, Shanghai, China, 26–28 May 2014; ASCE: Reston, VA, USA, 2014. [Google Scholar]
- Chang, I.; Im, J.; Lee, S.-W.; Cho, G.-C. Strength durability of gellan gum biopolymer-treated Korean sand with cyclic wetting and drying. Constr. Build. Mater. 2017, 143, 210–221. [Google Scholar] [CrossRef]
- Cabalar, A.; Wiszniewski, M.; Skutnik, Z. Effects of Xanthan Gum Biopolymer on the Permeability, Odometer, Unconfined Compressive and Triaxial Shear Behavior of a Sand. Soil Mech. Found. Eng. 2017, 54, 356–361. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, S.R.; Cameotra, S.S. Potential commercial application of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 92. [Google Scholar] [CrossRef]
- Karunakaran, E.; Mukherjee, J.; Ramalingam, B.; Biggs, C.A. “Biofilmology”: A multidisciplinary review of the study of microbial biofilms. Appl. Microbiol. Biotechnol. 2011, 90, 1869–1881. [Google Scholar] [CrossRef]
- Xue, Y.; Rossi, F.; Colica, G.; Deng, S.; De Philippis, R.; Chen, L. Use of Cyanobacterial Polysaccharides to Promote Shrub Performances in Desert Soils: A Potential Approach for the Restoration of Desertified Areas. Biol. Fertil. Soils 2012, 49, 143–152. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, D.; Hu, C.; Rao, B. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol. Biochem. 2009, 41, 926–929. [Google Scholar] [CrossRef]
- Li, P.; Harding, S.E.; Liu, Z. Cyanobacterial exopolysaccharides: Their nature and potential biotechnological applications. Biotechnol. Genet. Eng. Rev. 2001, 18, 375–404. [Google Scholar] [CrossRef]
- Fischer, T.; Veste, M.; Wiehe, W.; Lange, P. Water repellency and pore clogging at early successional stages of microbiotic crusts on inland dunes, Brandenburg, NE Germany. Catena 2010, 80, 47–52. [Google Scholar] [CrossRef]
- Malam Issa, O.; Défarge, C.; Trichet, J.; Valentin, C.; Rajot, J.L. Microbiotic soil crusts in the Sahel of Western Niger and their influence on soil porosity and water dynamics. Catena 2009, 77, 48–55. [Google Scholar] [CrossRef]
- Potts, M. Desiccation Tolerance of Prokaryotes. Microbiol. Rev. 1994, 58, 755–805. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Potrafka, R.M.; Garcia Pichel, F.; De Philippis, R. The role of the exopolysaccharides in enhancing hydraulic conductivity of biological soil crusts. Soil Biol. Biochem. 2012, 46, 33–40. [Google Scholar] [CrossRef]
- Tripathi, L.; Irorere, V.U.; Marchant, R.; Banat, I.M. Marine derived biosurfactants: A vast potential future resource. Biotechnol. Lett. 2018, 40, 1441–1457. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, C.; Lentini, V.; Spanò, A.; Maugeri, T. New bacilli from shallow hydrothermal vents of Panarea Island (Italy) and their biotechnological potential. J. Appl. Microbiol. 2012, 112, 1102–1112. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Panico, A.; Lama, L.; Gambacorta, A.; Nicolaus, B. A Halophilic thermotolerant Bacillus isolated from a marine hot spring able to produce a new exopolysaccharide. Biotechnol. Lett. 2002, 24, 515–519. [Google Scholar] [CrossRef]
- Spanò, A.; Gugliandolo, C.; Lentini, V.; Maugeri, T.L.; Anzelmo, G.; Poli, A.; Nicolaus, B. A Novel EPS-producing Strain of Bacillus licheniformis isolated from a shallow vent Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [Google Scholar] [CrossRef]
