Adsorption Study of Congo Red Dye from Synthetic Wastewater at Different Concentrations Using Zinc Sulfide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Calculation of Catalytic Constants
3. Results and Discussions
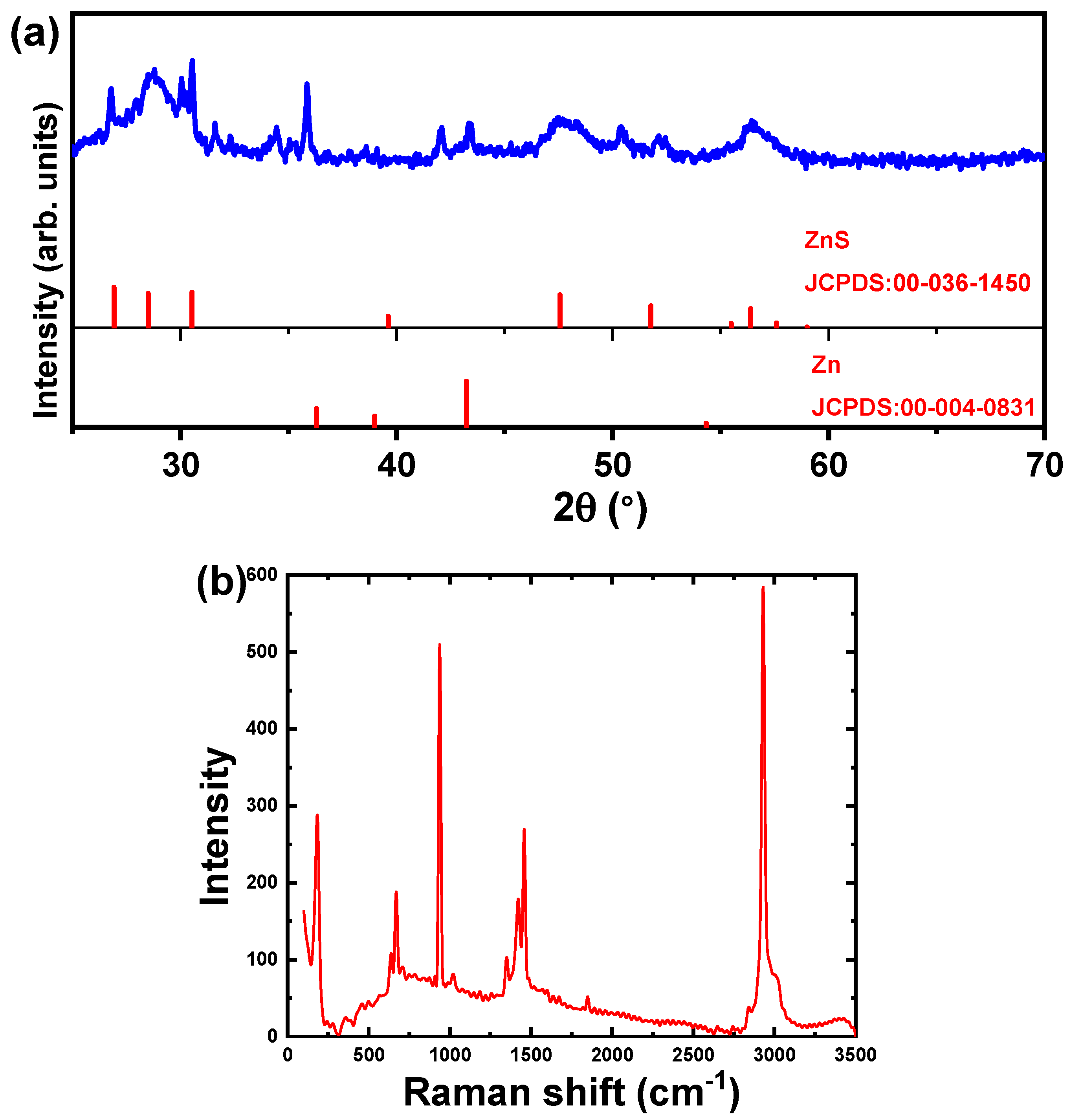
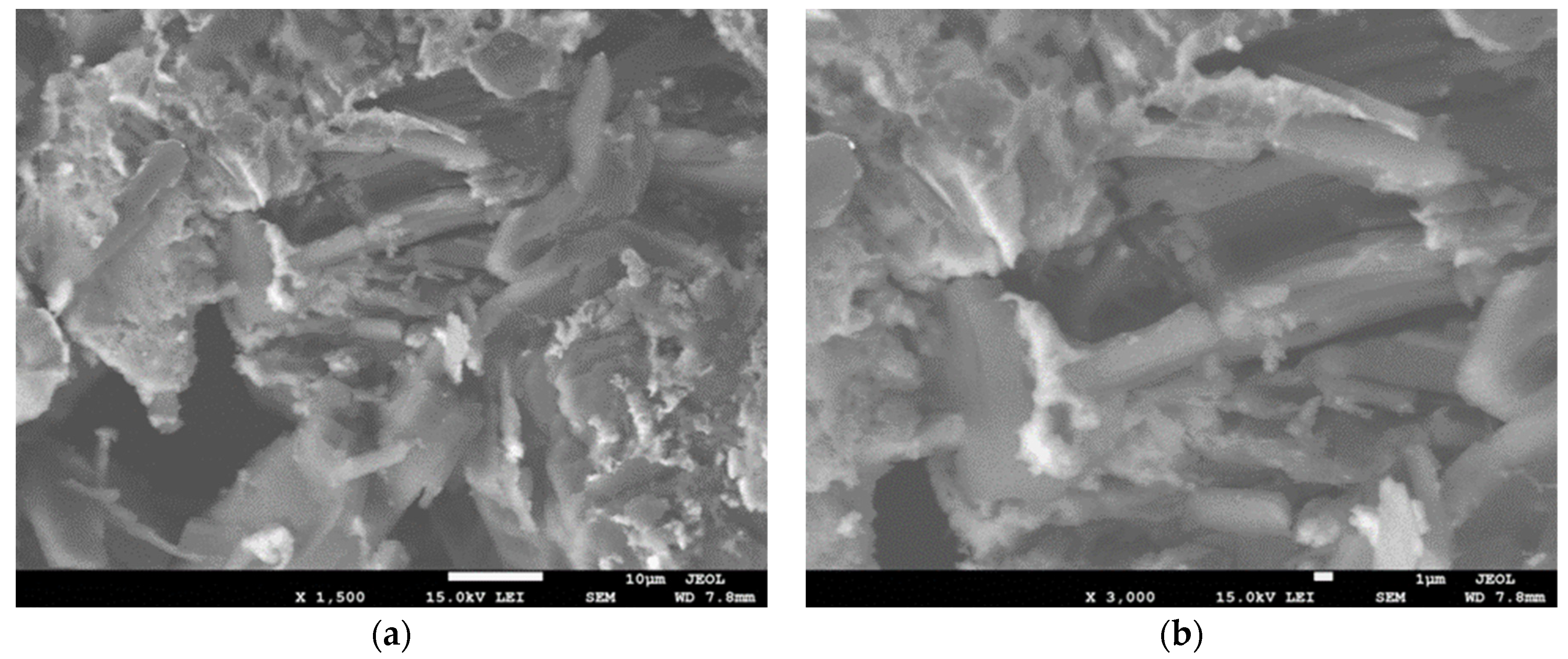
3.1. Structure and Morphology
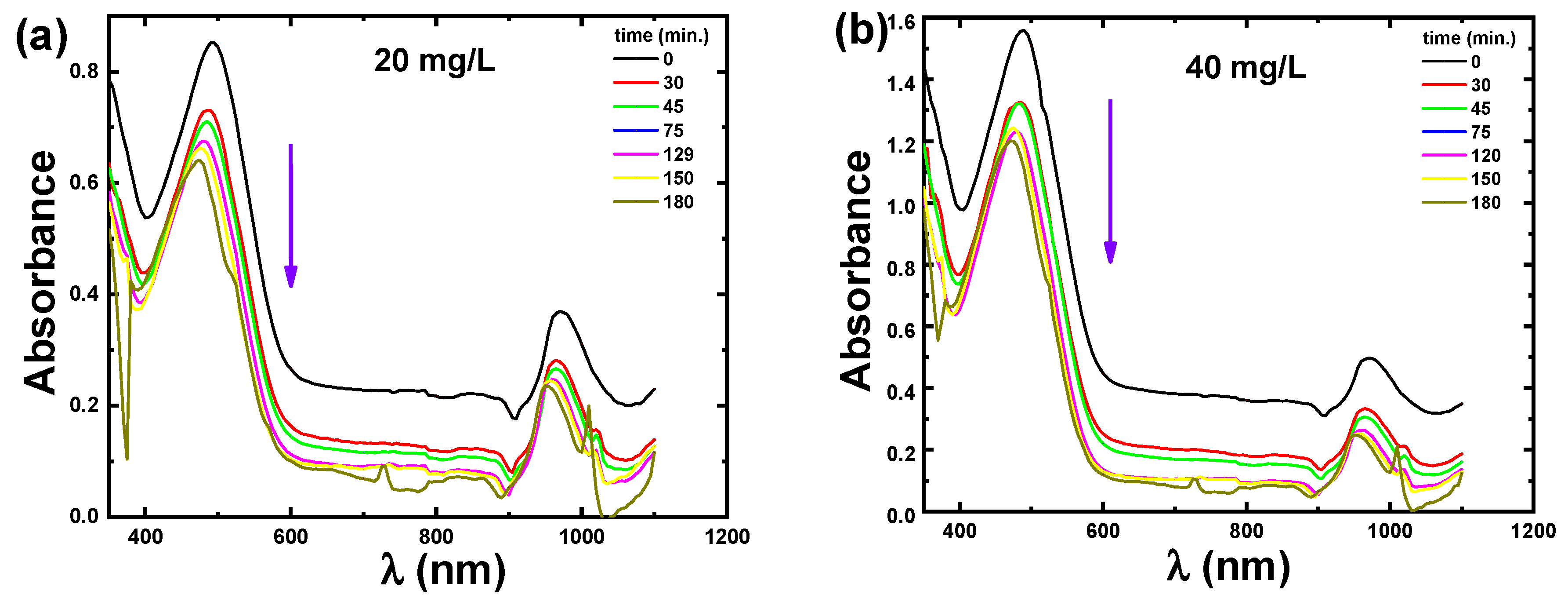
3.2. Catalytic Adsorption Studies
3.2.1. Catalytic Adsorption of CR Dye
3.2.2. Comparison of the Catalytic Performance Using ZnS Nanoparticles
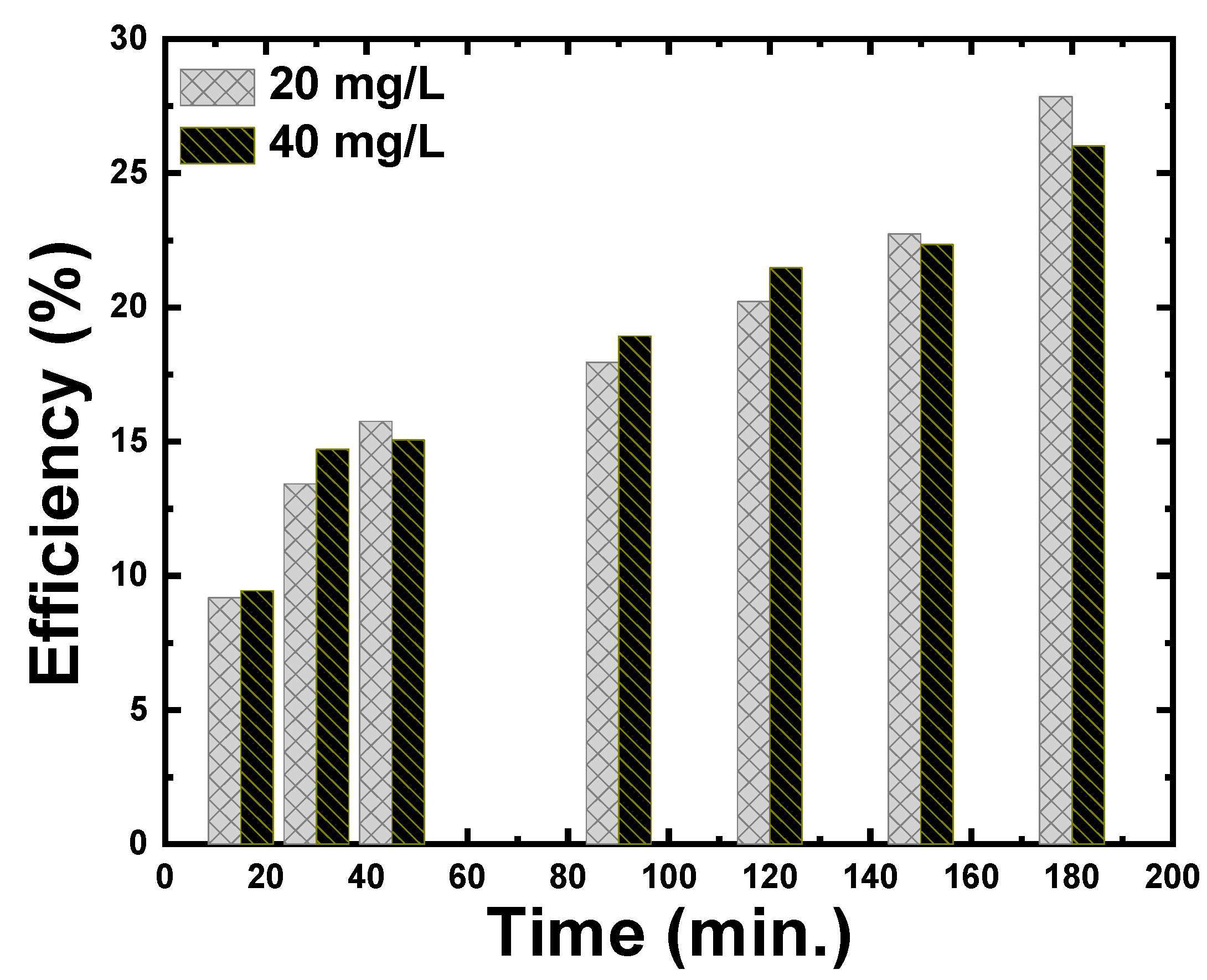
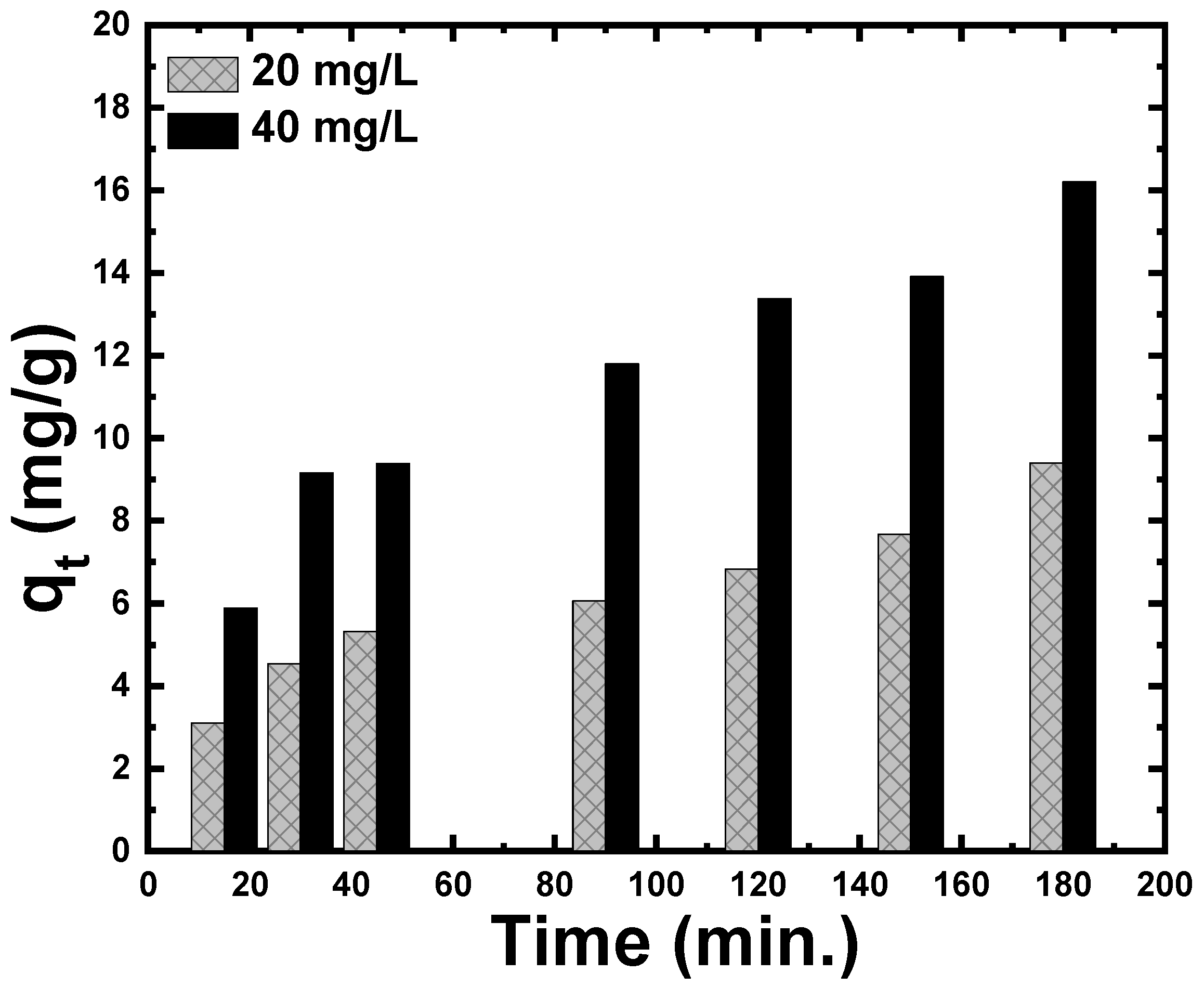
3.2.3. Effect of the Contact Time and the Initial Concentration on Adsorption of CR Dye
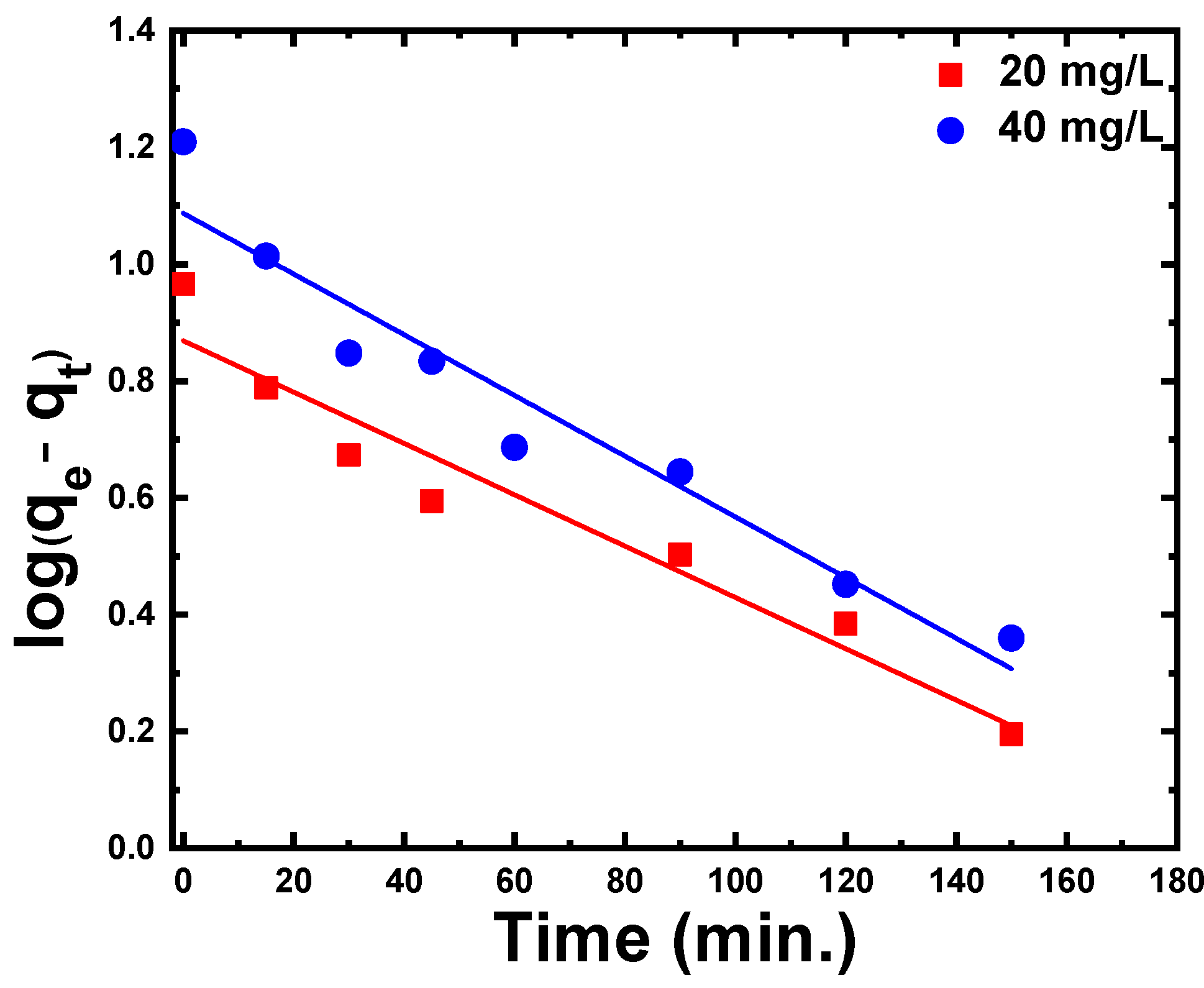
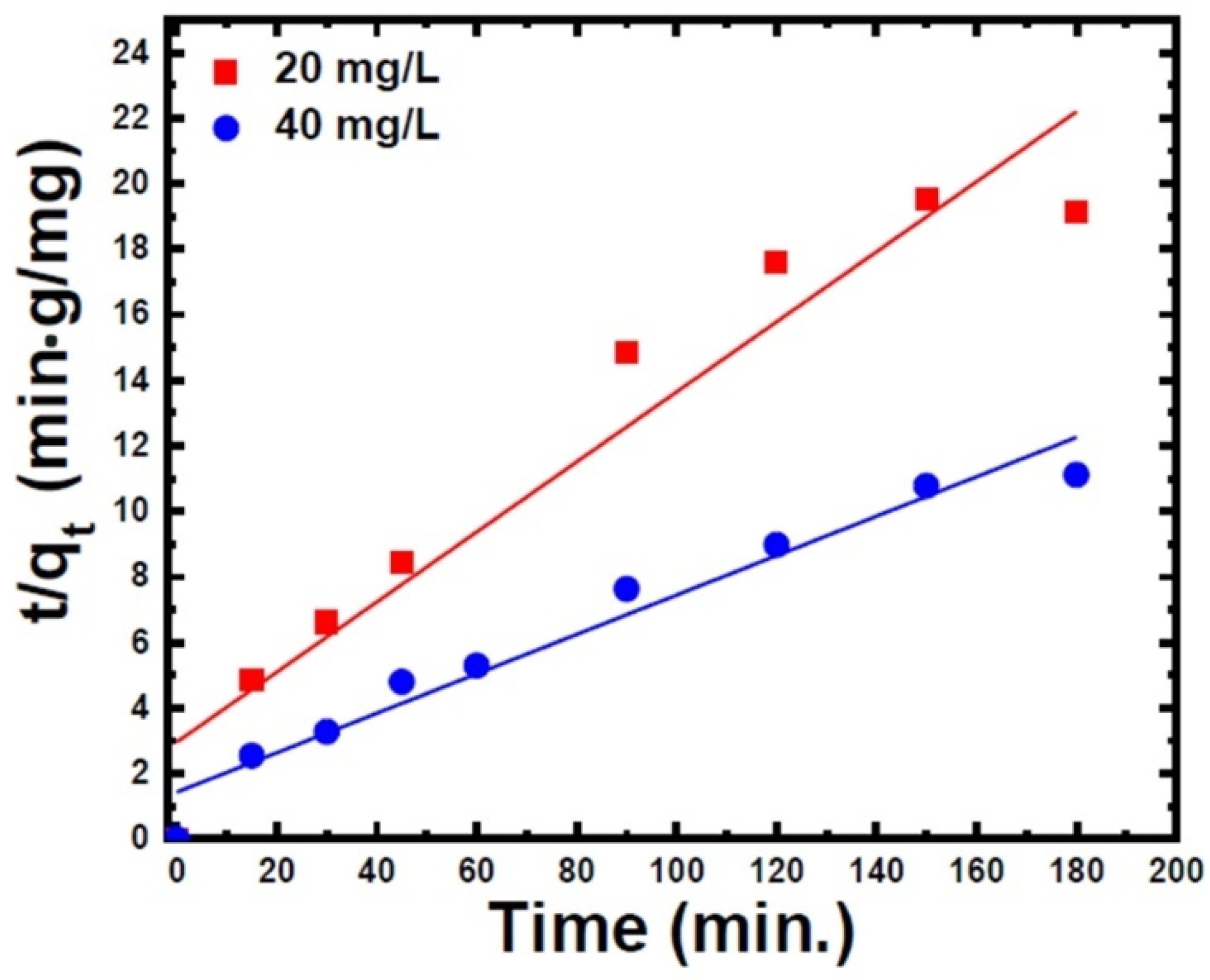
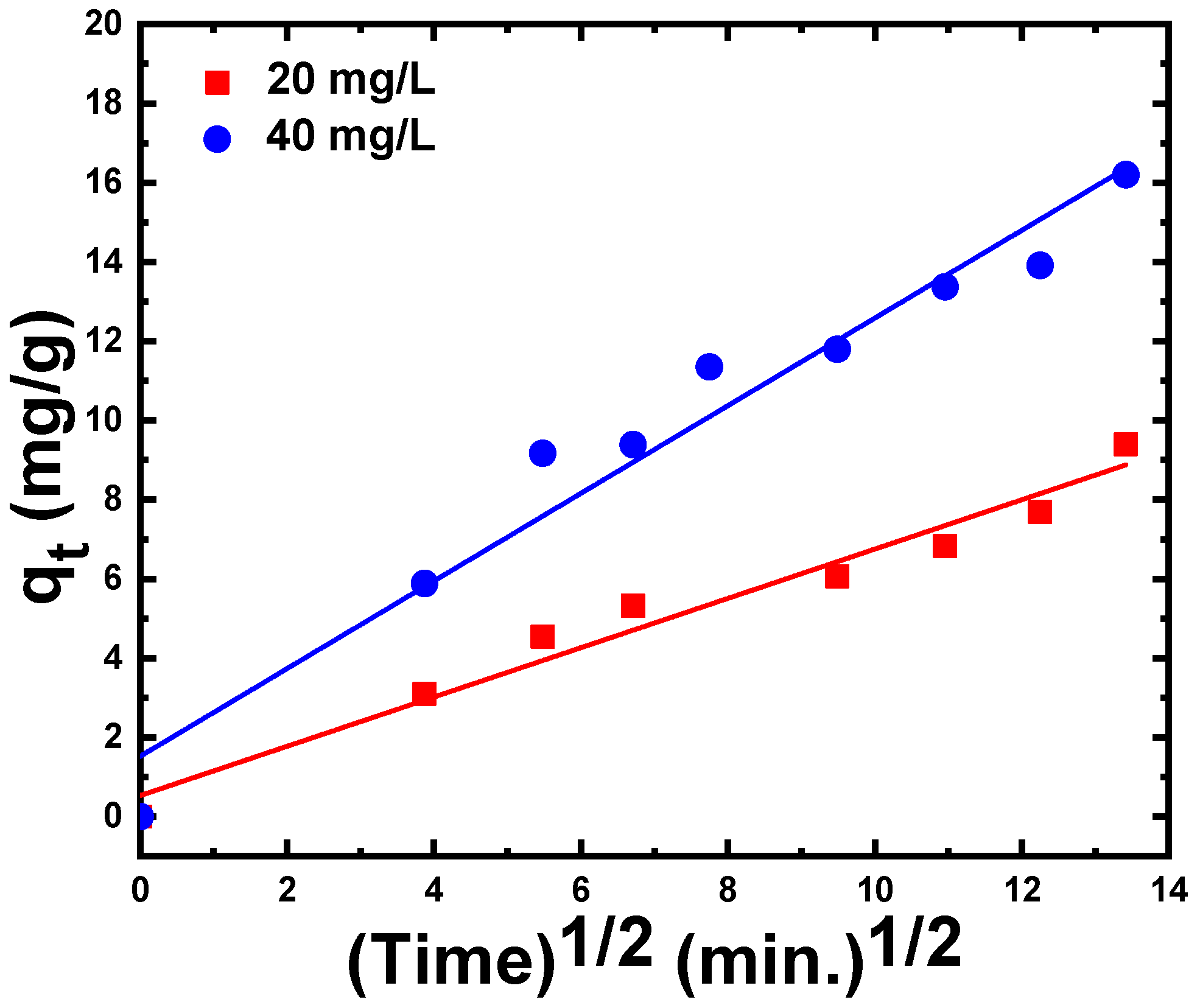
3.2.4. Adsorption Kinetic Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gayou, V.L.; Salazar-Hernandez, B.; Constantino, M.E.; Andrés, E.R.; Díaz, T.; Macuil, R.D.; López, M.R. Structural studies of ZnS thin films grown on GaAs by RF magnetron sputtering. Vacuum 2010, 84, 1191–1194. [Google Scholar] [CrossRef]
- Xiang, D.; Zhu, Y.; He, Z.; Liu, Z.; Luo, J. A simple one-step synthesis of ZnS nanoparticles via salt-alkali-composited-mediated method and investigation on their comparative photocatalytic activity. Mater. Res. Bull. 2013, 48, 188–193. [Google Scholar] [CrossRef]
- Dubey, K.K.; Nayar, V.; Choudhary, P.S. Synthesis, Structural and Optical Properties of ZnS Nano-Particles. Mater. Sci. Res. 2010, 7, 195–200. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Jin, C.Q.; Gao, F.; Wu, X.L.; Zhong, W.; Li, S.H.; Chu, P.K. Raman scattering study of zinc blende and wurtzite ZnS. J. Appl. Phys. 2009, 106, 123505. