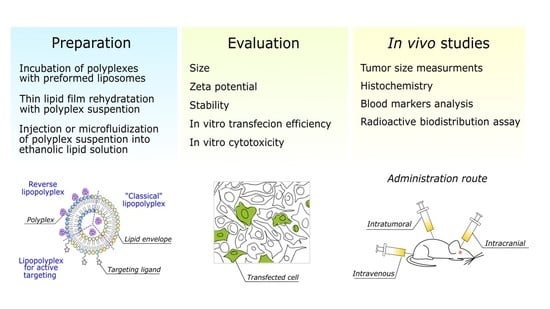
Polyethyleneimine-Based Lipopolyplexes as Carriers in Anticancer Gene Therapies
Abstract
:1. Introduction
2. Polyethyleneimine-Based Lipopolyplexes as Nucleic Acid Carriers
3. Composition of an Effective Lipopolyplex Based on Polyethyleneimine

4. Selected In Vivo Studies on Anticancer PEI-Based Lipopolyplexes
5. Possible Directions of Development of Lipopolyplexes
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Grillone, K.; Riillo, C.; Scionti, F.; Rocca, R.; Tradigo, G.; Guzzi, P.H.; Alcaro, S.; Di Martino, M.T.; Tagliaferri, P.; Tassone, P. Non-coding RNAs in cancer: Platforms and strategies for investigating the genomic ‘dark matter’. J. Exp. Clin. Cancer Res. 2020, 39, 117. [Google Scholar] [CrossRef]
- Hashemi, A.; Gorji-Bahri, G. MicroRNA: Promising roles in cancer therapy. Curr. Pharm. Biotechnol. 2020, 21, 1186–1203. [Google Scholar] [CrossRef]
- Shahryari, A.; Saghaeian Jazi, M.; Mohammadi, S.; Razavi Nikoo, H.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and clinical translation of approved gene therapy products for genetic disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.C.F.; Orgel, L.E. The stability of different forms of double-stranded decoy DNA in serum and nuclear extracts. Nucleic Acids Res. 1992, 20, 5857–5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral vectors for gene therapy: Translational and clinical outlook. Annu. Rev. Biomed. Eng. 2018, 17, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.-A.; Merrics, E.P.; Long, C.T.; et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2020, 39, 47–55. [Google Scholar] [CrossRef]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Orkin, S.H.; Reilly, P. Medicine. Paying for future success in gene therapy. Science 2016, 352, 1059–1061. [Google Scholar] [CrossRef]
- Lundstorm, K. Viral vectors in gene therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: Recent development, challenges, and prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Sainz-Ramos, M.; Gallego, I.; Villate-Beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How far are non-viral vectors to come of age and reach clinical translation in gene therapy? Int. J. Mol. Sci. 2021, 22, 7545. [Google Scholar] [CrossRef]
- Torres-Vanegas, J.D.; Cruz, J.C.; Reyes, L.H. Delivery systems for nucleic acids and proteins: Barriers, cell capture pathways and nanocarriers. Pharmaceutics 2021, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Dalby, B.; Cates, S.; Harris, A.; Ohki, E.C.; Tilkins, M.L.; Price, P.J.; Ciccarone, V.C. Advanced transfection with lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods 2004, 33, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef]
- Berger, M.; Lechanteur, A.; Evrard, B.; Piel, G. Innovative lipoplexes formulations with enhanced siRNA efficacy for cancer treatment: Where are we now? Int. J. Pharm. 2021, 605, 120851. [Google Scholar] [CrossRef] [PubMed]
- Wahane, A.; Waghmode, A.; Kapphahn, A.; Dhuri, K.; Gupta, A.; Bahal, R. Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules 2020, 25, 2866. [Google Scholar] [CrossRef]
- Chytła, A.; Gajdzik-Nowak, W.; Biernatowska, A.; Sikorski, A.F.; Czogalla, A. High-level expression of palmitoylated MPP1 recombinant protein in mammalian cells. Membranes 2021, 11, 715. [Google Scholar] [CrossRef]
- Juszkiewicz, K.; Sikorski, A.F.; Czogalla, A. Building blocks to design liposomal delivery systems. Int. J. Mol. Sci. 2020, 21, 9559. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Kircheis, R.; Wightman, L.; Wagner, E. Design and gene delivery activity of modified polyethylenimines. Adv. Drug Deliv. Rev. 2001, 53, 341–358. [Google Scholar] [CrossRef]
