Functionalization of Se-Te Nanorods with Au Nanoparticles for Enhanced Anti-Bacterial and Anti-Cancer Activities
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Chemicals
2.2. Synthesis of Se-Te Alloy Nanorods
2.3. Synthesis of Se-Te@Au Alloy Nanorods
2.4. Nanoparticle Characterization
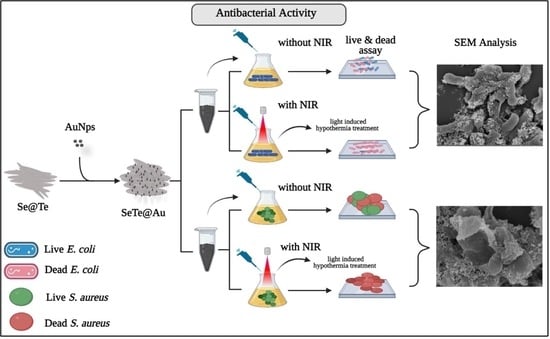
2.5. In Vitro Anti-Bacterial Activity
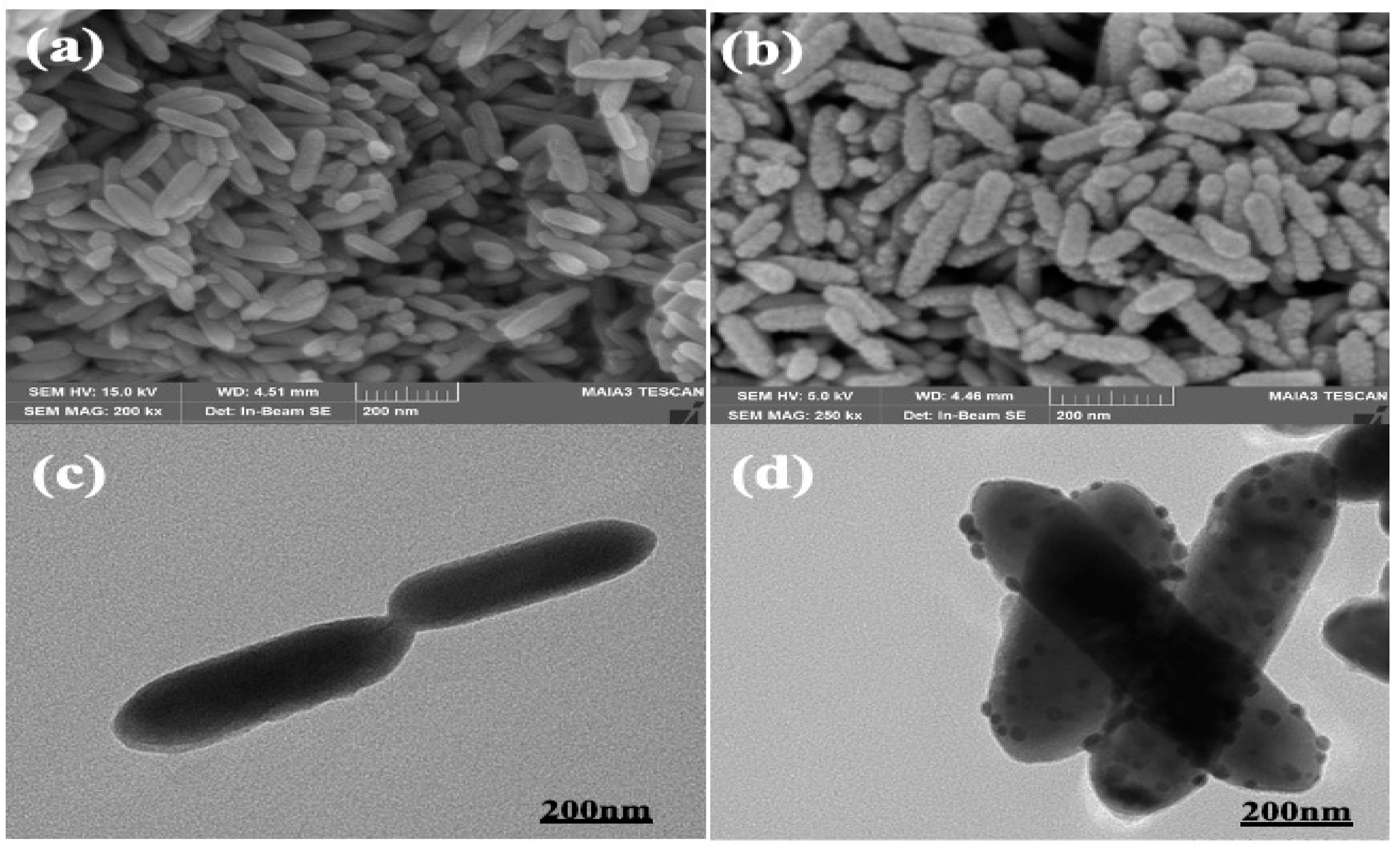
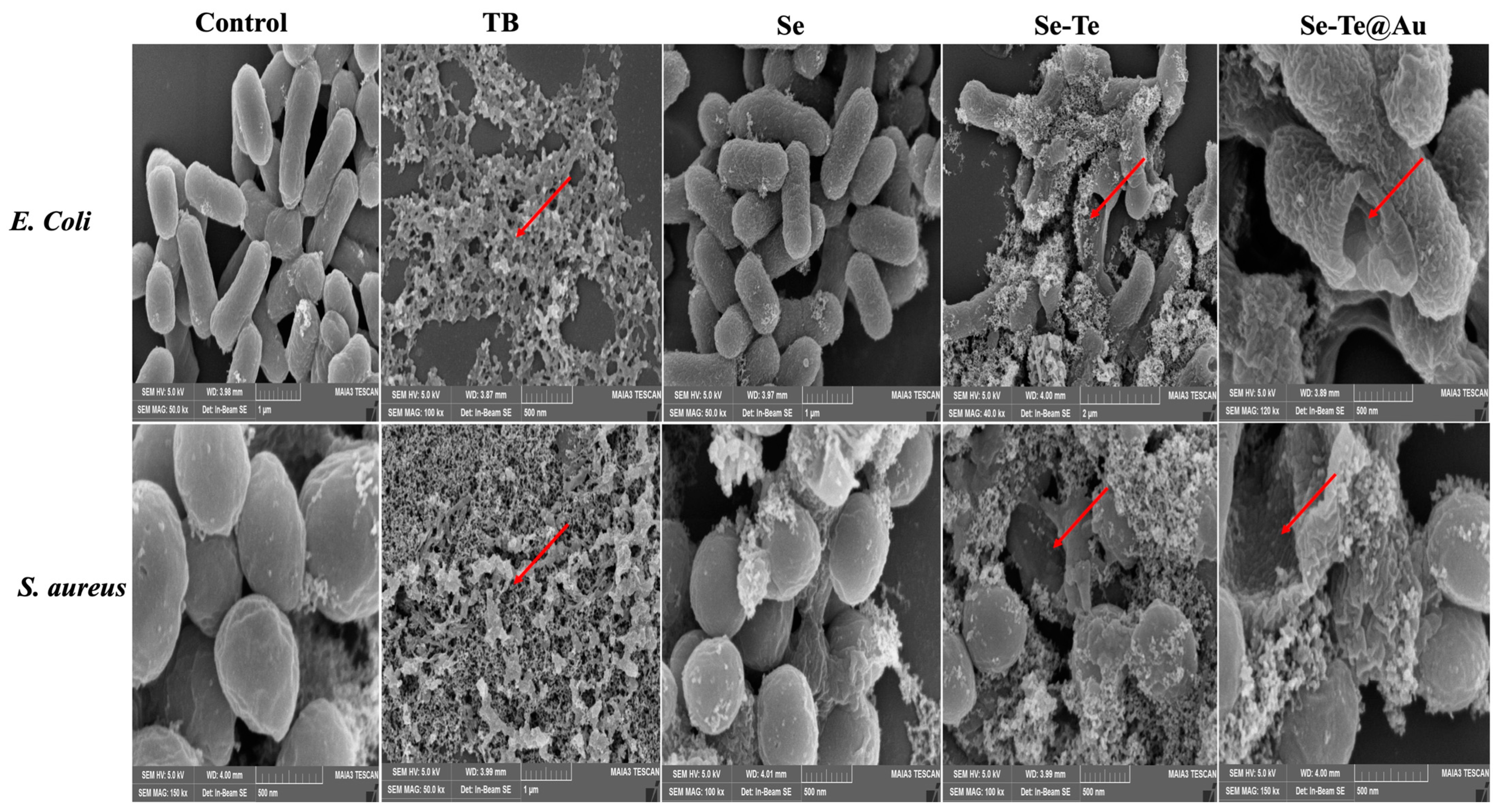
2.6. Morphological Characterization of Bacteria
3. Result and Discussion
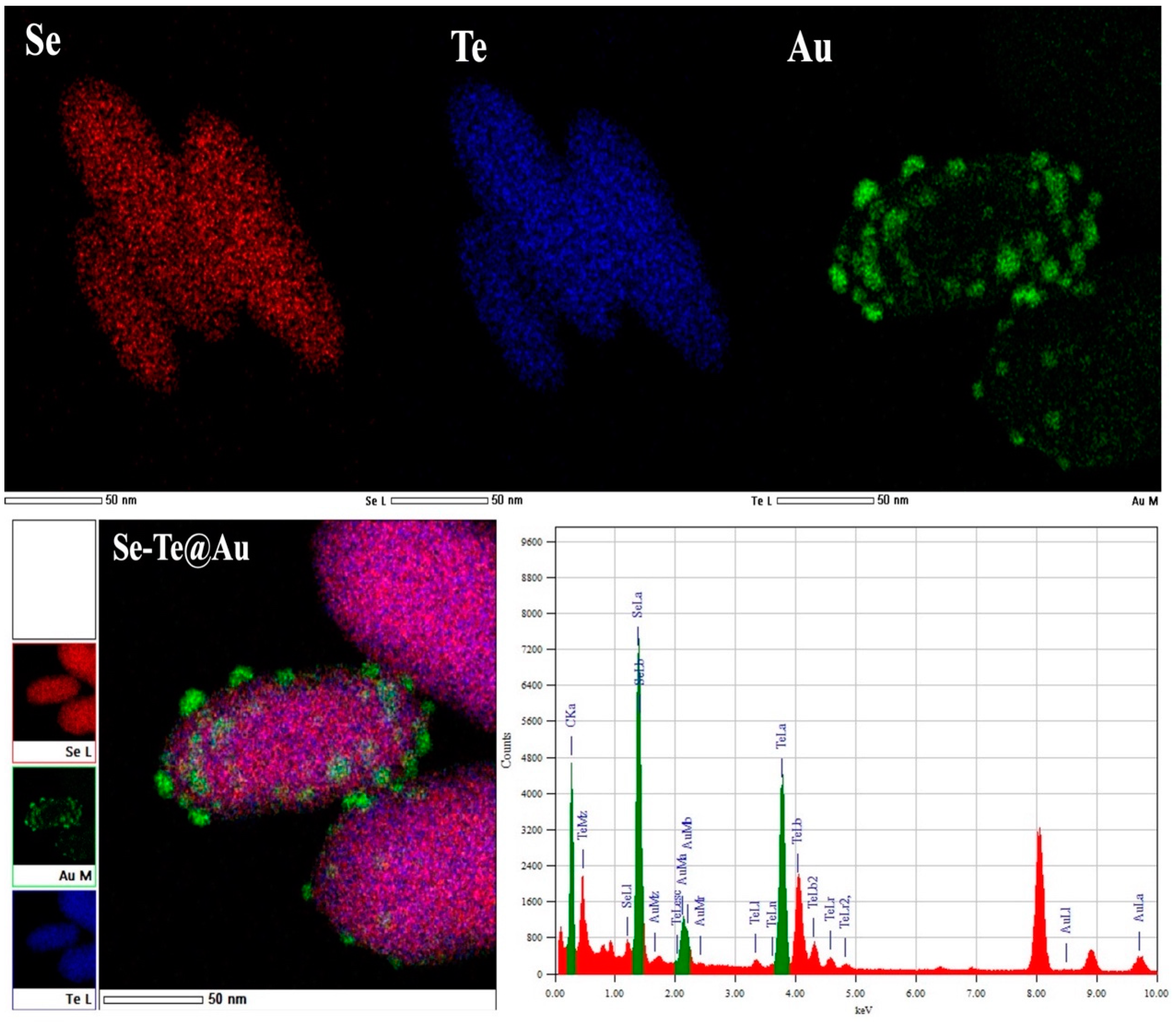
3.1. Characterization
3.1.1. Electron Microscopy of Se-Te Nanorods
3.1.2. Scanning Electron Microscopy of Se-Te@Au Nanorods
3.1.3. XPS Spectral Analysis
3.2. Anti-Bacterial Activity
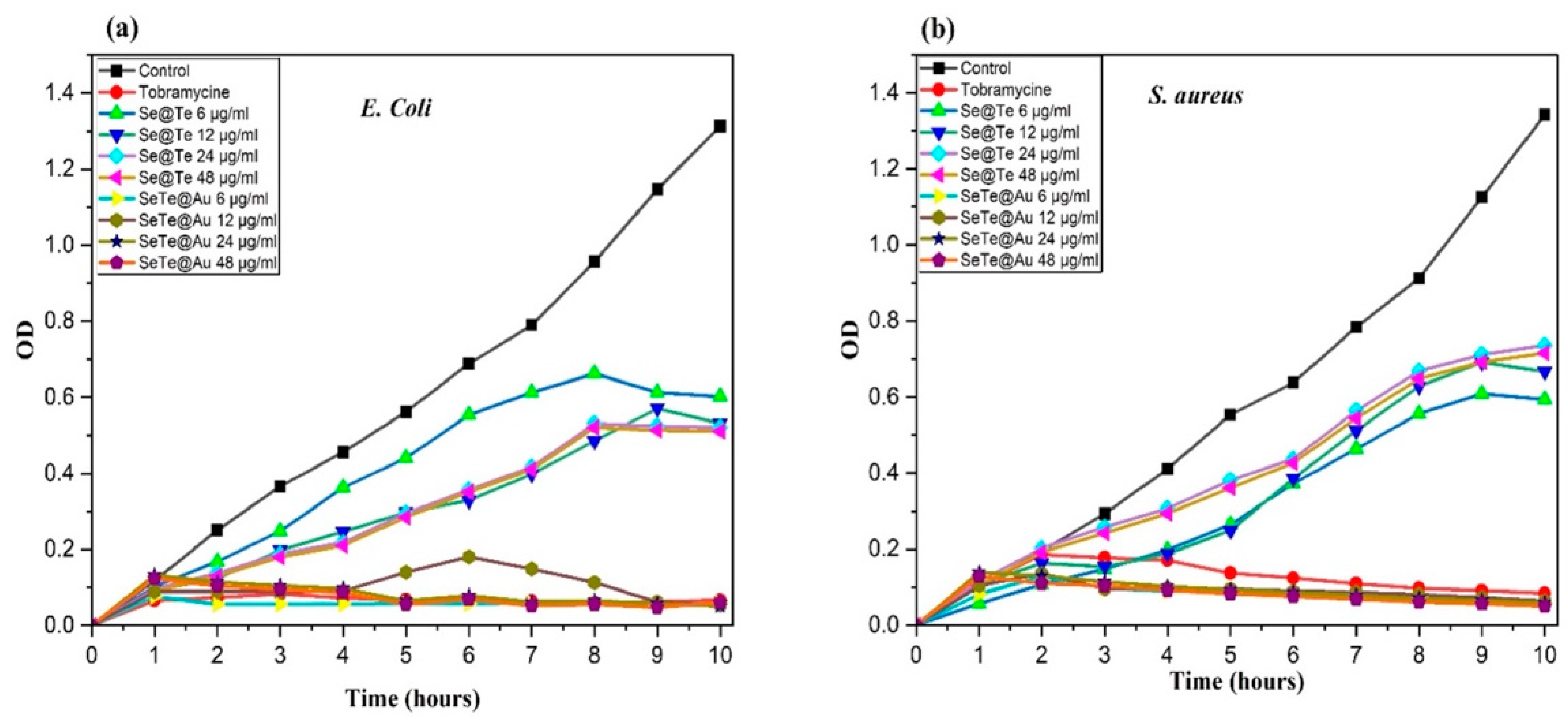
3.2.1. Growth Curves of Microbial Cells Treated with Different Concentrations of NPs
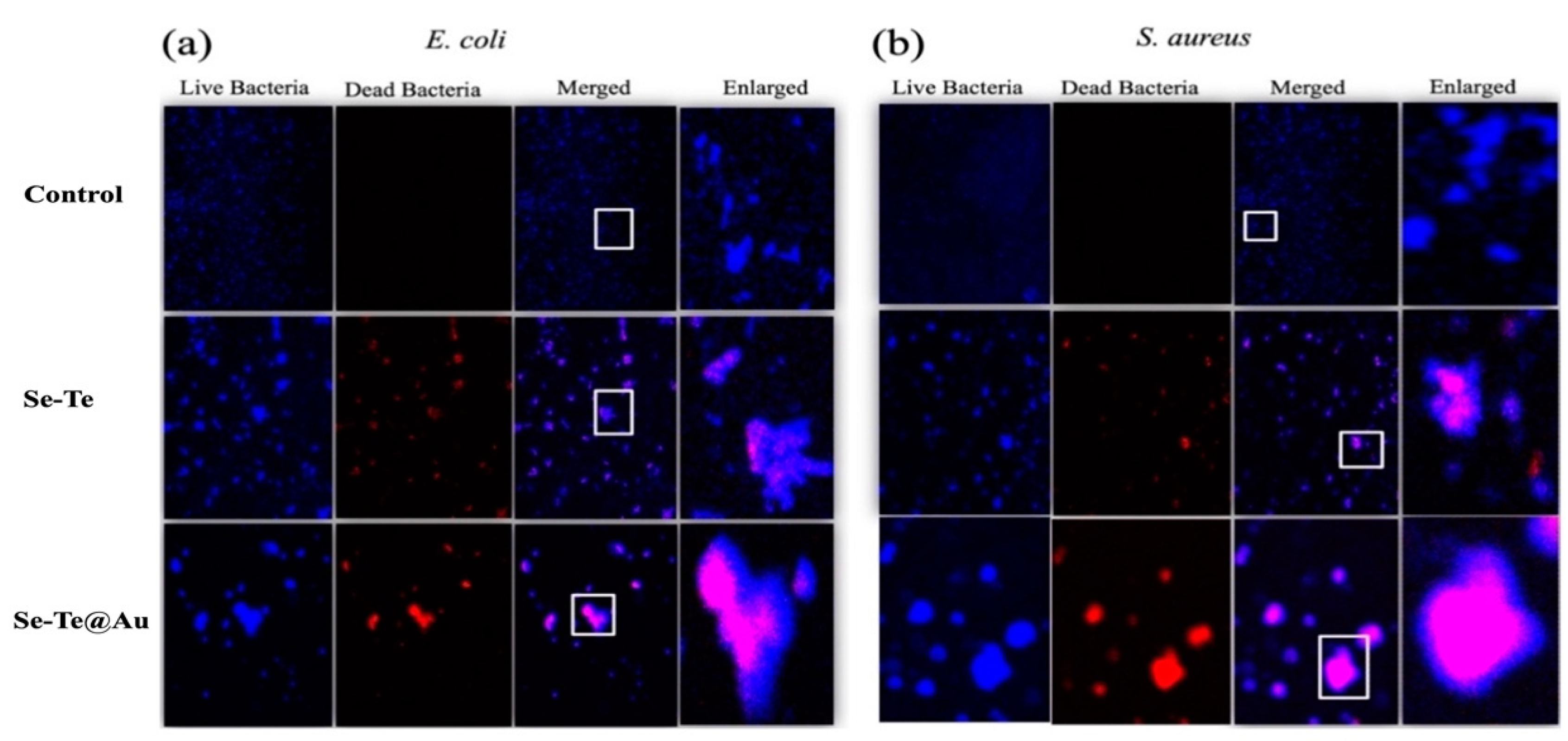
3.2.2. Live–Dead Assay
3.2.3. Membrane Disruption
3.2.4. Photo-Thermal Efficacy of Se-Te and Se-Te@Au Nanorods
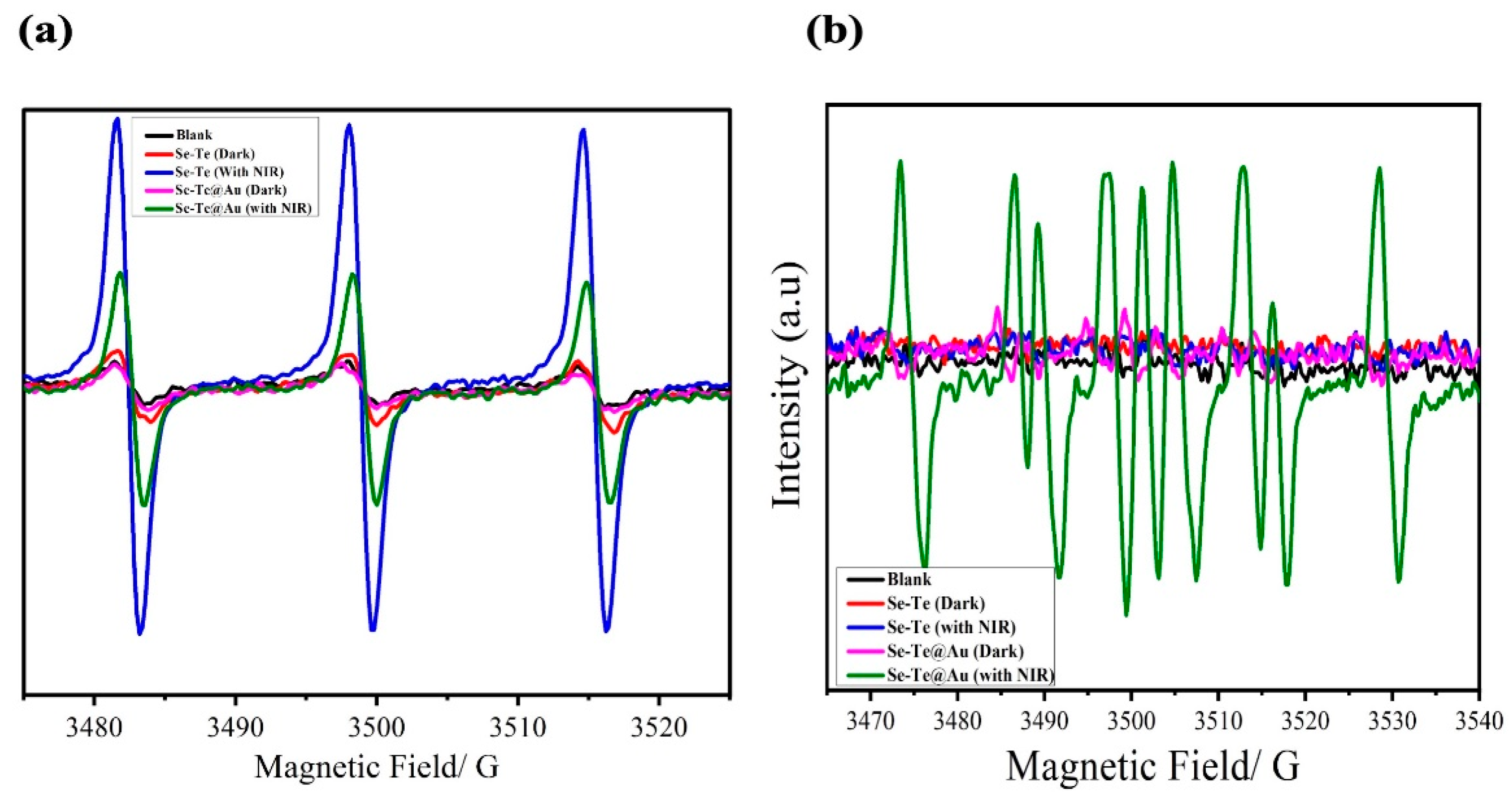
3.2.5. Investigation of ROS Production
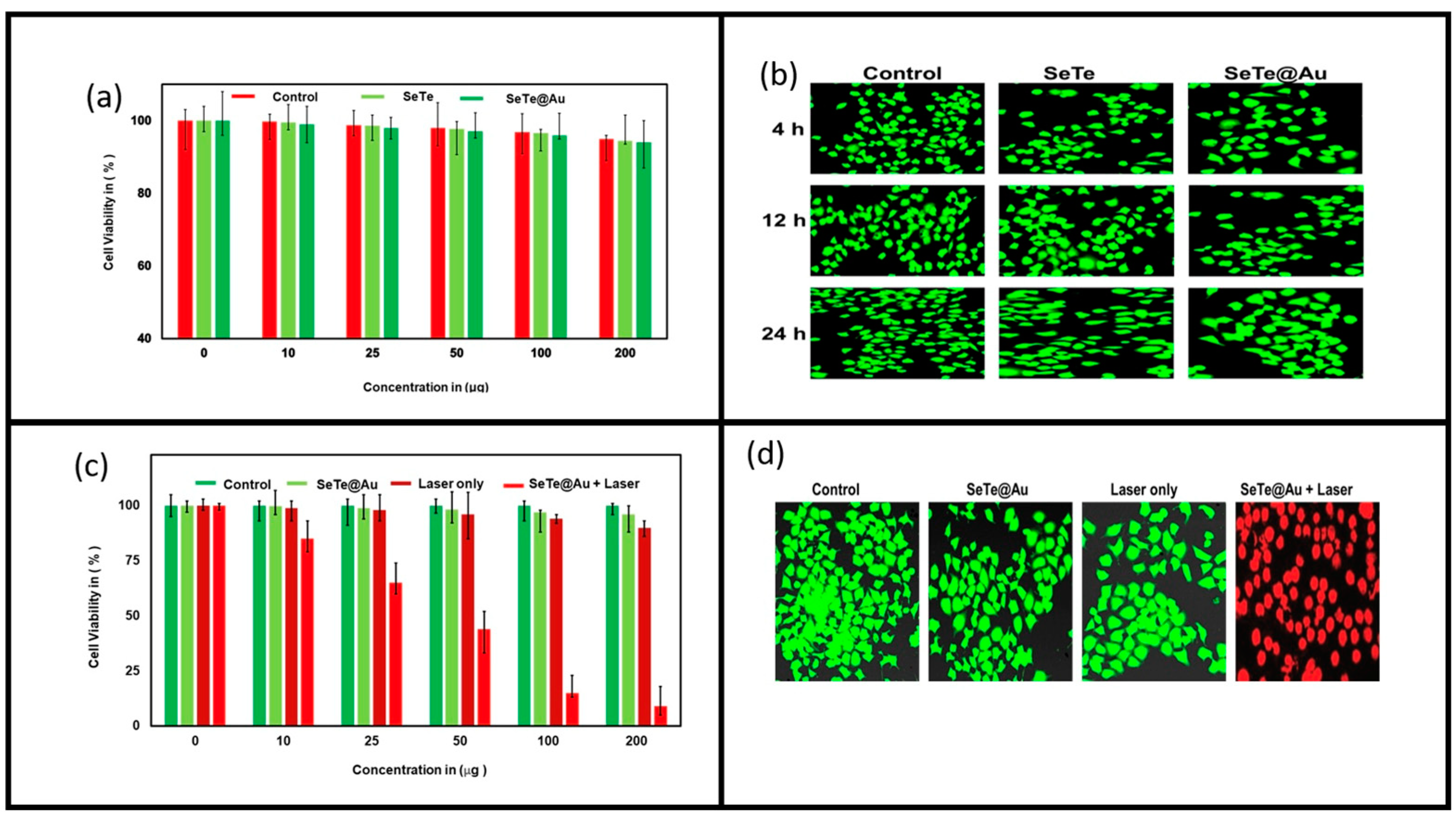
3.3. Anti-Cancer Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schierholz, J.M.; Beuth, J. Implant Infections: A Haven for Opportunistic Bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, I.; Prieto, I.; Albornoz, A.; Sanz, V.; Weis, C.; Turon, P.; Quidant, R. Plasmon-Based Biofilm Inhibition on Surgical Implants. Nano Lett. 2019, 19, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H. The Empiric Treatment of Nosocomial Intra-Abdominal Infections. Int. J. Infect. Dis. 2007, 11, S1–S6. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.S.; Ullah, I.; Ullah, S.; An, R.; Xu, H.; Nie, K.; Liu, C.; Liu, L. Recent Advances in the Surface Functionalization of Nanomaterials for Antimicrobial Applications. Materials 2021, 14, 6932. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022—2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar] [CrossRef]