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- La Porta, F.D.A.; Nogueira, A.E.; Gracia, L.; Pereira, W.S.; Botelho, G.; Mulinari, T.A.; Andrés, J.; Longo, E. An experimental and theoretical investigation on the optical and photocatalytic properties of ZnS nanoparticles. J. Phys. Chem. Solids 2017, 103, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Jain, T.; Singh, S.; Pandey, O.P. Photocatalytic degradation of organic dyes under UV–Visible light using capped ZnS nanoparticles. Sol. Energy 2012, 86, 626–633. [Google Scholar] [CrossRef]
- Wu, X.; Li, K.; Wang, H. Facile synthesis of ZnS nanostructured spheres and their photocatalytic properties. J. Alloy. Compd. 2009, 487, 537–544. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Kong, J.; Jia, H. Tunable synthesis, characterization and photocatalytic properties of various ZnS nanostructures. Ceram. Int. 2016, 42, 2854–2860. [Google Scholar] [CrossRef]
- Rahaman, S.; Sunil, M.A.; Singha, M.K.; Ghosh, K. Optimization and fabrication of low cost Cu2SnS3/ZnS thin film heterojunction solar cell using ultrasonic spray pyrolysis. Opt. Mater. 2022, 123, 111838. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Bhatt, V.; Mukherjee, S.; Kumar, S.; Sharma, H.; Yadav, M.K.; Tomar, S.; Yun, J.-H.; Choubey, R.K. Highly responsive and low-cost ultraviolet sensor based on ZnS/p-Si heterojunction grown by chemical bath deposition. Sens. Actuators A 2021, 331, 112988. [Google Scholar] [CrossRef]
- Milekhin, A.G.; Yeryukov, N.A.; Sveshnikova, L.L.; Duda, T.A.; Himcinschi, C.; Zenkevich, E.I.; Zahn, D.R.T. Resonant Raman scattering of ZnS, ZnO, and ZnS/ZnO core/shell quantum dots. Appl. Phys. A 2012, 107, 275–278. [Google Scholar] [CrossRef]