- Zakeri, A.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Beigi, V.; Mousavi, S.M.; Hashemi, S.A.R.; Zade, A.K.; Amani, A.M.; Savardashtaki, A.; Mirzaei, E.; et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: A developing horizon. Nano Rev. Exp. 2018, 9, 1488497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, L.L.; Pepin, J.; Kucharski, C.; Lin, X.; Xu, Z.; Uludag, H. A comparison of the effectiveness of cationic polymers poly-l-lysine (PLL) and polyethylenimine (PEI) for non-viral delivery of plasmid DNA to bone marrow stromal cells (BMSC). Eur. J. Pharm. Biopharm. 2007, 65, 388–397. [Google Scholar] [CrossRef]
- Pandey, A.P.; Sawant, K.K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C 2016, 68, 904–918. [Google Scholar] [CrossRef]
- Brunner, S.; Fürtbauer, E.; Sauer, T.; Kursa, M.; Wagner, E. Overcoming the nuclear barrier: Cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol. Ther. 2002, 5, 80–86. [Google Scholar] [CrossRef]
- Moret, I.; Peris, J.E.; Guillem, V.M.; Benet, M.; Revert, F.; Dasí, F.; Crespo, A.; Alino, S.F. Stability of PEI-DNA and DOTAP-DNA complexes: Effect of alkaline pH, heparin and serum. J. Control. Release 2001, 11, 169–181. [Google Scholar] [CrossRef]
- Von Gersdorff, K.; Sanders, N.N.; Vandenbroucke, R.; de Smedt, S.C.; Wagner, E.; Ogris, M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol. Ther. 2006, 14, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Tong, H.; Shi, Q.; Fernandes, J.C.; Jin, T.; Dai, K.; Zhang, X. Uptake mechanisms of non-viral gene delivery. J. Control. Release 2012, 158, 371–378. [Google Scholar] [CrossRef]
- Yue, Y.; Jin, F.; Deng, R.; Cai, J.; Dai, Z.; Lin, M.C.; Kung, H.F.; Mattebjerg, M.A.; Andersen, T.L.; Wu, C. Revisit complexation between DNA and polyethylenimine—Effect of length of free polycationic chains on gene transfection. J. Control. Release 2011, 152, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.Y.; Sharma, R.; Konieczny, S.F. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. J. Control. Release 2009, 139, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, L.M.P.; de Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc. Natl. Acad. Sci. USA 1999, 96, 5155–5181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Chen, J.; Li, Z.; Wang, Z.; Zhang, W.; Liu, J. Recent advances in the development of polyethylenimine-based gene vectors for safe and efficient gene delivery. Expert Opin. Drug Deliv. 2019, 16, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yan, H.; Zhou, Z.; Tang, J.; Liu, X.; Hartmann, R.; Parak, W.J.; Feliu, N.; Shen, Y. Detailed investigation on how the protein corona modulates the physicochemical properties and gene delivery of polyethylenimine (PEI) polyplexes. Biomater. Sci. 2018, 6, 1800–1817. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, F.G.; Hu, P.; Chen, Z. Interaction of polyethylenimine with model cell membranes studied by linear and nonlinear spectroscopic techniques. J. Phys. Chem. C 2014, 118, 12195–12205. [Google Scholar] [CrossRef]
- Morille, M.; Passirani, C.; Vonarbourg, A.; Clavreul, A.; Benoit, J.P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Feng, J.; Liu, Y.; Che, J.; Bai, G.; Dong, X.; Wu, F.; Jin, T. A synthetic carrier of nucleic acids structured as a neutral phospholipid envelope tightly assembled on polyplex surface. Adv. Healthc. Mater. 2020, 9, 1901705. [Google Scholar] [CrossRef]
- Plank, C.; Mechtler, K.; Szoka, F.C.; Wagner, E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum. Gene Ther. 1996, 7, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, G.; Ingle, N.P.; Reineke, T.M. Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: Implications for a mechanism of cytotoxicity. Mol. Pharm. 2011, 8, 1709–1719. [Google Scholar] [CrossRef]
