Cashew Gum Polysaccharide Nanoparticles Grafted with Polypropylene Glycol as Carriers for Diclofenac Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cashew Gum Polysaccharide Isolation
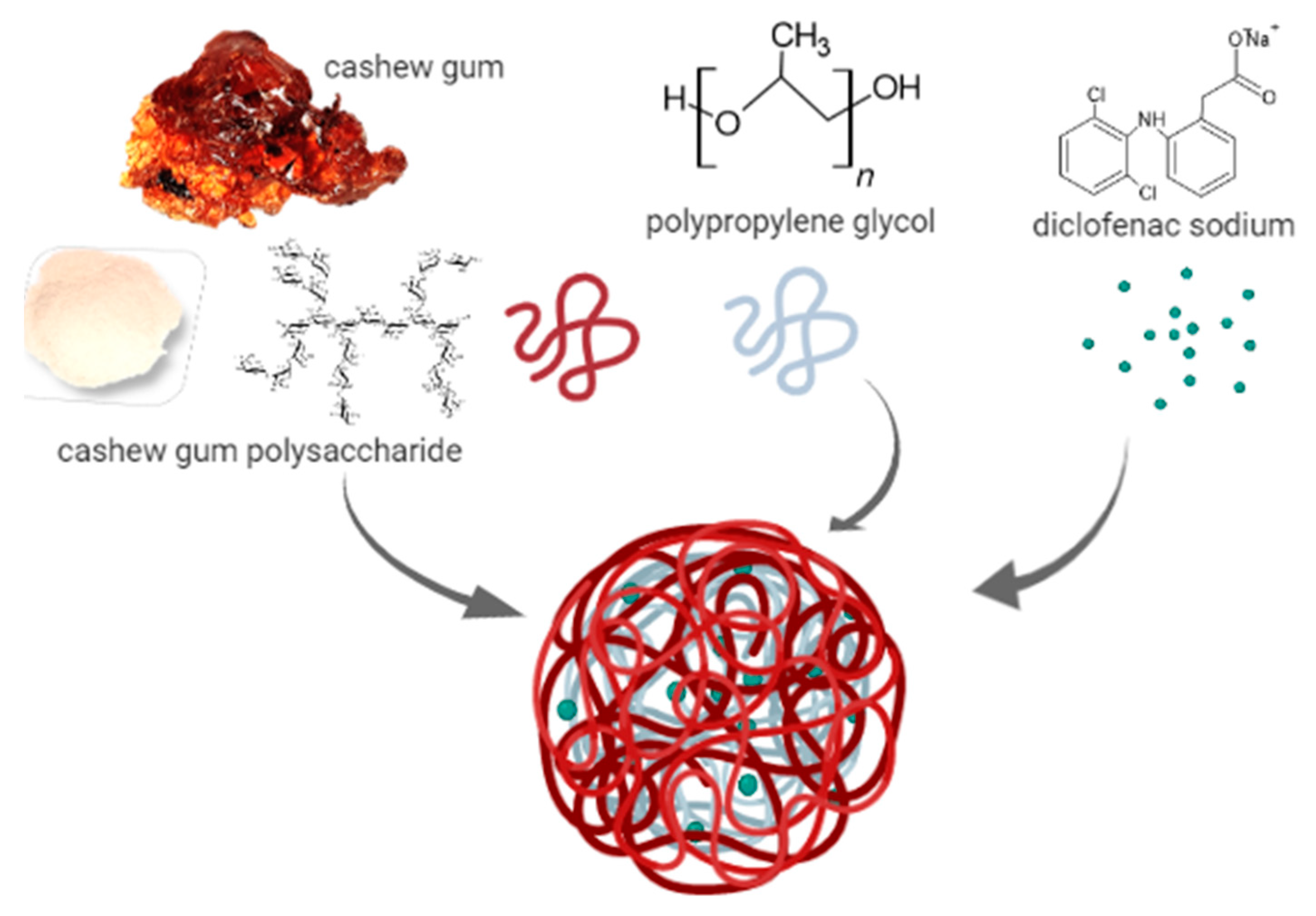
2.3. Nanoparticles Preparation
2.4. Entrapment Efficiency
2.5. Hydrodynamic Diameter and Zeta Potential
2.6. Scanning Electron Microscopy (SEM)