- Choudhury, H.G.; Tong, Z.; Mathavan, I.; Li, Y.; Iwata, S.; Zirah, S.; Rebuffat, S.; Van Veen, H.W.; Beis, K. Structure of an Antibacterial Peptide ATP-Binding Cassette Transporter in a Novel Outward Occluded State. Proc. Natl. Acad. Sci. USA 2014, 111, 9145–9150. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Lopez, S.; Kim, H.S.; Choi, E.C.; Delgado, M.; Granja, J.R.; Khasanov, A.; Kraehenbuehl, K.; Long, G.; Weinberger, D.A.; Wilcoxen, K.M.; et al. Antibacterial Agents Based on the Cyclic D, L-α-Peptide Architecture. Nature 2001, 412, 452–455. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhu, X.; Shi, Z.L.; Neoh, K.G.; Kang, E.T. Polymer Microspheres with Permanent Antibacterial Surface from Surface-Initiated Atom Transfer Radical Polymerization. Ind. Eng. Chem. Res. 2005, 44, 7098–7104. [Google Scholar] [CrossRef]
- Magennis, E.P.; Fernandez-Trillo, F.; Sui, C.; Spain, S.G.; Bradshaw, D.J.; Churchley, D.; Mantovani, G.; Winzer, K.; Alexander, C. Bacteria-Instructed Synthesis of Polymers for Self-Selective Microbial Binding and Labelling. Nat. Mater. 2014, 13, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Shen, D.; Xu, W. Synthesis and Antibacterial Activities of Quaternary Ammonium Salt of Chitosan. Carbohydr. Res. 2001, 333, 1–6. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Saddik, M.S.; Elsayed, M.M.A.; El-Mokhtar, M.A.; Sedky, H.; Abdel-Aleem, J.A.; Abu-Dief, A.M.; Al-Hakkani, M.F.; Hussein, H.L.; Al-Shelkamy, S.A.; Meligy, F.Y.; et al. Tailoring of Novel Azithromycin-Loaded Zinc Oxide Nanoparticles for Wound Healing. Pharmaceutics 2022, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; Alrashedee, F.M.M.; Emran, K.M.; Al-Abdulkarim, H.A. Development of Some Magnetic Metal–Organic Framework Nano Composites for Pharmaceutical Applications. Inorg. Chem. Commun. 