- Fairbrother, A.; Izquierdo-Roca, V.; Fontané, X.; Ibáñez, M.; Cabot, A.; Saucedo, E.; Pérez-Rodríguez, A. ZnS grain size effects on near-resonant Raman scattering: Optical non-destructive grain size estimation. Cryst. Eng. Comm. 2014, 16, 4120–4125. [Google Scholar] [CrossRef]
- Mostafa, M.; El Nady, J.; Ebrahim, S.M.; Elshaer, A.M. Synthesis, structural, and optical properties of Mn2+ doped ZnS quantum dots for biosensor application. Opt. Mater. 2021, 112, 110732. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Jeyakumar, S.J.; Praveen, P.; Punithavathy, I.K.; Richard, J.P. Synthesis and enhanced photocatalytic property of Ni doped ZnS nanoparticles. Sol. Energy 2018, 159, 434–443. [Google Scholar] [CrossRef]
- Peng, H.; Liuyang, B.; Lingjie, Y.; Jinlin, L.; Fangli, Y.; Yunfa, C. Shape-controlled synthesis of ZnS nanostructures: A simple and rapid method for one-dimensional materials by plasma. Nanoscale Res. Lett. 2009, 4, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Prasad, N.; Karthikeyan, B. Resonant and Off-Resonant Phonon Properties of Wurtzite ZnS: Effect of Morphology on Fröhlich Coupling and Phonon Lifetime. J. Phys. Chem. C 2018, 122, 18117–18123. [Google Scholar] [CrossRef]
- Safian, M.T.-U.; Umar, K.; Mohamad Ibrahim, M.N. Synthesis and scalability of graphene and its derivatives: A journey towards sustainable and commercial material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef] [Green Version]
- Bushra, R.; Shahadat, M.; Ahmad, A.; Nabi, S.A.; Umar, K.; Oves, M.; Raeissi, A.S.; Muneer, M. Synthesis, characterization, antimicrobial activity and applications of polyanilineTi(IV)arsenophosphate adsorbent for the analysis of organic and inorganic pollutants. J. Hazard. Mater. 2014, 264, 481–489. [Google Scholar] [CrossRef]