- Kafil, V.; Omidi, Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. BioImpacts 2011, 1, 23–30. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Okon, E.U.; Hammed, G.; Abu El Wafa, P.; Abraham, O.; Case, N.; Henry, E. In-vitro cytotoxicity of polyethyleneimine on HeLa and Vero cells. Int. J. Innov. Appl. Stud. 2014, 5, 192–199. [Google Scholar]
- Zhang, Q.-Y.; Ho, P.Y.; Tu, M.J.; Jilek, J.L.; Chen, Q.X.; Zeng, S.; Yu, A.M. Lipidation of polyethylenimine-based polyplex increases serum stability of bioengineered RNAi agents and offers more consistent tumoral gene knockdown in vivo. Int. J. Pharm. 2018, 547, 537. [Google Scholar] [CrossRef]
- Ewe, A.; Schaper, A.; Barnert, S.; Schubert, R.; Temme, A.; Bakowsky, U.; Aigner, A. Storage stability of optimal liposome-polyethylenimine complexes (lipopolyplexes) for DNA or siRNA delivery. Acta Biomater. 2014, 10, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Meissner, J.M.; Toporkiewicz, M.; Czogalla, A.; Matusewicz, L.; Kuliczkowski, K.; Sikorski, A.F. Novel antisense therapeutics delivery systems: In vitro and in vivo studies of liposomes targeted with anti-CD20 antibody. J. Control. Release 2015, 220, 515–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schäfer, J.; Bakowsky, U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101. [Google Scholar] [CrossRef]
- Kwolek, U.; Jamróz, D.; Janiczek, M.; Nowakowska, M.; Wydro, P.; Kepczynski, M. Interactions of polyethylenimines with zwitterionic and anionic lipid membranes. Langmuir 2016, 32, 5004–5018. [Google Scholar] [CrossRef]
- Elizondo, E.; Moreno, E.; Cabrera, I.; Córdoba, A.; Sala, S.; Veciana, J.; Ventosa, N. Liposomes and other vesicular systems: Structural characteristics, methods of preparation, and use in nanomedicine. Prog. Mol. Biol. Transl. Sci. 2011, 104, 1–52. [Google Scholar] [CrossRef]
- Hanzlíková, M.; Soininen, P.; Lampela, P.; Männistö, P.T.; Raasmaja, A. The role of PEI structure and size in the PEI/liposome-mediated synergism of gene transfection. Plasmid 2009, 61, 15–21. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewe, A.; Panchal, O.; Pinnapireddy, S.R.; Bakowsky, U.; Przybylski, S.; Temme, A.; Aigner, A. Liposome-polyethylenimine complexes (DPPC-PEI lipopolyplexes) for therapeutic siRNA delivery in vivo. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar] [CrossRef]
- Needham, D.; McIntosh, T.J.; Lasic, D.D. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. BBA Biomembr. 1992, 1108, 40–48. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Linder, B.; Weirauch, U.; Ewe, A.; Uhmann, A.; Seifert, V.; Mittelbronn, M.; Harter, P.N.; Aigner, A.; Kögel, D. Therapeutic targeting of stat3 using lipopolyplex nanoparticle-formulated sirna in a syngeneic orthotopic mouse glioma model. Cancers 2019, 11, 333. [Google Scholar] [CrossRef] [Green Version]
- Toporkiewicz, M.; Meissner, J.; Matusewicz, L.; Czogalla, A.; Sikorski, A.F. Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: Principles, hopes, and challenges. Int. J. Nanomed. 2015, 10, 1399–1414. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Bofinger, R.; Zaw-Thin, M.; Mitchell, N.J.; Patrick, P.S.; Stowe, C.; Gomez-Ramirez, A.; Hailes, H.C.; Kalber, T.L.; Tabor, A.B. Development of lipopolyplexes for gene delivery: A comparison of the effects of differing modes of targeting peptide display on the structure and transfection activities of lipopolyplexes. J. Pept. Sci. 2018, 24, e3131. [Google Scholar] [CrossRef]