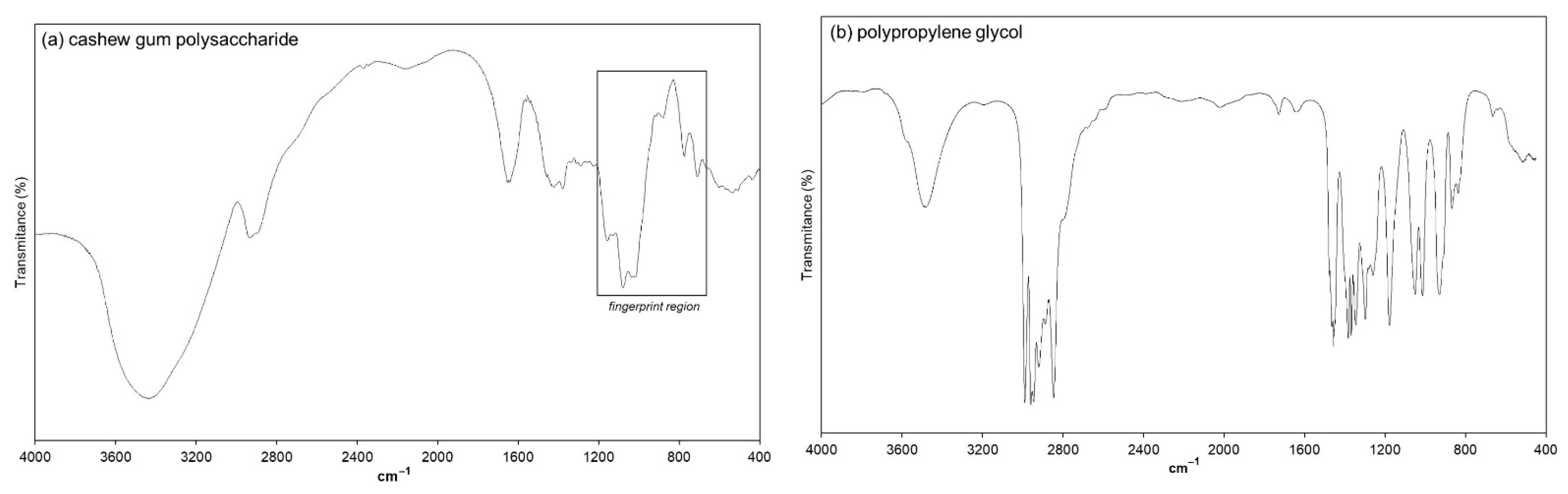
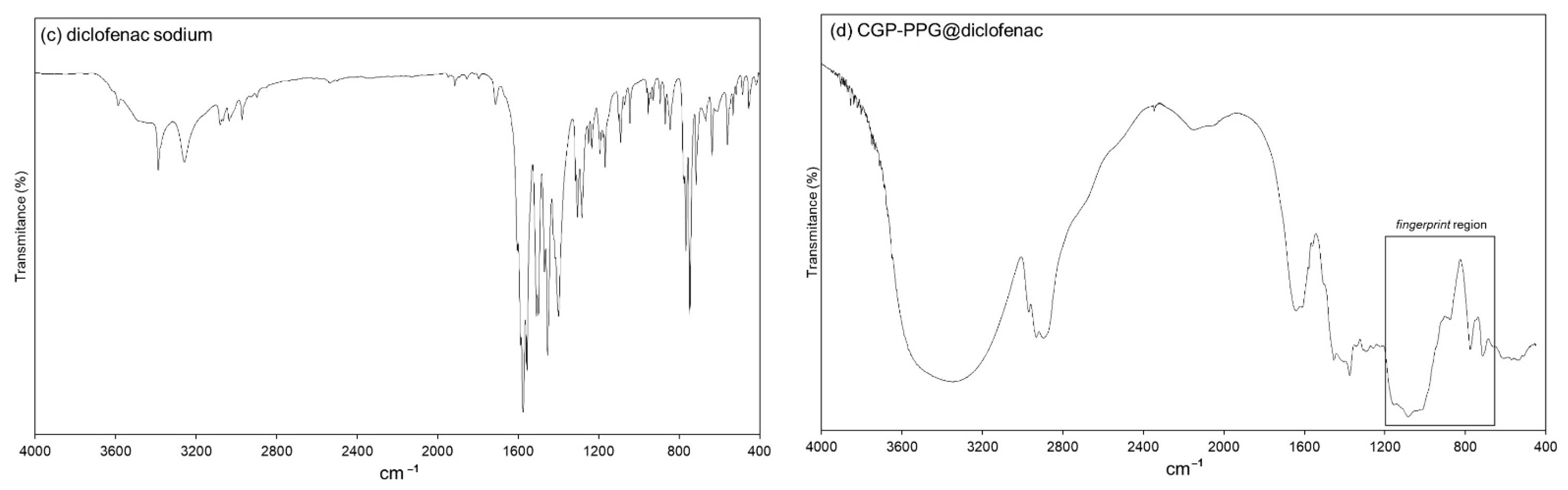
2.7. Fourier-Transform Infrared Spectroscopy (FTIR)
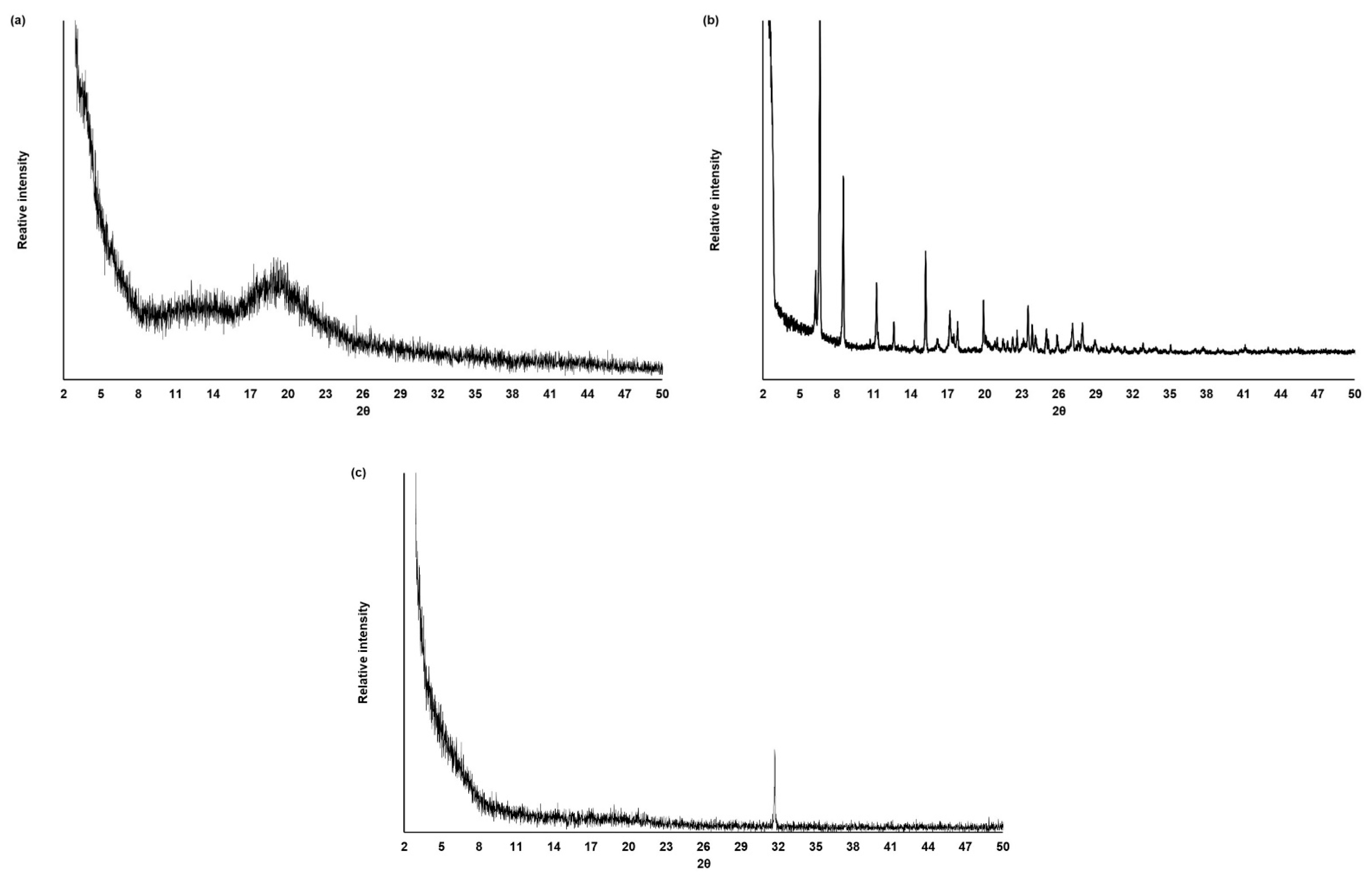