2022, 138, 109251. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abd-El Sayed, M.A.; Zikry, M.M.; Nafady, A. Green Synthesis of AgNPs() Ultilizing Delonix Regia Extract as Anticancer and Antimicrobial Agents. ChemistrySelect 2020, 5, 13263–13268. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Hartemann, P. Silver as an Antimicrobial: Facts and Gaps in Knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain Specificity in Antimicrobial Activity of Silver and Copper Nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable Synthesis of ZnO Nanoparticles and Their Morphology-Dependent Antibacterial and Optical Properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Hu, X.; Yao, Q.; Hu, Q.; Amini, S.; Miserez, A.; Tang, G. Engineering Bioinspired Bacteria-Adhesive Clay Nanoparticles with a Membrane-Disruptive Property for the Treatment of Helicobacter Pylori Infection. Nanoscale 2016, 8, 16486–16498. [Google Scholar] [CrossRef] [PubMed]
- De Abajo, F.J.G. Microscopy: Plasmons Go Quantum. Nature 2012, 483, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.Y.; Chang, W.S.; Link, S. Optical Characterization of Single Plasmonic Nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zheng, T.; Ai, B.; Gu, P.; Guan, Y.; Wang, Y.; Zhao, Z.; Zhang, G. Multiple Plasmonic Hot Spots Platform: Nanogap Coupled Gold Nanoparticles. Appl. Surf. Sci. 2022, 593, 153388. [Google Scholar] [CrossRef]
- Rakhshani, M.R. Narrowband Plasmonic Absorber Using Gold Nanoparticle Arrays for Refractive Index Sensing. IEEE Sens. J. 2022, 22, 4043–4050. [Google Scholar] [CrossRef]
- Su, S.; Yu, T.; Hu, J.; Xianyu, Y. A Bio-Inspired Plasmonic Nanosensor for Angiotensin-Converting Enzyme through Peptide-Mediated Assembly of Gold Nanoparticles. Biosens. Bioelectron. 2022, 195, 113621. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, D.-W.; Pu, H.; Wei, Q.; Lin, X. Ti3C2Tx MXenes Loaded with Au Nanoparticle Dimers as a Surface-Enhanced Raman Scattering Aptasensor for AFB1 Detection. Food Chem. 2022, 372, 131293. [Google Scholar] [CrossRef]
