Enhancement of Domain Wall Pinning in High-Temperature Resistant Sm2Co17 Type Magnets by Addition of Y2O3
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Magnetic Properties
3.2. Analysis of Elements’ Diffusion, Cellular Structure and Phases
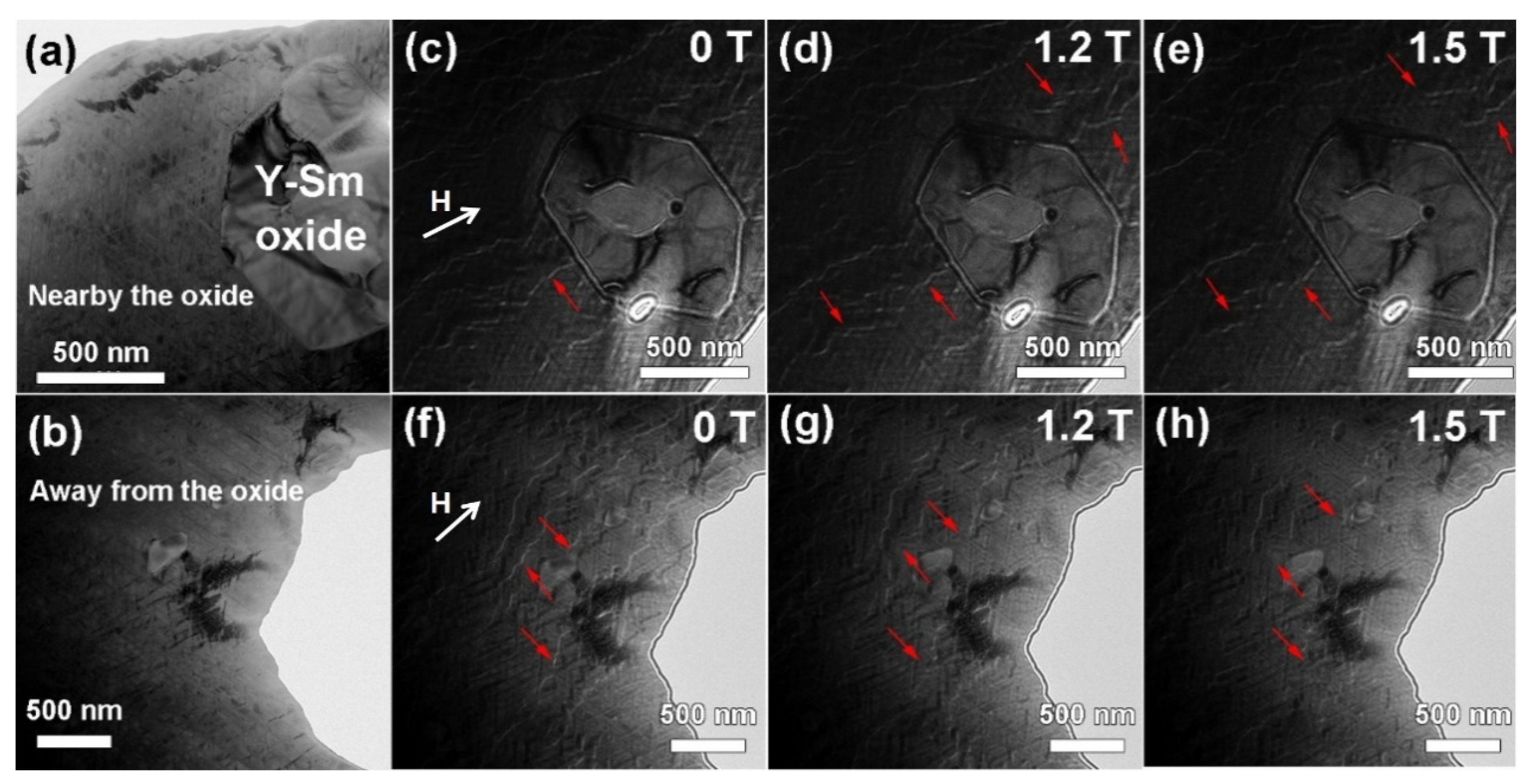
3.3. Analysis of Antimagnetization Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strnat, K.J.; Strnat, R.M.W. Rare earth-cobalt permanent magnets. J. Magn. Magn. Mater. 1991, 100, 38–56. [Google Scholar] [CrossRef]
- Mishra, R.K.; Thomas, G.; Yoneyama, T.; Fukuno, A.; Ojima, T. Microstructure and properties of step aged rare earth alloy magnets. J. Appl. Phys. 1981, 52, 2517–2519. [Google Scholar] [CrossRef] [Green Version]
- Walmer, M.S.; Chen, C.H.; Walmer, M.H. A new class of Sm-TM magnets for operating temperatures up to 550 °C. IEEE Transact. Magn. 2000, 36, 3376–3381. [Google Scholar] [CrossRef]
- Chen, C.H.; Walmer, M.; Walmer, M.; Liu, S.; Kuhl, E.; Simon, G. Sm2(Co,Fe,Cu,Zr) 17 magnets for use at temperatures ≥400 °C. J. Appl. Phys. 1998, 83, 6706–6708. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Goll, D.; Kronmüller, H.; Stadelmaier, H.H. Micromagnetism and the microstructure of high-temperature permanent magnets. J. Appl. Phys. 2004, 96, 6534–6545. [Google Scholar] [CrossRef]
- Goll, D.; Stadelmaier, H.H.; Kronmüller, H. Samarium-cobalt 2:17 magnets: Analysis of the coercive field of Sm2(CoFeCuZr)17 high-temperature permanent magnets. Scr. Mater. 2010, 63, 243–245. [Google Scholar] [CrossRef]
- Feng, H.; Chen, H.; Guo, Z.; Yu, R.; Li, W. Twinning structure in Sm(Co,Fe,Cu,Zr) z permanent magnet. Intermetallics 2010, 18, 1067–1071. [Google Scholar] [CrossRef]
- Sepehri-Amin, H.; Thielsch, J.; Fischbacher, J.; Ohkubo, T.; Schrefl, T.; Gutfleisch, O.; Hono, K. Correlation of microchemistry of cell boundary phase and interface structure to the coercivity of Sm(Co0.784Fe0.1Cu0.088Zr0.028)7.19 sintered magnets. Acta Mater. 2017, 126, 1–10. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Ohkubo, T.; Koyama, T.; Ohashi, K.; Tawara, Y.; Hono, K. The microstructure of sintered Sm(Co0.72Fe0.20Cu0.055Zr0.025)7.5 permanent magnet studied by atom probe. Acta Mater. 2004, 52, 737–748. [Google Scholar] [CrossRef]