- Zou, L.; Wang, X.; Xu, X.; Wang, H. Reduced graphene oxide wrapped CdS composites with enhanced photocatalytic performance and high stability. Ceram. Int. 2016, 42, 372–378. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Wang, M.; Zhang, X.; Liao, L.; Fang, T.; Li, B. Photocatalytic degradation of Congo red by using the Cu2O/alpha-Fe2O3 composite catalyst. Desalination Water Treat. 2021, 215, 222–231. [Google Scholar] [CrossRef]
- Xiong, J.; Luo, Q.; Zhao, K.; Zhang, M.; Han, C.; Cheng, G. Facilely Anchoring Cu nanoparticles on WO3 Nanocubes for Enhanced Photocatalysis through Efficient Interface Charge Transfer. J. Inorg. Mater. 2021, 36, 325. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Kannan, K.; Thiripuranthagan, S. Photocatalytic degradation of textile reactive dyes—A comparative study using nano silver decorated titania-silica composite photocatalysts. J. Nanosci. Nanotechnol. 2018, 18, 2921–2930. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.A.A.; Rashad, M.; AL-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye. Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Byon, C.; Jeon, S. Enhanced photocatalytic activity of ZnS nanoparticles loaded with MoS2 nanoflakes by self-assembly approach. Phys. B 2016, 502, 103–112. [Google Scholar] [CrossRef]
- Janani, B.; Okla, M.K.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Thomas, A.M.; Raju, L.L.; Al-Qahtani, W.H.; Khan, S.S. CuO loaded ZnS nanoflower entrapped on PVA-chitosan matrix for boosted visible light photocatalysis for tetracycline degradation and anti-bacterial application. J. Environ. Manag. 2022, 306, 114396. [Google Scholar] [CrossRef]