- Aldosari, F.M.M. Characterization of Labeled Gold Nanoparticles for Surface-Enhanced Raman Scattering. Molecules 2022, 27, 892. [Google Scholar] [CrossRef] [PubMed]
- Pothipor, C.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A Gold Nanoparticle-Dye/Poly(3-Aminobenzylamine)/Two Dimensional MoSe2/Graphene Oxide Electrode towards Label-Free Electrochemical Biosensor for Simultaneous Dual-Mode Detection of Cancer Antigen 15-3 and MicroRNA-21. Colloids Surf. B Biointerfaces 2022, 210, 112260. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Sharma, P.; Pilloton, R.; Khanuja, M.; Narang, J. Colorimetric Biosensor for the Naked-Eye Detection of Ovarian Cancer Biomarker PDGF Using Citrate Modified Gold Nanoparticles. Biosens. Bioelectron. X 2022, 11, 100142. [Google Scholar] [CrossRef]
- Jain, P.K.; ElSayed, I.H.; El-Sayed, M.A. Au Nanoparticles Target Cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for Photothermal Therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khan, S.S.; Ren, Y.; Zhang, X.; Qin, M.; Xiong, X.; Krastev, R.; Jan, A.U.; Liu, L.; Yuan, Q. Near-Infrared Laser 808-Nm Excitable Palladium Nano-Dots Loaded on Graphene Oxide Hybrid for the Antibacterial Activity. Appl. Organomet. Chem. 2021, 35, e6380. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J. Recent Nanotechnology Approaches for Prevention and Treatment of Biofilm-Associated Infections on Medical Devices. Biomed Res. Int. 2016, 2016, 1851242. [Google Scholar] [CrossRef] [Green Version]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.B. Antimicrobial Surface Functionalization of Plastic Catheters by Silver Nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Atapour, A.; Khajehzadeh, H.; Shafie, M.; Abbasi, M.; Mosleh-Shirazi, S.; Kasaee, S.R.; Amani, A.M. Gold Nanoparticle-Based Aptasensors: A Promising Perspective for Early-Stage Detection of Cancer Biomarkers. Mater. Today Commun. 2022, 30, 103181. [Google Scholar] [CrossRef]
