Fabrication and Investigation of PE-SiO2@PZS Composite Separator for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Synthesis of SiO2 Nanoparticles
2.4. Synthesis of Silica-Polyphosphazene Nanoparticles (SiO2@PZS)
2.5. Fabrication of PE-SiO2@PZS Composite Membrane
3. Results and Discussion
3.1. Characterization of SiO2@PZS Nanoparticles
3.2. Characterization of Membranes for LIBs
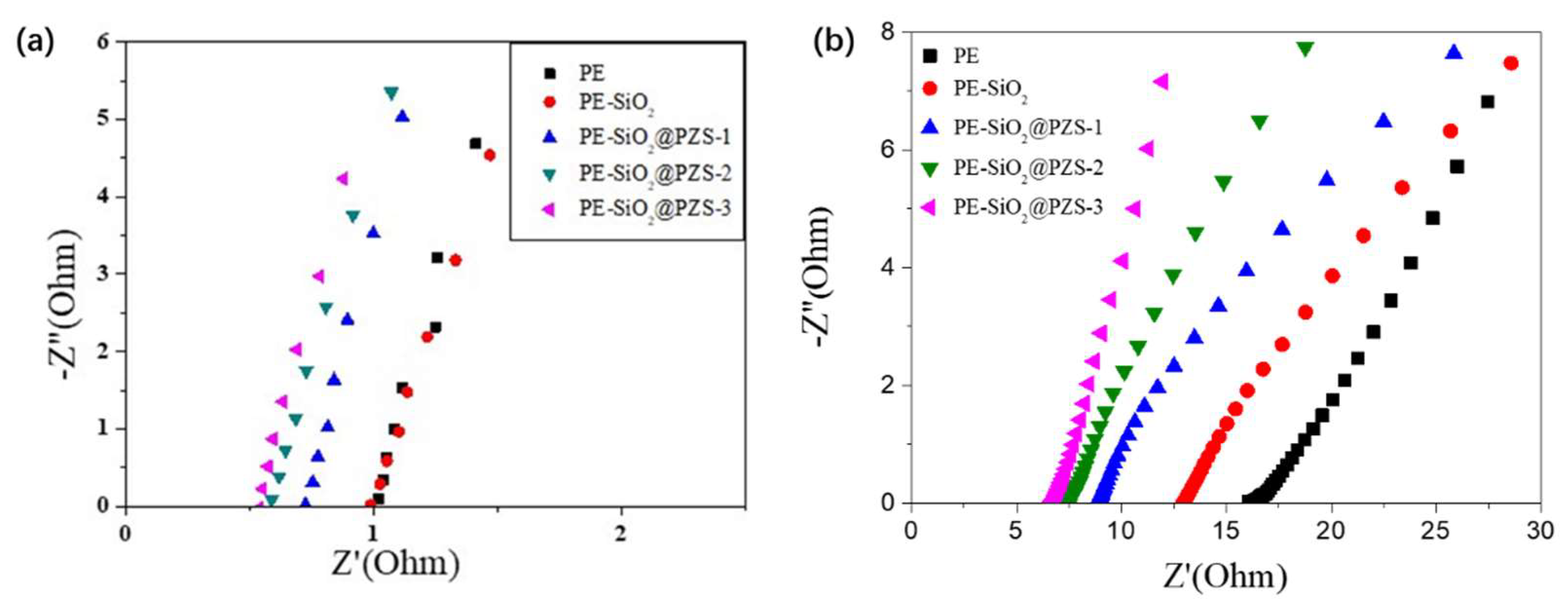
3.3. Characterization of Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Sun, Q.; Zhen, C.; Niu, Y.; Han, Y.; Zeng, G.; Chen, D.; Feng, C.; Chen, N.; Lv, W.; et al. Recent Progress in Flame-Retardant Separators for Safe Lithium-Ion Batteries. Energy Storage Mater. 2021, 37, 628–647. [Google Scholar] [CrossRef]
- Tong, X.; Tian, Z.; Sun, J.; Tung, V.; Kaner, R.B.; Shao, Y. Self-Healing Flexible/stretchable Energy Storage Devices. Mater. Today 2021, 44, 78–104. [Google Scholar] [CrossRef]
- Son, H.B.; Shin, M.; Song, W.-J.; Han, D.-Y.; Choi, S.; Cha, H.; Nam, S.; Cho, J.; Choi, S.; Yoo, S.; et al. A Dry Room-Free High-Energy Density Lithium-ion Batteries Enabled by Impurity Scavenging Separator Membrane. Energy Storage Mater. 2021, 36, 355–364. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Xu, Y.; Zhang, G.; Li, S.; Li, C.; Zhang, X.; Sun, X.; Ge, X.; Ma, Y. Strategies to Boost Ionic Conductivity and Interface Compatibility of Inorganic-Organic Solid Composite Electrolytes. Energy Storage Mater. 2021, 36, 291–308. [Google Scholar] [CrossRef]
- Pang, Y.; Pan, J.; Yang, J.; Zheng, S.; Wang, C. Electrolyte/Electrode Interfaces in All-Solid-State Lithium Batteries: A Review. Electrochem. Energy Rev. 2021, 4, 169–193. [Google Scholar] [CrossRef]
- Nagaoka, K.; Naruse, H.; Shinohara, I.; Watanabe, M. High Ionic Conductivity in Poly(dimethyl siloxane-co-ethylene oxide) Dissolving Lithium Perchlorate. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 659–663. [Google Scholar] [CrossRef]