- Kronmüller, H.; Goll, D. Micromagnetic analysis of pinning-hardened nanostructured, nanocrystalline Sm2Co17 based alloys. Scr. Mater. 2002, 47, 545–550. [Google Scholar] [CrossRef]
- Ray, A.E. Metallurgical behavior of Sm(Co,Fe,Cu,Zr)z alloys. J. Appl. Phys. 1984, 55, 2094–2096. [Google Scholar] [CrossRef]
- Ray, A.E. A revised model for the metallurgical behavior of 2:17-type permanent magnet alloys. J. Appl. Phys. 1990, 67, 4972–4974. [Google Scholar] [CrossRef]
- Rabenberg, L.; Mishra, R.K.; Thomas, G. Microstructure of precipitation-hardened SmCo permanent magnets. J. Appl. Phys. 1982, 53, 2389–2391. [Google Scholar] [CrossRef] [Green Version]
- Maury, C.; Rabenberg, L.; Allibert, C.H. Genesis of the cell microstructure in the Sm(Co,Fe,Cu,Zr)z permanent magnets with 2:17 type. Phys. Stat. Solidi A 1993, 140, 57–72. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, Z.; Li, M.; Liu, L.; Li, T.; Chen, R.; Lee, D.; Yan, A. The evolution of phase constitution and microstructure in iron-rich 2:17-type Sm-Co magnets with high magnetic performance. Sci. Rep. 2018, 8, 9103. [Google Scholar] [CrossRef]
- Wu, H.C.; Zhang, C.Y.; Liu, Z.; Wang, G.Q.; Lu, H.M.; Chen, G.X.; Li, Y.; Chen, R.J.; Yan, A.R. Nanoscale short-range ordering induced cellular structure and microchemistry evolution in Sm2Co17-type magnets. Acta Mater. 2020, 200, 883–892. [Google Scholar] [CrossRef]
- Hadjipanayis, G.C.; Tang, W.; Zhang, Y.; Chui, S.T.; Liu, J.F.; Chen, C.; Kronmuller, H. High temperature 2:17 magnets: Relationship of magnetic properties to microstructure and processing. IEEE Transact. Magn. 2000, 36, 3382. [Google Scholar] [CrossRef]
- Liu, J.F.; Ding, Y.; Zhang, Y.; Dimitar, D.; Zhang, F.; Hadjipanayis, G.C. New rare-earth permanent magnets with an intrinsic coercivity of 10 kOe at 500 °C. J. Appl. Phys. 1999, 85, 5660. [Google Scholar] [CrossRef]