- Milan, J.; Niemczyk, K.; Kus-Liśkiewicz, M. Treasure on the Earth—Gold Nanoparticles and Their Biomedical Applications. Materials 2022, 15, 3355. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.A. Fundamentals and Applications Plasmonics: Fundamentals and Applications; Sringer: Berlin/Heidelberg, Germany, 2004; Volume 677, ISBN 9780387331508. [Google Scholar]
- Baffou, G.; Quidant, R. Thermo-Plasmonics: Using Metallic Nanostructures as Nano-Sources of Heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Utsav; Khanna, S.; Makani, N.H.; Paneliya, S.; Mukhopadhyay, I.; Banerjee, R. Thermal Crowning Mechanism in Gold-Silica Nanocomposites: Plasmonic-Photonic Pairing in Archetypal Two-Dimensional Structures. Phys. Chem. Chem. Phys. 2021, 23, 17197–17207. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-Mediated near-Infrared Thermal Therapy of Tumors under Magnetic Resonance Guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quek, C.H.; Leong, K.W. Near-Infrared Fluorescent Nanoprobes for in Vivo Optical Imaging. Nanomaterials 2012, 2, 92–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Gómez, B.; Arregui, L.; Serrano, S.; Santos, A.; Pérez-Corona, T.; Madrid, Y. Selenium and tellurium-based nanoparticles as interfering factors in quorum sensing-regulated processes: Violacein production and bacterial biofilm formation. Metallomics 2019, 11, 1104–1114. [Google Scholar] [CrossRef]
- Surendran-Nair, M.; Lau, P.; Liu, Y.; Venkitanarayanan, K. Efficacy of Selenium in Controlling Acinetobacter Baumannii Associated Wound Infections. Wound Med. 2019, 26, 100165. [Google Scholar] [CrossRef]