2.8. X-ray Diffraction (XRD) Analysis
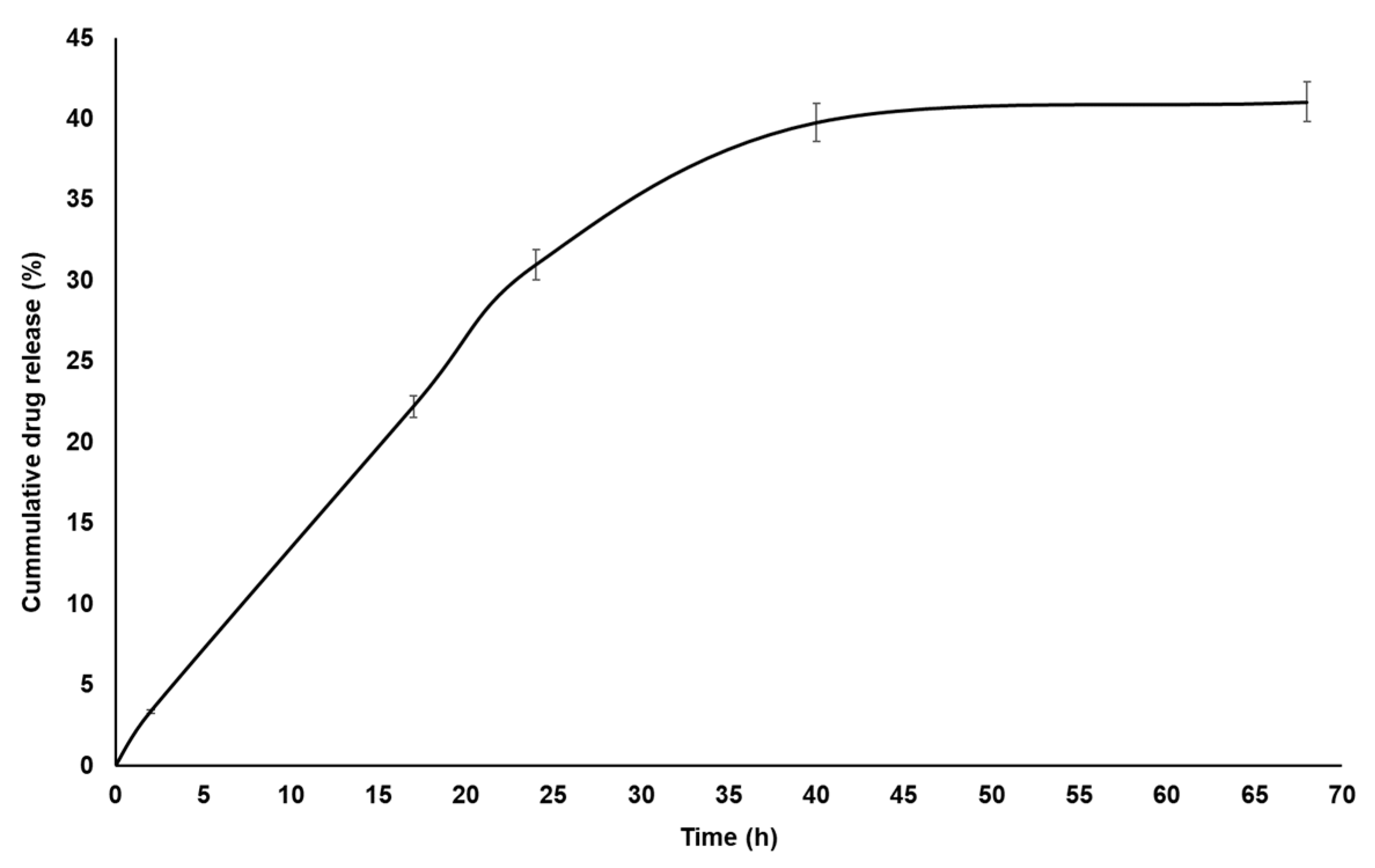
2.9. In Vitro Drug Release Studies
2.10. Drug Release Kinetics
3. Results and Discussion