- Yu, N.J.; Zhu, M.G.; Fang, Y.K.; Song, L.W.; Sun, W.; Song, K.K.; Wang, Q.; Li, W. The microstructure and magnetic characteristics of Sm(CobalFe0.1Cu0.09Zr0.03)7.24 high temperature permanent magnets. Scr. Mater. 2017, 132, 44–48. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yue, M.; Wu, D.; Zhang, D.T.; Liu, W.Q.; Zhang, H.G. Microstructure modification induced giant coercivity enhancement in Sm(CoFeCuZr)z permanent magnets. Scr. Mater. 2018, 146, 231. [Google Scholar] [CrossRef]
- Yan, G.H.; Liu, Z.; Xia, W.X.; Zhang, C.Y.; Wang, G.Q.; Chen, R.J.; Lee, D.; Liu, J.P.; Yan, A.R. Grain boundary modification induced magnetization reversal process and giant coercivity enhancement in 2:17 type SmCo magnets. J. Alloys Compd. 2019, 785, 429–435. [Google Scholar] [CrossRef]
- Wang, G.Q.; Liu, Z.; Zhang, C.Y.; Yang, Q.Q.; Wu, H.C.; Chen, R.J.; Yan, A.R. Cellular structure regulation and coercivity enhancement induced by La2O3 doping in 2:17 type SmCo magnet. J. Alloys Compd. 2020, 849, 156589. [Google Scholar] [CrossRef]
- Tang, W.; Gabay, A.M.; Zhang, Y.; Hadjipanayis, G.C.; Kronmüller, H. Temperature dependence of coercivity and magnetization reversal mechanism in Sm(CobalFe0.1CuyZr0.04)7.0 magnets. IEEE Transact. Magn. 2001, 37, 2515. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Doyle, G.; Knhl, G.E.; Chen, C.; Walmer, M.; Walmer, M. New sintered high temperature Sm-Co based permanent magnet materials. IEEE Transact. Magn. 1999, 35, 3325. [Google Scholar]
- Duerrschnabel, M.; Yi, M.; Uestuener, K.; Liesegang, M.; Katter, M.; Kleebe, H.J.; Xu, B.; Gutflfleisch, O.; Molina-Luna, L.; Duerrschnabel, M. Atomic structure and domain wall pinning in samarium-cobalt-based permanent magnets. Nat. Commun. 2017, 8, 54. [Google Scholar] [CrossRef]
- Lectard, E.; Allibert, C.H.; Ballou, R. Saturation magnetization and anisotropy fields in the Sm(Co1-xCux)5 phases. J. Appl. Phys. 1994, 75, 6277–6279. [Google Scholar] [CrossRef]







| Addition Amount | 300 K | 373 K | 473 K | 573 K | 673 K | 773 K | 823 K | |
|---|---|---|---|---|---|---|---|---|