- Medina Cruz, D.; Tien-Street, W.; Zhang, B.; Huang, X.; Vernet Crua, A.; Nieto-Argüello, A.; Cholula-Díaz, J.L.; Martínez, L.; Huttel, Y.; González, M.U.; et al. Citric Juice-Mediated Synthesis of Tellurium Nanoparticles with Antimicrobial and Anticancer Properties. Green Chem. 2019, 21, 1982–1998. [Google Scholar] [CrossRef]
- Rahman, A.U.; Wei, Y.; Ahmad, A.; Khan, A.U.; Ali, R.; Ullah, S.; Ahmad, W.; Yuan, Q. Selenium Nanorods Decorated Gold Nanostructures: Synthesis, Characterization and Biological Applications. J. Clust. Sci. 2020, 31, 727–737. [Google Scholar] [CrossRef]
- Ullah, S.; Yasin, G.; Ahmad, A.; Qin, L.; Yuan, Q.; Khan, A.U.; Khan, U.A.; Rahman, A.U.; Slimani, Y. Construction of Well-Designed 1D Selenium-Tellurium Nanorods Anchored on Graphene Sheets as a High Storage Capacity Anode Material for Lithium-Ion Batteries. Inorg. Chem. Front. 2020, 7, 1750–1761. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S. Antibacterial Activity and Mechanism of Lacidophilin from Lactobacillus Pentosus Against Staphylococcus Aureus and Escherichia Coli. Front. Microbiol. 2020, 11, 582349. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, J.; Wang, L.; Liu, Q.; Fan, Y.; Li, B.; Yu, Y.L.; Chen, C.; Li, Y.F. Using Nano-Selenium to Combat Coronavirus Disease 2019 (COVID-19)? Nano Today 2021, 36, 101037. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, L.; Zhao, J.; He, L.; Cui, L.; Gao, Y.; Chen, C.; Fan, Y.; Li, B.; Li, Y.F. Nanosafety Evaluation through Feces: A Comparison between Selenium Nanoparticles and Selenite in Rats. Nano Today 2021, 36, 101010. [Google Scholar] [CrossRef]
- Vasuki, K.; Manimekalai, R. NIR Light Active Ternary Modified ZnO Nanocomposites for Combined Cancer Therapy. Heliyon 2019, 5, e02729. [Google Scholar] [CrossRef]
- Song, J.M.; Lin, Y.Z.; Zhan, Y.J.; Tian, Y.C.; Liu, G.; Yu, S.H. Superlong high-quality tellurium nanotubes: Synthesis, characterization, and optical property. Cryst. Growth Des. 2008, 8, 1902–1908. [Google Scholar] [CrossRef]