- Shabana, A.M.; Xu, B.; Schneiderman, Z.; Ma, J.; Chen, C.C.; Kokkoli, E. Targeted liposomes encapsulating mir-603 complexes enhance radiation sensitivity of patient-derived glioblastoma stem-like cells. Pharmaceutics 2021, 13, 1115. [Google Scholar] [CrossRef]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2020, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Basnet, H.; Kaygusuz, Y.; Laughney, A.M.; He, L.; Sharma, R.; O’Rourke, K.P.; Reuter, V.P.; Huang, Y.-H.; Turkekul, M.; et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 2020, 1, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Mittal, V.; Ban, Y.; Lourenco, A.R.; Yomtoubian, S.; Lee, S. Metastatic tumor cells—Genotypes and phenotypes. Front. Biol. 2018, 13, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Dai, Z.; Wu, C. How does DNA complex with polyethylenimine with different chain lengths and topologies in their aqueous solution mixtures? Macromolecules 2012, 45, 4346–4353. [Google Scholar] [CrossRef]
- Choosakoonkriang, S.; Lobo, B.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Biophysical characterization of PEI/DNA complexes. J. Pharm. Sci. 2003, 92, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-viral in vitro gene delivery: It is now time to set the bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual salt and pH induced changes in polyethylenimine solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef] [Green Version]
- Reisinger, H.; Steinfellner, W.; Katinger, H.; Kunert, R. Serum-free transfection of CHO cells with chemically defined transfection systems and investigation of their potential for transient and stable transfection. Cytotechnology 2009, 60, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275. [Google Scholar] [CrossRef]
- Wagner, M.; Rinkenauer, A.C.; Schallon, A.; Schubert, U.S. Opposites attract: Influence of the molar mass of branched poly(ethylene imine) on biophysical characteristics of siRNA-based polyplexese. RSC Adv. 2013, 3, 12774–12785. [Google Scholar] [CrossRef]
- Lampela, P.; Soininen, P.; Urtti, A.; Männistö, P.T.; Raasmaja, A. Synergism in gene delivery by small PEIs and three different nonviral vectors. Int. J. Pharm. 2004, 270, 175–184. [Google Scholar] [CrossRef]
- Lampela, P.; Elomaa, M.; Ruponen, M.; Urtti, A.; Männistö, P.T.; Raasmaja, A. Different synergistic roles of small polyethylenimine and Dosper in gene delivery. J. Control. Release 2003, 88, 173–183. [Google Scholar] [CrossRef]
- Kunath, K.; von Harpe, A.; Fischer, D.; Petersen, H.; Bickel, U.; Voigt, K.; Kissel, T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: Comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J. Control. Release 2003, 89, 113–125. [Google Scholar] [CrossRef]
- Xun, M.M.; Xiao, Y.P.; Zhang, J.; Liu, Y.H.; Peng, Q.; Guo, Q.; Wu, W.-X.; Xu, Y.; Yu, X.-Q. Low molecular weight PEI-based polycationic gene vectors via Michael addition polymerization with improved serum-tolerance. Polymer 2015, 65, 45–54. [Google Scholar] [CrossRef]
- Eigner, A.; Fischer, D.; Merdan, T.; Brus, C.; Kissel, T.; Czubayko, F. Delivery of unmodified bioactive ribozymes by an RNA stabilizing polyethylenimine LMW PEI efficiently down regulates gene expression. Gene Ther. 2002, 9, 1700–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itaka, K.; Harada, A.; Yamasaki, Y.; Nakamura, K.; Kawaguchi, H.; Kataoka, K. In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J. Gene Med. 2004, 6, 76–84. [Google Scholar] [CrossRef]