3.1. Production of CGP-PPG@Diclofenac Nanoparticles
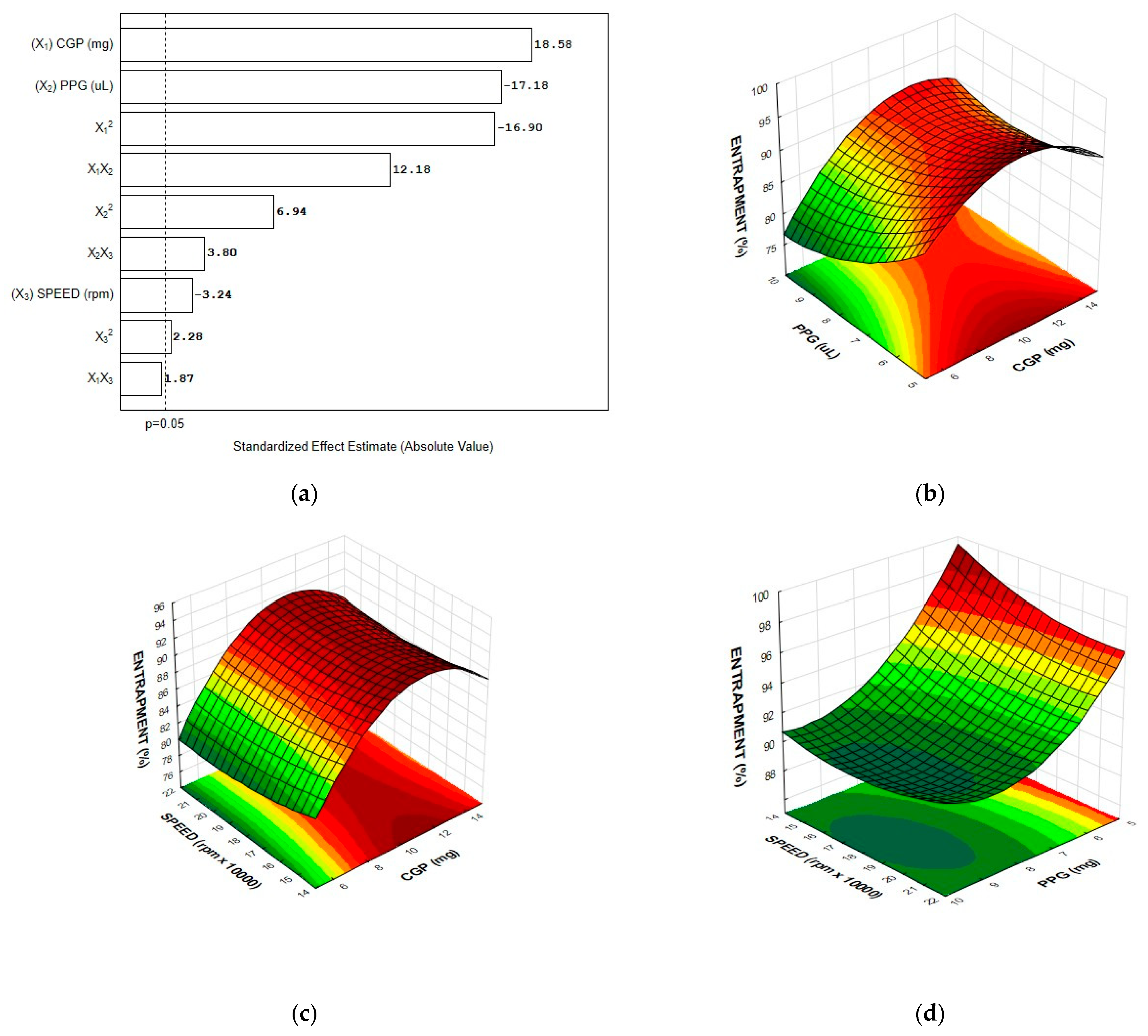
3.2. Entrapment Efficiency
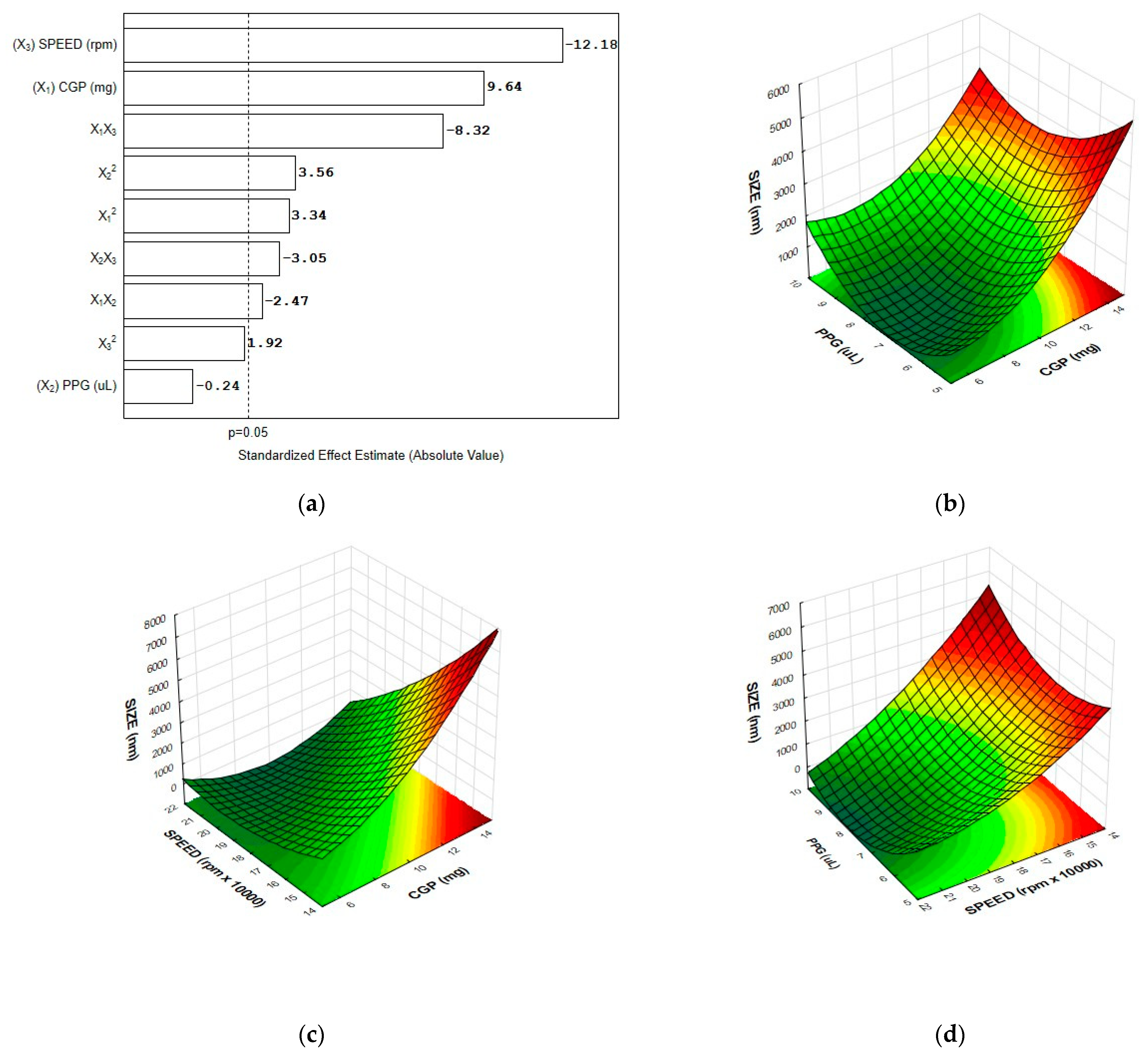