- Chamakura, K.; Perez-Ballestero, R.; Luo, Z.; Bashir, S.; Liu, J. Comparison of Bactericidal Activities of Silver Nanoparticles with Common Chemical Disinfectants. Colloids Surf. B Biointerfaces 2011, 84, 88–96. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia Coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef]
- Carlson, C.; Hussein, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-Ion-Mediated Reactive Oxygen Species Generation Affecting Bactericidal Activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Sohail; Iqbal, Z.; Afzal, M.; Afzal, A.; Rahman, I.U.; Shad, S.; Ahmed, B.; Anjum, N.; Qureshi, K.; Bibi, A. In Vitro Antibacterial Study of Taraxacum Officinale Leaves Extracts against Different Bacterial Pathogenic Strains. J. Pharmacogn. Phytochem. 2014, 3, 15–17. [Google Scholar]
- Haghniaz, R.; Rabbani, A.; Vajhadin, F.; Khan, T.; Kousar, R.; Khan, A.R.; Montazerian, H.; Iqbal, J.; Libanori, A.; Kim, H.J.; et al. Anti-bacterial and Wound Healing-promoting Effects of Zinc Ferrite Nanoparticles. J. Nanobiotechnol. 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria Viz. Escherichia Coli and Pseudomonas Aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef] [PubMed]
- Zada, S.; Ahmad, A.; Khan, S.; Iqbal, A.; Ahmad, S.; Ali, H.; Fu, P. Biofabrication of Gold Nanoparticles by Lyptolyngbya JSC-1 Extract as Super Reducing and Stabilizing Agents: Synthesis, Characterization and Antibacterial Activity. Microb. Pathog. 2018, 114, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Setyawati, M.I.; Lim, T.P.; Leong, D.T.; Xie, J. Antimicrobial Cluster Bombs: Silver Nanoclusters Packed with Daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef]