- Nicolaus, B.; Panico, A.; Manca, M.C.; Lama, L.; Gambacorta, A.; Maugeri, T.L.; Gugliandolo, C.; Caccamo, D. A thermophilic Bacillus isolated from an Eolian shallow hydrothermal vent, able to produce exopolysaccharides. Syst. Appl. Microbiol. 2000, 23, 426–432. [Google Scholar] [CrossRef]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. Three novel halotolerant and thermophilic Geobacillus strains from shallow marine vents. Syst. Appl. Microbiol. 2002, 25, 450–455. [Google Scholar] [CrossRef]
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and immunomodulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 2006, 6, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Spanò, A.; Laganà, P.; Visalli, G.; Maugeri, T.L.; Gugliandolo, C. In vitro antibiofilm activity of an exopolysaccharide from the marine thermophilic Bacillus licheniformis T14. Curr. Microbiol. 2016, 72, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Spano, A.; Arena, A. Bacterial Exopolysaccharide of Shallow Marine Vent Origin as Agent in Counteracting Immune Disorders Induced by Herpes Virus. J. Immunoass. Immunochem. 2016, 37, 251–260. [Google Scholar] [CrossRef]
- Zammuto, V.; Spanò, A.; Nicolò, M.S.; Grillo, E.; Caccamo, M.T.; Magazù, S.; Cappello, S.; Gugliandolo, C. Thermophilic hydrocarbon-utilizing bacilli from marine shallow hydrothermal vents as producers of biosurfactants. J. Mar. Sci. Eng. 2022, 10, 1077. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Zammuto, V.; Gugliandolo, C.; Madeleine-Perdrillat, C.; Spanò, A.; Magazù, S. Thermal restraint of a bacterial exopolysaccharide of shallow vent origin. Int. J. Biol. Macromol. 2018, 114, 649–655. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2019, 145, 77–83. [Google Scholar] [CrossRef]
- Bridelli, M.G.; Crippa, P.R. Infrared and water sorption studies of the hydration structure and mechanism in natural and synthetic melanin. J. Phys. Chem. B 2010, 114, 9381–9390. [Google Scholar] [CrossRef]
- Maisano, G.; Majolino, D.; Migliardo, P.; Venuto, S.; Aliotta, F.; Magazú, S. Sound velocity and hydration phenomena in aqueous polymeric solutions. Mol. Phys. 1993, 78, 421–435. [Google Scholar] [CrossRef]
- Bridelli, M.G. Fourier Transform Infrared Spectroscopy in the Study of Hydrated Biological Macromolecules (Chapter). In Fourier Transforms-High-Tech Application and Current Trends; Nikolic, G.S., Cakic, M.D., Cvetkovic, D.J., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Magazù, S.; Maisano, G.; Migliardo, P.; Villari, V. Experimental Simulation of Macromolecules in Trehalose Aqueous solutions: A Photon Correlation Spectroscopy Study. J. Chem. Phys. 1999, 111, 9086. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and multivariate analysis to study the effect of bulk and nano copper oxide on peanut plant leaves. J. Sci. Adv. Mat. Dev. 2016, 1, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Migliardo, F.; Magazù, S.; Caccamo, M.T. Infrared, Raman and INS Studies of Poly-Ethylene Oxide Oligomers. J. Mol. Struct. 2013, 1048, 261–266. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Hofko, B.; Alavi, M.Z.; Grothe, H.; Jones, D.; Harvey, J. Repeatability and sensitivity of FTIR ATR spectral analysis methods for bituminous binders. Mater. Struct. 2017, 50, 187. [Google Scholar] [CrossRef]
- Migliardo, F.; Affouard, F.; Bordat, P.; Descamps, M.; Lerbret, A.; Magazù, S.; Ramirez-Cuesta, A.J.; Telling, M.F.T. A Combined Neutron Scattering and Simulation Study on Bioprotectant Systems. Chem. Phys. 2005, 317, 258. [Google Scholar]
- Branca, C.; Magazù, S.; Maisano, G.; Migliardo, F. Vibrational and Relaxational Contributions in Disaccharide/H2O Glass Formers. Phys. Rev. B 2001, 64, 224204. [Google Scholar] [CrossRef]