- Wang, C.; Sakai, T.; Watanabe, O.; Hirahara, K.; Nakanishi, T. All Solid-State Lithium-Polymer Battery Using a Self-Cross-Linking Polymer Electrolyte. J. Electrochem. Soc. 2003, 150, A1166–A1170. [Google Scholar] [CrossRef]
- Javier, A.E.; Patel, S.N.; Hallinan, D.T., Jr.; Srinivasan, V.; Balsara, N.P. Simultaneous Electronic and Ionic Conduction in A Block Copolymer: Application in Lithium Battery Electrodes. Angew. Chem. Int. Ed. Engl. 2011, 50, 9848–9851. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for Solid-State Lithium Rechargeable Batteries: Recent Advances and Perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB Triblock Copolymers as Highly Efficient Electrolytes for Lithium-Metal Batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Liang, Y.; Dong, H.; Hao, F.; Wang, A.; Zhu, Y.; Cui, X.; Yao, Y. Hyperbranched PEO-Based Hyperstar Solid Polymer Electrolytes with Simultaneous Improvement of Ion Transport and Mechanical Strength. ACS Appl. Energy Mater. 2019, 2, 1608–1615. [Google Scholar] [CrossRef]
- Meng, N.; Lian, F.; Cui, G. Macromolecular Design of Lithium Conductive Polymer as Electrolyte for Solid-State Lithium Batteries. Small 2021, 17, 2005762. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; He, X.; Fang, M.; Gao, J.; Li, J.; Deng, L.; Chen, H.; Tian, G.; Wang, J.; et al. In Situ Prepared Nano-Crystalline TiO2–Poly(methyl methacrylate) Hybrid Enhanced Composite Polymer Electrolyte for Li-ion Batteries. J. Mater. Chem. A 2013, 1, 233–240. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Shang, Y.; Wang, L.; He, X.; Li, J.; Zhao, P.; Wang, Y. Nanocomposite Polymer Membrane Derived from Nano TiO2-PMMA and Glass Fiber Nonwoven: High Thermal Endurance and Cycle Stability in Lithium Ion Battery Applications. J. Mater. Chem. A 2015, 3, 17697–17703. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO Based Polymer-Ceramic Hybrid Solid Electrolytes: A Review. Nano Converg. 2021, 8, 102–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Guo, W.; Fu, Y. Advances in Composite Polymer Electrolytes for Lithium Batteries and Beyond. Adv. Energy Mater. 2020, 11, 467–479. [Google Scholar] [CrossRef]
- Sun, G.; Guo, J.; Niu, H.; Chen, N.; Zhang, M.; Tian, G.; Qi, S.; Wu, D. The Design of A Multifunctional Separator Regulating the Lithium Ion Flux for Advanced Lithium-Ion Batteries. RSC Adv. 2019, 9, 40084–40091. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Mi, W.; Yu, L.; Jin, Y.; Lin, Y.S. Zeolite Coated Polypropylene Separators with Tunable Surface Properties for Lithium-Ion Batteries. Microporous Mesoporous Mater. 2016, 226, 406–414. [Google Scholar] [CrossRef]
- Qiu, Z.; Yuan, S.; Wang, Z.; Shi, L.; Jo, J.H.; Myung, S.-T.; Zhu, J. Construction of Silica-Oxygen-Borate Hybrid Networks on Al2O3-Coated Polyethylene Deparators Realizing Multifunction for High-Performance Lithium Ion Batteries. J. Power Sources 2020, 472, 228445. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Shang, Y.; Fang, M.; Deng, L.; Gao, J.; Li, J.; Chen, H.; He, X. Dispersibility of Nano-TiO2 on Performance of Composite Polymer Electrolytes for Li-Ion Batteries. Electrochim. Acta 2013, 111, 674–679. [Google Scholar] [CrossRef]
- Fu, D.; Luan, B.; Argue, S.; Bureau, M.N.; Davidson, I.J. Nano SiO2 Particle Formation and Deposition on Polypropylene Separators for Lithium-Ion Batteries. J. Power Sources 2012, 206, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-H.; Hsieh, C.-T.; Ke, W.-J.; Lee, L.-W.; Lin, Y.-F.; Liu, H.-W.; Gu, S.; Fu, C.-C.; Juang, R.-S.; Mallick, B.C.; et al. Roll-to-Roll Atomic Layer Feposition of Titania Coating on Polymeric Separators for Lithium Ion Batteries. J. Power Sources 2021, 482, 675–687. [Google Scholar] [CrossRef]