- Altaf, A.; Aldawsari, H.; Banjar, Z.M.; Iohara, D.; Anraku, M.; Uekama, K.; Hirayama, F. Potential Use of C60 /2-Hydroxypropyl-β-Cyclodextrin Nanoparticles as a New Photosensitizer in the Treatment of Cancer. Int. J. Photoenergy 2014, 2014, 570506. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Gu, H.; Xu, B. Multifunctional Magnetic Nanoparticles: Synthesis Modification and Biomedical Applications. Prog. Chem. 2009, 42, 1097–1107. [Google Scholar]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu 9S 5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells In Vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef]
- Zada, S.; Dai, W.; Kai, Z.; Lu, H.; Meng, X.; Zhang, Y.; Cheng, Y.; Yan, F.; Fu, P.; Zhang, X.; et al. Algae Extraction Controllable Delamination of Vanadium Carbide Nanosheets with Enhanced Near-Infrared Photothermal Performance. Angew. Chem.—Int. Ed. 2020, 59, 6601–6606. [Google Scholar] [CrossRef]



| Strain | Nanoparticle | Zone of Inhibition (Current Study) | Zone of Inhibition (Previously Reported Studies) |
|---|---|---|---|
| E. coli | Se-Te | 24 ± 2 mm | 13 ± 0.5 mm [60]; 9.1 ± 1.6 mm [61], 18 ± 2 mm [62] |
| Se-Te@Au | 25 ± 2 mm | ||
| S. aureus | Se-Te | 21 ± 2 mm | 10.0 ± 1.2 mm [60]; 9.2 ± 1 mm [61], 14 ± 2 mm [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.S.; Ullah, I.; Zada, S.; Ahmad, A.; Ahmad, W.; Xu, H.; Ullah, S.; Liu, L. Functionalization of Se-Te Nanorods with Au Nanoparticles for Enhanced Anti-Bacterial and Anti-Cancer Activities. Materials 2022, 15, 4813. https://doi.org/10.3390/ma15144813
Khan SS, Ullah I, Zada S, Ahmad A, Ahmad W, Xu H, Ullah S, Liu L. Functionalization of Se-Te Nanorods with Au Nanoparticles for Enhanced Anti-Bacterial and Anti-Cancer Activities. Materials. 2022; 15(14):4813. https://doi.org/10.3390/ma15144813
Chicago/Turabian StyleKhan, Shahin Shah, Irfan Ullah, Shah Zada, Aftab Ahmad, Waqar Ahmad, Haijun Xu, Sadeeq Ullah, and Luo Liu. 2022. "Functionalization of Se-Te Nanorods with Au Nanoparticles for Enhanced Anti-Bacterial and Anti-Cancer Activities" Materials 15, no. 14: 4813. https://doi.org/10.3390/ma15144813