- Tuleva, B.; Christova, N.; Jordanov, B.; Nikolova-Damyanova, B.; Petrov, P. Naphthalene degradation and biosurfactant activity by Bacillus cereus 28BN. Z. Naturforsch. C J. Biosci. 2005, 60, 577–582. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacilllus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Daerr, A.; Mogne, A. Pendent_Drop: An ImageJ Plugin to Measure the Surface Tension from an Image of a Pendent Drop. J. Open Res. Softw. 2016, 4, 2–6. [Google Scholar] [CrossRef]
- Raddadi, N.; Giacomucci, L.; Marasco, R.; Daffonchio, D.; Cherif, A.; Fava, F. Bacterial polyextremotolerant bioemulsifiers from arid soils improve water retention capacity and humidity uptake in sandy soil. Microb. Cell Fact. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Lombardo, D.; Magazù, S. Self-assembly processes in hydrated montmorillonite by FTIR investigations. Materials 2020, 13, 1100. [Google Scholar] [CrossRef]
- Magazù, S.; Calabrò, E.; Caccamo, M.T. Experimental study of thermal restraint in bio-protectant disaccharides by FTIR spectroscopy. Open Biotechnol. 2018, 12, 123–133. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Tagging the oligomer-to-polymer crossover on EG and PEGs by infrared and Raman spectroscopies and by wavelet cross-correlation spectral analysis. Vib. Spectr. 2016, 85, 222–227. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Calabrò, E.; Cannuli, A.; Magazù, S. Wavelet Study of Meteorological Data Collected by Arduino-Weather Station: Impact on Solar Energy Collection Technology. MATEC Web Conf. 2016, 55, 02004. [Google Scholar] [CrossRef]
- Li, G.; Jing, W.; Wen, Z.-Q. Identification of unknown mixtures of materials from biopharmaceutical manufacturing processes by microscopic-FTIR and library searching. Am. Pharm. Rev. 2011, 14, 60–64. [Google Scholar]
- Liu, G.-L.; Kazarian, S.G. Recent advances and applications to cultural heritage using ATR-FTIR spectroscopy and ATR-FTIR spectroscopic imaging. Analyst 2022, 147, 1777–1797. [Google Scholar] [CrossRef]
- Ramani, K.; Jain, S.C.; Mandal, A.B.; Sekaran, G. Microbial induced lipoprotein biosurfactant from slaughterhouse lipid waste and its application to the removal of metal ions from aqueous solution. Colloids Surf. B 2012, 97, 254–263. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Hou, D.J.; Yu, X.L. Facile preparation of FITC-modified silicon nanodots for ratiometric pH sensing and imaging. Spectrochim. Acta Part A 2020, 234, 118276. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Cannuli, A.; Calabrò, E.; Magazù, S. Acoustic Levitator Power Device: Study of Ethylene-Glycol Water Mixtures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 199, 012119. [Google Scholar] [CrossRef]
- Zammuto, V.; Fuchs, F.M.; Fiebrandt, M.; Stapelmann, K.; Ulrich, N.J.; Maugeri, T.L.; Pukall, R.; Gugliandolo, C.; Moeller, R. Comparing Spore Resistance of Bacillus strains isolated from hydrothermal vents and Spacecraft Assembly Facilities to Environmental Stressors and Decontamination Treatments. Astrobiology 2018, 18, 1425–1434. [Google Scholar] [CrossRef]
- Zammuto, V.; Rizzo, M.G.; De Plano, L.M.; Franco, D.; Guglielmino, S.; Caccamo, M.T.; Magazù, S.; Fujimori, A.; Lo Giudice, A.; Guglielmin, M.; et al. Effects of Heavy Ion Particle Irradiation on Spore Germination of Bacillus spp. from Extremely Hot and Cold Environments. Life 2020, 10, 264. [Google Scholar] [CrossRef]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptide lipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–494. [Google Scholar] [CrossRef]