- Rashad, M.; Al-Ghamdi, S.A.; Alzahrani, A.O.M.; Al-Tabaa, K.; Al-Osemi, S.; Al-Atawi, O.; Al-Anzi, N.; Issa, S.A.M.; Abd-Elnaiem, A.M. Zinc oxide nanoparticles for adsorption of potassium permanganate from wastewater using shaking method. Desalination Water Treat. 2021, 229, 227–234. [Google Scholar] [CrossRef]
- Rashad, M.; Al-Aoh, H.A. Promising adsorption studies of bromophenol blue using copper oxide nanoparticles. Desalination Water Treat. 2019, 139, 360–368. [Google Scholar] [CrossRef]
- Boudiaf, S.; Nasrallah, N.; Mellal, M.; Belhamdi, B.; Belabed, C.; Djilali, M.A.; Trari, M. Kinetic studies of Congo Red Photodegradation on the hetero-system CoAl2O4/ZnO with a stirred reactor under solar light. J. Environ. Chem. Eng. 2021, 9, 105572. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, Y.; Qian, J.; Qiao, Y.; Liu, J.; Li, S.; Dai, L.; Sun, K.; Guo, H.; Sui, G.; et al. Sorption of organochlorine pesticides on polyethylene microplastics in soil suspension. Ecotoxicol. Environ. Saf. 2021, 223, 112591. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, X.; Hou, Z.; Ding, T. Sorption of diclofenac by polystyrene microplastics: Kinetics, isotherms and particle size effects. Chemosphere 2022, 290, 133311. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Idrees, M.; Bilal, M. Revealing and elucidating chemical speciation mechanisms for lead and nickel adsorption on zeolite in aqueous solutions. Colloids Surf. A 2021, 623, 126711. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, R.; Falyouna, O.; Bensaida, K.; Sugihara, Y.; Eljamal, O. Insights into kinetics, isotherms, and thermodynamics of phosphorus sorption onto nanoscale zero-valent iron. J. Mol. Liq. 2021, 328, 115402. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. Pseudo-second-order kinetic models for the sorption of Hg(II) onto dead biomass of marine Aspergillus niger: Comparison of linear and non-linear methods. Colloids Surf. A 2008, 328, 40–43. [Google Scholar] [CrossRef]
- Szostak, K.; Banach, M. Sorption and photocatalytic degradation of methylene blue on bentonite-ZnO-CuO nanocomposite. J. Mol. Liq. 2019, 286, 110859. [Google Scholar] [CrossRef]
- Wang, P.; Tyndall, S.; Rahman, T.; Roy, P.; Jahromi, H.; Adhikari, S.; Boersma, M. Sorption and recovery of phenolic compounds from aqueous phase of sewage sludge hydrothermal liquefaction using bio-char. Chemosphere 2022, 287, 131934. [Google Scholar] [CrossRef]
- Torres, J.; Gu, Y.; Samyn, P.; Yperman, J. Fe-TiO2/AC and Co-TiO2/AC Composites: Novel Photocatalysts Prepared from Waste Streams for the Efficient Removal and Photocatalytic Degradation of Cibacron Yellow F-4G D. Catalysts 2021, 11, 1137. [Google Scholar]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Prasad, N.; Karthikeyan, B. A Raman spectral probe on polar w-ZnS nanostructures and surface optical phonon modes in nanowires. Nanoscale 2019, 11, 4948–4958. [Google Scholar] [CrossRef]
- Nilsen, W.G. Raman spectrum of cubic ZnS. Phys. Rev. 1969, 182, 838–850. [Google Scholar] [CrossRef]
- Trajić, J.; Kostić, R.; Romčević, N.; Romčević, M.; Mitrić, M.; Lazović, V.; Balaž, P.; Stojanović, D. Raman spectroscopy of ZnS quantum dots. J. Alloys Compd. 2015, 637, 401–406. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Rajput, B.D.; Browne, D.A. Lattice dynamics of II-VI materials using the adiabatic bond-charge model. Phys. Rev. B 1996, 53, 9052. [Google Scholar] [CrossRef] [Green Version]