| 0 wt.% | Br (kGs) | 9.45 | 9.17 | 8.81 | 8.31 | 7.65 | 6.72 | 6.26 |
| Hcj (kOe) | 21.34 | 18.76 | 16.19 | 13.77 | 10.72 | 7.01 | 5.14 | |
| (BH)max (MGOe) | 20.97 | 19.71 | 17.93 | 15.92 | 13.04 | 9.87 | 7.79 | |
| 0.5 wt.% | Br (kGs) | 9.35 | 9.13 | 8.85 | 8.39 | 7.90 | 7.25 | 6.78 |
| Hcj (kOe) | 27.13 | 23.78 | 19.87 | 16.07 | 12.09 | 8.15 | 6.27 | |
| (BH)max (MGOe) | 20.61 | 19.82 | 18.3 | 16.36 | 14.27 | 11.64 | 9.86 | |
| 1 wt.% | Br (kGs) | 9.19 | 8.89 | 8.60 | 8.06 | 7.45 | 6.66 | 6.22 |
| Hcj (kOe) | 27.42 | 23.81 | 19.65 | 15.41 | 10.78 | 6.27 | 4.49 | |
| (BH)max (MGOe) | 19.71 | 18.59 | 17.05 | 15.14 | 12.54 | 9.64 | 7.52 | |
| 2 wt.% | Br (kGs) | 9.10 | 8.84 | 8.36 | 7.89 | 7.26 | 6.60 | 6.24 |
| Hcj (kOe) | 24.37 | 21.32 | 17.95 | 14.65 | 10.97 | 7.11 | 5.32 | |
| (BH)max (MGOe) | 19.51 | 18.57 | 16.54 | 14.39 | 11.94 | 9.64 | 7.72 | |
| 3 wt.% | Br (kGs) | 9.02 | 8.90 | 8.52 | 8.16 | 7.62 | 6.68 | 6.22 |
| Hcj (kOe) | 21.43 | 18.88 | 16.25 | 13.72 | 10.64 | 7 | 5.14 | |
| (BH)max (MGOe) | 19.48 | 18.95 | 16.86 | 15 | 12.77 | 9.72 | 7.59 | |
| Phase | Composition | K1 (MJ/m3) | Ms (T) | A (pJ/m) | |
|---|---|---|---|---|---|
| Cell phase | Sm2Co17 | 4.2 | 1.22 | 14 | |
| Cell boundary phase | original magnet | Sm(Co0.7Cu0.3)5 | 8.7 | 0.56 | 6.6 |
| magnet with 0.5 wt.% addition | Sm(Co0.72Cu0.28)5 | 9.36 | 0.588 | 6.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, C.; Wu, H.; Chen, R.; Yan, A. Enhancement of Domain Wall Pinning in High-Temperature Resistant Sm2Co17 Type Magnets by Addition of Y2O3. Materials 2022, 15, 5160. https://doi.org/10.3390/ma15155160
Liu Z, Zhang C, Wu H, Chen R, Yan A. Enhancement of Domain Wall Pinning in High-Temperature Resistant Sm2Co17 Type Magnets by Addition of Y2O3. Materials. 2022; 15(15):5160. https://doi.org/10.3390/ma15155160
Chicago/Turabian StyleLiu, Zhuang, Chaoyue Zhang, Haichen Wu, Renjie Chen, and Aru Yan. 2022. "Enhancement of Domain Wall Pinning in High-Temperature Resistant Sm2Co17 Type Magnets by Addition of Y2O3" Materials 15, no. 15: 5160. https://doi.org/10.3390/ma15155160