- Gogia, A.; Wang, Y.; Rai, A.K.; Bhattacharya, R.; Subramanyam, G.; Kumar, J. Binder-Free, Thin-Film Ceramic-Coated Separators for Improved Safety of Lithium-Ion Batteries. ACS Omega 2021, 6, 4204–4211. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, P.; Huang, S.; He, X.; Yang, P.; Wu, D.; Sun, D.; Zhao, J. Functional Separator Consisted of Polyimide Nonwoven Fabrics and Polyethylene Coating Layer for Lithium-Ion Batteries. J. Power Sources 2015, 298, 158–165. [Google Scholar] [CrossRef]
- Heidari, A.A.; Mahdavi, H. Recent Development of Polyolefin-Based Microporous Separators for Li-Ion Batteries: A Review. Chem. Rec. 2020, 20, 570–595. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, F.; Chen, C.; Shi, L.; Yuan, S.; Sun, L.; Zhu, J. Self-assembly of PEI/SiO2 on Polyethylene Separators for Li-Ion Batteries with Enhanced Rate Capability. ACS Appl. Mater. Interfaces 2015, 7, 3314–3322. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, P.; Shi, C.; Chen, L.; Dai, J.; Zhao, J. The Functional Separator Coated with Core–Shell Structured Silica–Poly(methyl methacrylate) Sub-Microspheres for Lithium-Ion Batteries. J. Membr. Sci. 2015, 474, 148–155. [Google Scholar] [CrossRef]
- Fu, W.; Xu, R.; Zhang, X.; Tian, Z.; Huang, H.; Xie, J.; Lei, C. Enhanced Wettability and Electrochemical Performance of Separators for Lithium-Ion Batteries by Coating Core-Shell Structured Silica-Poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) Particles. J. Power Sources 2019, 436, 567–576. [Google Scholar] [CrossRef]