- Serrano, J.; Cantarero, A.; Cardona, M.; Garro, N.; Lauck, R.; Tallman, R.E.; Ritter, T.M.; Weinstein, B.A. Raman scattering in β-ZnS. Phys. Rev. B 2004, 69, 014301. [Google Scholar] [CrossRef]
- Shaohua, W.; Pan, H.; Jingsong, Z.; Yuejin, Z.; Lihe, Z.; Rukun, D. TTG-CVD based ZnS material preparation. Infrared Laser Eng. 2021, 50, 20210004. [Google Scholar]
- Guo, M.; Song, M.; Li, S.; Yin, Z.; Song, X.; Bu, Y. Facile and economical synthesis of ZnS nanotubes and their superior adsorption performance for organic dyes. CrystEngComm 2017, 19, 2380–2393. [Google Scholar] [CrossRef]

| Preparation Method | Dye (Symbol) | Dye Concentration (mg/L) | Catalytic Efficiency (%) | Catalyst Amount (g/L) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| Solid state reaction | Methylene blue (MB) | 10 | 72.13 | 0.3 | 180 | [15] |
| Salt-alkali-composited-mediated | Methyl orange (MO) | 20 | 97.67 for synthesized ZnS& 26.61 for commercial ZnS | 0.2 | 840 | [2] |
| Chemical precipitation | MB, xylenol orange (XO), Methyl orange (MO), and methyl red (MR) | 50 | 78.41, 81.22, 90.90 & 95.10 | 0.5 | 120 | [5] |
| Microwave-assisted solvothermal | Rhodamine B (RhB) | 10 | 97, 82, and 56 prepared ZnS from zinc nitrate, zinc chloride, and zinc acetate | 0.75 | 210 | [6] |
| Chemical precipitation | Bromophenol blue (BPB) | 10 | 42.5 | 2.5 | 180 | [7] |
| Hydrothermal | Rhodamine B (RhB) | 5 | 90 | 1.0 | 60 | [8] |
| Hydrothermal | Methylene blue (MB) | 12 | 92 | 0.1 | 198 | [9] |
| Direct precipitation | Congo red (CR) | 25 | 96.3 | 0.2 | 60 | [48] |
| Aniline blue (AB) | 87.2 | |||||
| Brilliant blue (G250) | 61.6 | |||||
| Methylene blue (MB) | 83.4 | |||||
| Azophloxine (AR1) | 6.5 | |||||