- Dai, Z.; Gjetting, T.; Mattebjerg, M.A.; Wu, C.; Andresen, T.L. Elucidating the interplay between DNA-condensing and free polycations in gene transfection through a mechanistic study of linear and branched PEI. Biomaterials 2011, 32, 8626–8634. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, D.D.; Maggi, A.; Soria, M.R.; Monaco, L. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997, 25, 3095–3101. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, S.; Lee, L.K.; Roth, C.M. Interplay of polyethyleneimine molecular weight and oligonucleotide backbone chemistry in the dynamics of antisense activity. Nucleic Acids Res. 2007, 35, 4396–4408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehner, R.; Wang, X.; Hunziker, P. Plasmid linearization changes shape and efficiency of transfection complexes. Eur. J. Nanomed. 2013, 5, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Kwok, A.; Hart, S.L. Comparative structural and functional studies of nanoparticle formulations for DNA and siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Feng, C.L.; Zheng, W.S.; Huang, S.; Zhang, W.X.; Wu, H.N.; Zhan, Y.; Han, Y.-X.; Wu, S.; Jiang, J.-D. Tumor-selective lipopolyplex encapsulated small active RNA hampers colorectal cancer growth in vitro and in orthotopic murine. Biomaterials 2017, 141, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Palmer, L.; Chan, K.; Giesbrecht, C.; Jeffs, L.; MacLachlan, I. Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Mol. Ther. 2007, 15, 713–720. [Google Scholar] [CrossRef]
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoellerbauer, P.; Mims, A.; Klisovic, R.; Walker, A.R.; Chan, K.K.; et al. Targeted delivery of microRNA-29b by transferrin-conjugated anionic lipopolyplex nanoparticles: A novel therapeutic strategy in acute myeloid leukemia. Clin. Cancer Res. 2013, 19, 2355–2367. [Google Scholar] [CrossRef] [Green Version]
- Perche, F.; Clemençon, R.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Pichon, C. Neutral lipopolyplexes for in vivo delivery of conventional and replicative RNA vaccine. Mol. Ther. Nucleic Acids 2019, 17, 767–775. [Google Scholar] [CrossRef]
- Ko, Y.T.; Bhattacharya, R.; Bickel, U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J. Control. Release 2009, 133, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.; Höbel, S.; Bakowsky, U.; Aigner, A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials 2010, 31, 6892–6900. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Munye, M.M.; Tagalakis, A.D.; Manunta, M.D.I.; Hart, S.L. The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci. Rep. 2014, 4, 7107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control. Release 2016, 236, 1–14. [Google Scholar] [CrossRef]
- Jilek, J.L.; Zhang, Q.Y.; Tu, M.J.; Ho, P.Y.; Duan, Z.; Qiu, J.X.; Yu, A.M. Bioengineered let-7c inhibits orthotopic hepatocellular carcinoma and improves overall survival with minimal immunogenicity. Mol. Ther. Nucleic Acids 2019, 14, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wang, G.; He, B.; Li, L.; Li, C.; Lai, Y.; Xu, X.; Gu, Z. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int. J. Nanomed. 2012, 7, 4637–4648. [Google Scholar] [CrossRef] [Green Version]
- Petrek, H.; Ho, P.Y.; Batra, N.; Tu, M.J.; Zhang, Q.; Qiu, J.X.; Yu, A.M. Single bioengineered ncRNA molecule for dual-targeting toward the control of non-small cell lung cancer patient-derived xenograft tumor growth. Biochem. Pharmacol. 2021, 189, 114392. [Google Scholar] [CrossRef] [PubMed]
- García, L.; Buñuales, M.; Düzgüneş, N.; de Ilarduya, C.T. Serum-resistant lipopolyplexes for gene delivery to liver tumour cells. Eur. J. Pharm. Biopharm. 2007, 67, 58–66. [Google Scholar] [CrossRef]