- Cho, J.; Jung, Y.-C.; Lee, Y.S.; Kim, D.-W. High Performance Separator Coated with Amino-Functionalized SiO2 Particles for Safety Enhanced Lithium-Ion Batteries. J. Membr. Sci. 2017, 535, 151–157. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Lee, S.-Y. Closely Packed SiO2 Nanoparticles/Poly(vinylidene fluoride-hexafluoropropylene) Layers-Coated Polyethylene Deparators for Lithium-Ion Batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Oh, S.W.; Myung, S.-T.; Oh, S.-M.; Yoon, C.S.; Amine, K.; Sun, Y.-K. Polyvinylpyrrolidone-Assisted Synthesis of Microscale C-LiFePO4 with High Tap Density as Positive Electrode Materials for Lithium Batteries. Electrochim. Acta 2010, 55, 1193–1199. [Google Scholar] [CrossRef]
- Rodriguez-Fernández, D.; Langer, J.; Henriksen-Lacey, M.; Liz-Marzan, L.M. Hybrid Au-SiO2 Core-Satellite Colloids as Switchable SERS Tags. Chem. Mater. 2015, 27, 2540–2545. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Meng, L.; Lu, Q.; Fu, J.; Huang, X. Superparamagnetic Submicro-Megranates: Fe3O4 Nanoparticles Coated with Highly Cross-Linked Organic/Inorganic Hybrids. Chem. Commun. 2009, 42, 6370–6372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, Y.; Yuan, W.; Xi, J.; Huang, X.; Tang, X.; Zheng, S. One-Pot Synthesis of Poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) Nanotubes via an In Situ Template Approach. Adv. Mater. 2006, 18, 2997–3000. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Wang, X. Synthesis, Characterization, Thermal Properties and Fame Retardancy of a Novel Nonflammable Phosphazene-Based Epoxy Resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wei, H.; Fu, J.; Huang, Y.; Tang, X. Enhanced electrochemical properties of polyethylene oxide-based composite solid polymer electrolytes with porous inorganic–organic hybrid polyphosphazene nanotubes as fillers. J. Solid State Chem. 2012, 16, 101–107. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wei, H.; Fu, J.; Huang, Y.; Tang, X. Novel PEO-based solid composite polymer electrolytes with inorganic–organic hybrid polyphosphazene microspheres as fillers. J. Appl. Electrochem. 2010, 40, 1475–1481. [Google Scholar] [CrossRef]