| Chemical precipitation | Congo red (CR) | 20 | 27.84 | 0.6 | 180 | This Paper |
| 40 | 26.01 |
| Model | Parameter | Dye Concentration (mg/L) | |
|---|---|---|---|
| 20 | 40 | ||
| Pseudo-first-order kinetic model | exp. (mg/g) | 9.24 | 16.19 |
| cal. (mg/g) | 7.38 | 12.19 | |
| exp.– cal. | 1.86 | 4 | |
| (min−1) | 0.01 | 0.012 | |
| R2 | 0.96 | 0.97 | |
| Pseudo-second-order kinetic model | cal. (mg/g) | 9.43 | 16.67 |
| exp.– cal. | 0.19 | 0.48 | |
| (g/mg·min) | 0.0038 | 0.00252 | |
| R2 | 0.96 | 0.97 | |
| Intra-particle diffusion kinetic model | (mg/min1/2·g) | 0.62 | 1.106 |
| C | 0.53 | 1.52 | |
| R2 | 0.98 | 0.96 | |
| Elovic kinetic model | α (mg/g·min) | 8.43 | 11.24 |
| β (g/mg) | 0.46 | 0.27 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashad, M.; Helali, S.; Issa, S.; Al-Ghamdi, S.; Alsharif, M.; Alzahrani, A.O.; Sobhi, M.; Ene, A.; Abd-Elnaiem, A.M. Adsorption Study of Congo Red Dye from Synthetic Wastewater at Different Concentrations Using Zinc Sulfide Nanoparticles. Materials 2022, 15, 5048. https://doi.org/10.3390/ma15145048
Rashad M, Helali S, Issa S, Al-Ghamdi S, Alsharif M, Alzahrani AO, Sobhi M, Ene A, Abd-Elnaiem AM. Adsorption Study of Congo Red Dye from Synthetic Wastewater at Different Concentrations Using Zinc Sulfide Nanoparticles. Materials. 2022; 15(14):5048. https://doi.org/10.3390/ma15145048
Chicago/Turabian StyleRashad, Mohamed, Saloua Helali, Shams Issa, Saleh Al-Ghamdi, Marwah Alsharif, Ahmed Obaid Alzahrani, Mohamed Sobhi, Antoaneta Ene, and Alaa M. Abd-Elnaiem. 2022. "Adsorption Study of Congo Red Dye from Synthetic Wastewater at Different Concentrations Using Zinc Sulfide Nanoparticles" Materials 15, no. 14: 5048. https://doi.org/10.3390/ma15145048