- Penacho, N.; Simões, S.; de Lima, M.C.P. Polyethylenimine of various molecular weights as adjuvant for transfection mediated by cationic liposomes. Mol. Membr. Biol. 2009, 26, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Salmon, H.; Distasio, N.; Do, H.D.; Scherman, D.; Alhareth, K.; Tabrizian, M.; Mignet, N. Viscous core liposomes increase siRNA encapsulation and provides gene inhibition when slightly positively charged. Pharmaceutics 2021, 13, 479. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, K.; Ewe, A.; Kohn, C.; Loth, T.; Aigner, A.; Hacker, M.C.; Schulz-Siegmund, M. Sustained delivery of siRNA poly- and lipopolyplexes from porous macromer-crosslinked gelatin gels. Int. J. Pharm. 2017, 526, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zhupanyn, P.; Ewe, A.; Büch, T.; Malek, A.; Rademacher, P.; Müller, C.; Reinert, A.; Jaimes, Y.; Aigner, A. Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J. Control. Release 2020, 319, 63–76. [Google Scholar] [CrossRef]
- Pelisek, J.; Gaedtke, L.; DeRouchey, J.; Walker, G.F.; Nikol, S.; Wagner, E. Optimized lipopolyplex formulations for gene transfer to human colon carcinoma cells under in vitro conditions. J. Gene Med. 2006, 8, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Dang, C.; Wang, X.; Ragan, R.; Kwon, Y.J. Mixing-sequence-dependent nucleic acid complexation and gene transfer efficiency by polyethylenimine. Biomater. Sci. 2015, 3, 1124–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Vulugundam, G.; Kondaiah, P.; Bhattacharya, S. Co-liposomes of redox-active alkyl-ferrocene modified low MW branched PEI and DOPE for efficacious gene delivery in serum. J. Mater. Chem. B 2015, 3, 2318–2330. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [Green Version]
- Sabín, J.; Vázquez-Vázquez, C.; Prieto, G.; Bordi, F.; Sarmiento, F. Double charge inversion in polyethylenimine-decorated liposomes. Langmuir 2012, 28, 10534–10542. [Google Scholar] [CrossRef] [PubMed]
- Opanasopit, P.; Paecharoenchai, O.; Rojanarata, T.; Ngawhirunpat, T.; Ruktanonchai, U. Type and composition of urfactants mediating gene transfection of polyethylenimine-coated liposomes. Int. J. Nanomed. 2011, 6, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; Shen, H.; Shen, S.; Xie, M.; Mao, C.; Qiu, L.; Jin, Y. Development of a successive targeting liposome with multi-ligand for efficient targeting gene delivery. J. Gene Med. 2011, 13, 290–301. [Google Scholar] [CrossRef]
- Samaddar, S.; Mazur, J.; Boehm, D.; Thompson, D.H. Development and in vitro characterization of bladder tumor cell targeted lipid-coated polyplex for dual delivery of plasmids and small molecules. Int. J. Nanomed. 2019, 14, 9547–9561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, R.; Wu, S.; An, J.; Liang, Y.; Hou, L.; Zhang, Z. Self-responsive co-delivery system for remodeling tumor intracellular microenvironment to promote PTEN-mediated anti-tumor therapy. Nanoscale 2020, 12, 9392–9403. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.P.; Sarisozen, C.; Luther, E.; Pan, J.; Torchilin, V.P. Surface-engineered polyethyleneimine-modified liposomes as novel carrier of siRNA and chemotherapeutics for combination treatment of drug-resistant cancers. Drug Deliv. 2019, 26, 443. [Google Scholar] [CrossRef] [Green Version]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Pinnapireddy, S.R.; el Assy, M.R.; Schlote, P.; Bakowsky, U. Glycosylated artificial virus-like hybrid vectors for advanced gene delivery. Polymers 2019, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Hao, F.; Li, Y.; Zhu, J.; Sun, J.; Marshall, B.; Lee, R.J.; Teng, L.; Yang, Z.; Xie, J. Polyethylenimine-based formulations for delivery of oligonucleotides. Curr. Med. Chem. 2019, 26, 2264–2284. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Gholami, L.; Asghari, F.; Askarian, S.; Barzegar, S.; Rezaee, M.; Oskuee, R.K. Viral vector mimicking and nucleus targeted nanoparticles based on dexamethasone polyethylenimine nanoliposomes: Preparation and evaluation of transfection efficiency. Colloids Surf. B Biointerfaces 2018, 165, 252–261. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Shah, H.; Tariq, I.; Engelhardt, K.; Bakowsky, U.; Pinnapireddy, S.R. Development and characterization of ultrasound activated lipopolyplexes for enhanced transfection by low frequency ultrasound in in vitro tumor model. Macromol. Biosci. 2020, 20, 2000173. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Deng, W.; Xu, X.; Zhao, X.; Vo, J.N.; Anwer, A.G.; Williams, T.C.; Cui, H.; Goldys, E.M. Photoresponsive endosomal escape enhances gene delivery using liposome–polycation–DNA (LPD) nanovectors. J. Mater. Chem. B 2018, 6, 5269–5281. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yan, G.; Quan, S.; Jin, E.; Quan, J.; Jin, G. MRI-visible liposome–polyethylenimine complexes for DNA delivery: Preparation and evaluation. Biosci. Biotechnol. Biochem. 2019, 83, 622–632. [Google Scholar] [CrossRef]
- Zhen, S.; Li, X. Liposomal delivery of CRISPR/Cas9. Cancer Gene Ther. 2020, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Le, Q.-V.; Wu, Y.; Park, J.; Oh, Y.-K. Nanovesicle-mediated delivery systems for CRISPR/Cas genome editing. Pharmaceutics 2020, 12, 1233. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle delivery of CRISPR/Cas9 for genome editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef] [PubMed]

| Nucleic Acid | Polyethyleneimine | Lipids | Targeting Ligand | Administration Route | Targeted Cells | Mode of Action | Reference |
|---|---|---|---|---|---|---|---|
| saRNA | 2 kDa bPEI | PE | HA | Local (injection to the rectum) | Human colorectal tumor xenografts | Stimulation of p21 expression | [88] |
| siRNA | PEI F25-LMW | DPPC | - | Intracranial | Tu2449 murine glioma cells | Knockdown of STAT3 gene expression | [58] |
| siRNA | PEI F25-LMW | DPPC | - | Intravenous | PC3 prostate carcinoma xenografts | Knockdown of survivin gene expression | [54] |
| siRNA | PEI F25-LMW | ECV | - | Intravenous | PC3 prostate carcinoma xenografts | Knockdown of survivin gene expression | [103] |
| ODN | 2.5 kDa lPEI | HEPC, DOPE, DC-Chol, DSPE-PEG2000, DSPE-PEG-Mal | Anti-CD20 antibody conjugated via maleimide | Intravenous | Human Burkitt’s lymphoma Daudi cells | Reduction of the Bcl-2 protein level | [48] |
| pDNA | 25 kDa bPEI | DPPC, Chol, DSPE-PEI25kDa | - | Intravenous | PC3 prostate carcinoma xenografts | Reacquisition of PTEN functionality | [112] |
| siRNA | CDG—25 kDa PEI | PC, Chol | - | Intravenous | U-87 MG glioblastoma xenografts | Silencing of VEGF expression | [40] |
| BERA | 10 kDa bPEI | DOTMA, Chol, DMG-PEG2000 | - | Intravenous | Hepatocellular carcinoma xenografts | Selective modulation of the expression of several genes (LIN28B, ARID3B, Bcl-xl, c-Myc) | [96] |
| Intravenous | Non-small-cell lung carcinoma patient-derived xenografts | Selective modulation of the expression of several genes (RAS, VAMP3, CDK6) | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzykiewicz, J.; Czogalla, A. Polyethyleneimine-Based Lipopolyplexes as Carriers in Anticancer Gene Therapies. Materials 2022, 15, 179. https://doi.org/10.3390/ma15010179
Jerzykiewicz J, Czogalla A. Polyethyleneimine-Based Lipopolyplexes as Carriers in Anticancer Gene Therapies. Materials. 2022; 15(1):179. https://doi.org/10.3390/ma15010179
Chicago/Turabian StyleJerzykiewicz, Julia, and Aleksander Czogalla. 2022. "Polyethyleneimine-Based Lipopolyplexes as Carriers in Anticancer Gene Therapies" Materials 15, no. 1: 179. https://doi.org/10.3390/ma15010179