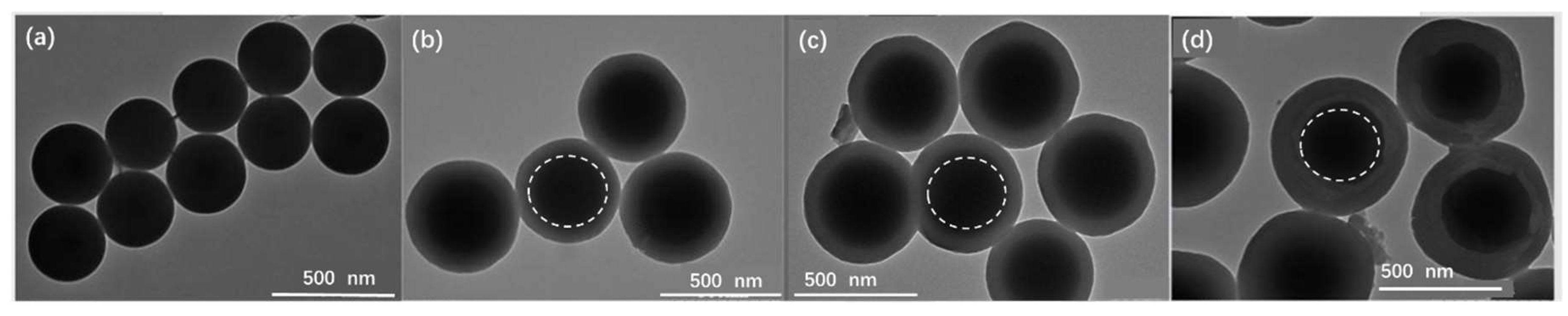
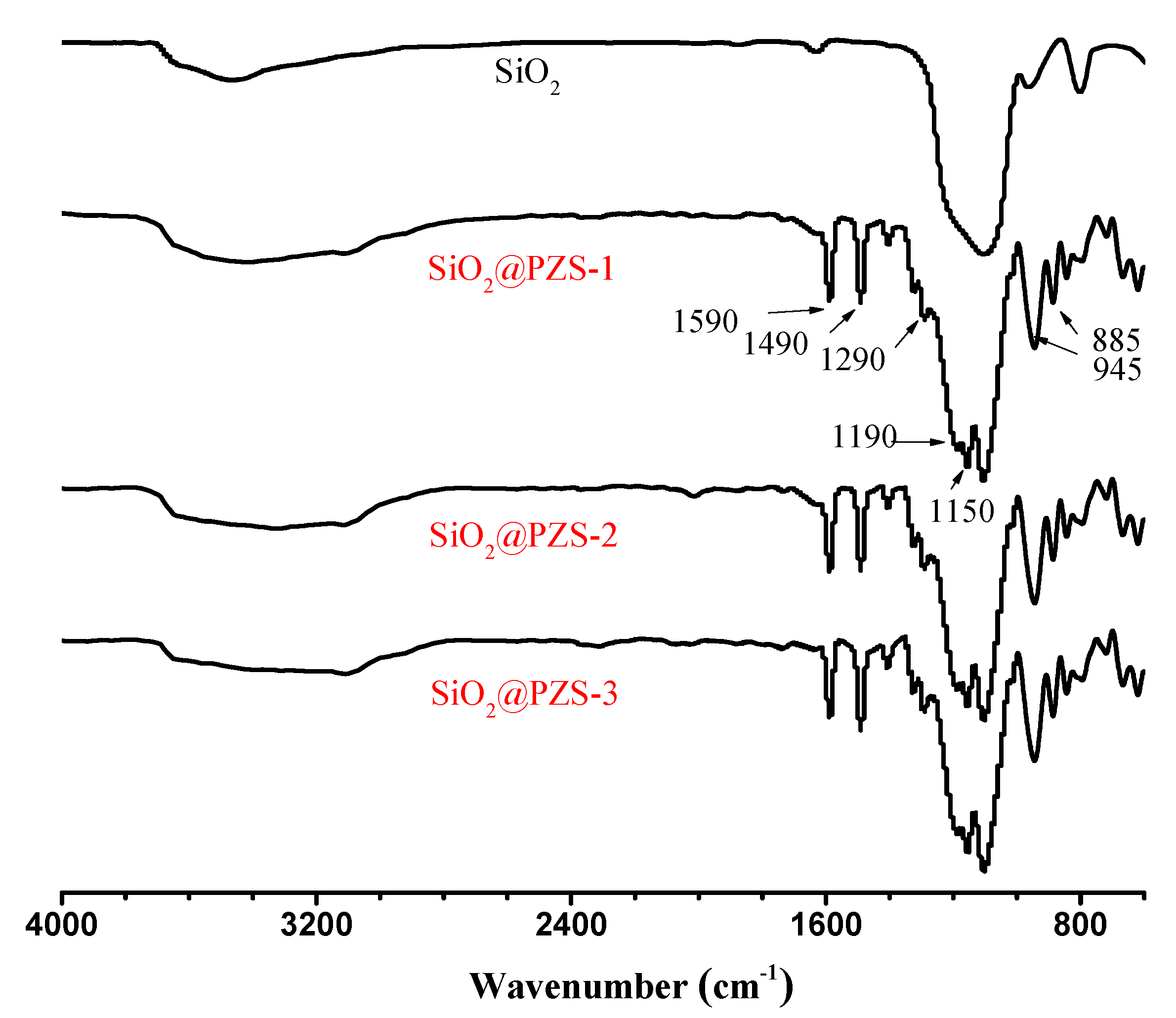
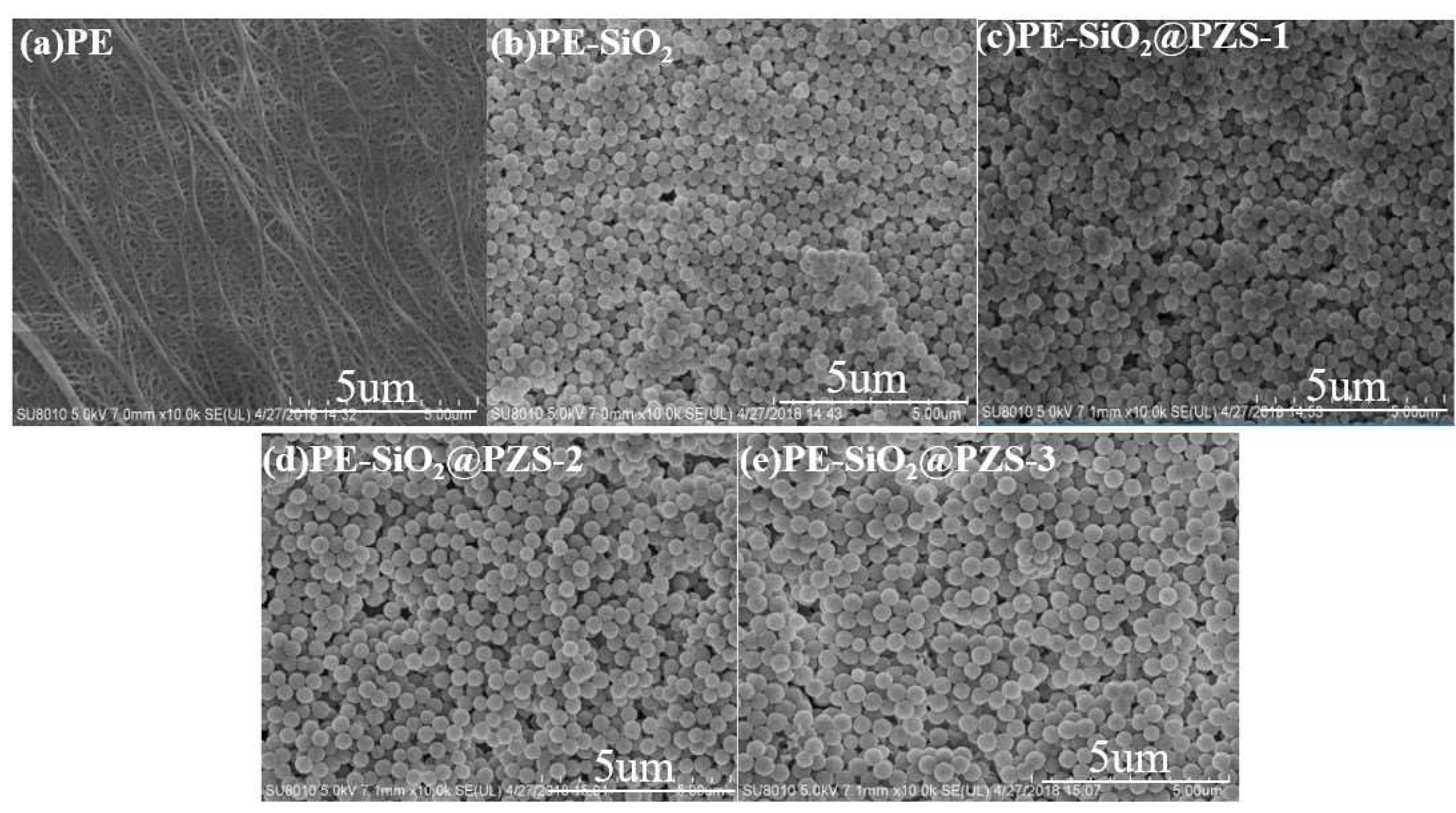

| Sample | SiO2 (g) | BPS (mol/L) | HCCP (mol/L) | CAN (mL) | TEA (mL) | Layer Thickness of PZS (nm) a |
|---|---|---|---|---|---|---|
| SiO2@PZS-1 | 0.5 | 0.03 | 0.01 | 100 | 2 | 65 ± 6 |
| SiO2@PZS-2 | 0.5 | 0.06 | 0.02 | 100 | 4 | 96 ± 6 |
| SiO2@PZS-3 | 0.5 | 0.12 | 0.04 | 100 | 8 | 128 ± 7 |
| Separator | Thickness (μm) | Gurley Value (s/100 mL) | Electrolyte Uptake (%)/1 h |
|---|---|---|---|
| PE | 18 | 203 ± 1.5 | 90.0 ± 2.2 |
| PE-SiO2 | 20.7 ± 0.5 | 215 ± 5.0 | 123.0 ± 9.0 |
| PE-SiO2@PZS-1 | 21.8 ± 1.3 | 206 ± 7.2 | 138.6 ± 11.6 |
| PE-SiO2@PZS-2 | 21.2 ± 1.3 | 204 ± 2.5 | 182.0 ± 14.5 |
| PE-SiO2@PZS-3 | 22.3 ± 0.9 | 210 ± 3.5 | 213.5 ± 16.9 |
| Separator | Rb (Ω) | Ionic Conductivity (S/cm) |
|---|---|---|
| PE | 1.00 | 5.8 × 10−4 |
| PE-SiO2 | 0.98 | 6.8 × 10−4 |
| PE-SiO2@PZS-1 | 0.71 | 9.9 × 10−4 |
| PE-SiO2@PZS-2 | 0.58 | 1.2 × 10−3 |
| PE-SiO2@PZS-3 | 0.53 | 1.4 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Chen, Y.; Liu, P.; Zhan, J. Fabrication and Investigation of PE-SiO2@PZS Composite Separator for Lithium-Ion Batteries. Materials 2022, 15, 4875. https://doi.org/10.3390/ma15144875
Xu L, Chen Y, Liu P, Zhan J. Fabrication and Investigation of PE-SiO2@PZS Composite Separator for Lithium-Ion Batteries. Materials. 2022; 15(14):4875. https://doi.org/10.3390/ma15144875
Chicago/Turabian StyleXu, Liguo, Yanwu Chen, Peijiang Liu, and Jianghua Zhan. 2022. "Fabrication and Investigation of PE-SiO2@PZS Composite Separator for Lithium-Ion Batteries" Materials 15, no. 14: 4875. https://doi.org/10.3390/ma15144875