- Ganesan, N.G.; Rangarajan, V. A kinetics study on surfactin production from Bacillus subtilis MTCC 2415 for application in green cosmetics. Biocatal. Agric. Biotechnol. 2021, 33, 102001. [Google Scholar] [CrossRef]
- Santos, V.S.V.; Silveira, E.; Pereira, B.B. Toxicity and Applications of Surfactin for Health and Environmental Biotechnology. J. Toxicol. Environ. Health Part. B Crit. Rev. 2018, 21, 382–399. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, Y.; Bhatt, P.; Chen, S. Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J. Hazard. Mater. 2021, 418, 126253. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and properties of two novel exopolysaccharides synthesized by a thermophilic bacterium Aeribacillus pallidus 418. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef]
- Amatayakul, T.; Halmos, A.; Sherkat, F.; Shah, N. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 2006, 16, 40–51. [Google Scholar] [CrossRef]
- Oleksy-Sobczak, M.; Klewicka, E. Optimization of Media Composition to Maximize the Yield of Exopolysaccharides Production by Lactobacillus rhamnosus Strains. Prob. Antimicrob. Proteins 2020, 12, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Y. Characterizing molecular structure of water adsorbed by cellulose nanofiber film using in situ micro-FTIR spectroscopy. J. Wood. Chem. Technol. 2017, 37, 383–392. [Google Scholar] [CrossRef]
- Célino, A.; Gonçalves, O.; Jacquemin, F.; Fréour, S. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef]
- Ping, Z.H.; Nguyen, Q.T.; Chen, S.M.; Zhou, J.Q.; Ding, Y.D. States of Water in Different Hydrophilic Polymers—DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yang, S.; Wang, J.; Gan, D.; Gama, M.; Yang, Z.; Zhu, Y.; Yao, F.; Luo, H. Scalable synthesis of robust and stretchable composite wound dressings by dispersing silver nanowires in continuous bacterial cellulose. Compos. B Eng. 2020, 199, 108259. [Google Scholar] [CrossRef]
- Disalvo, E.A.; Frias, M.A. Water State and Carbonyl Distribution Populations in Confined Regions of Lipid Bilayers Observed by FTIR Spectroscopy. Langmuir 2013, 29, 6969–6974. [Google Scholar] [CrossRef] [PubMed]



| Time | Surface Tension (mN m−1) | Emulsion Activity (E24) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CFS-SWY+SAC | CFS-MB+SAC | MB+SAC | SWY+SAC | CFS-SWY+SAC | CFS-MB+SAC | TritonX-100 | SWY+SAC | MB+SAC | |
| 24 | 63 ± 1.2 | 53 ± 1.1 * | 68.3 ± 1.2 | 67 ± 1.4 | 30 | 58 | 73 | 0 | 0 |
| 48 | 49 ± 0.6 * | 39 ± 1.1 ** | 68.3 ± 1.6 | 67± 1.4 | 28 | 61 | 73 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccamo, M.T.; Zammuto, V.; Spanò, A.; Gugliandolo, C.; Magazù, S. Hydrating Capabilities of the Biopolymers Produced by the Marine Thermophilic Bacillus horneckiae SBP3 as Evaluated by ATR-FTIR Spectroscopy. Materials 2022, 15, 5988. https://doi.org/10.3390/ma15175988
Caccamo MT, Zammuto V, Spanò A, Gugliandolo C, Magazù S. Hydrating Capabilities of the Biopolymers Produced by the Marine Thermophilic Bacillus horneckiae SBP3 as Evaluated by ATR-FTIR Spectroscopy. Materials. 2022; 15(17):5988. https://doi.org/10.3390/ma15175988
Chicago/Turabian StyleCaccamo, Maria Teresa, Vincenzo Zammuto, Antonio Spanò, Concetta Gugliandolo, and Salvatore Magazù. 2022. "Hydrating Capabilities of the Biopolymers Produced by the Marine Thermophilic Bacillus horneckiae SBP3 as Evaluated by ATR-FTIR Spectroscopy" Materials 15, no. 17: 5988. https://doi.org/10.3390/ma15175988