3.3. Optimization of the Nanoparticle Formulation and Model Validation
3.4. Nanoparticles Characterization
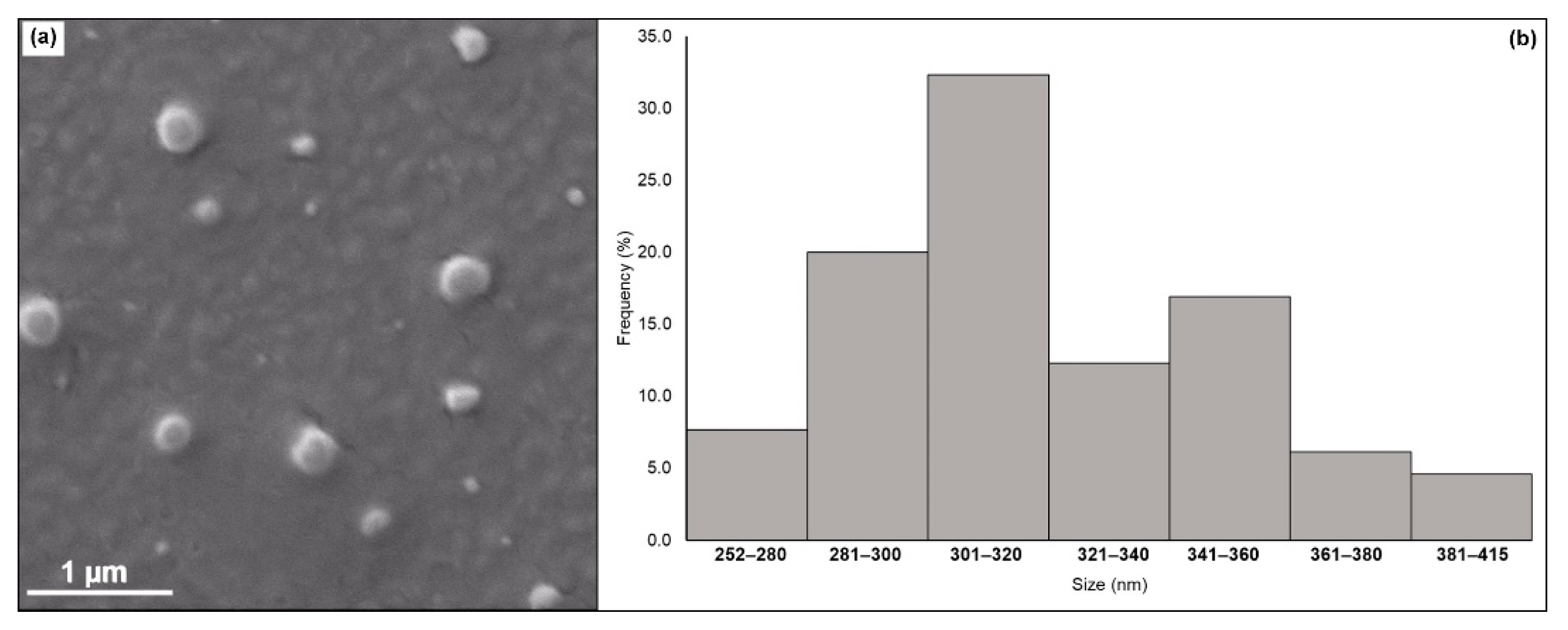
3.4.1. Scanning Electron Microscopy (SEM)
3.4.2. FTIR Spectroscopy Analysis
3.4.3. XRD Analysis
3.5. In Vitro Drug Release Study
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Gao, J.; An, Z.; Zhao, X.; Yao, H.; Zhang, M.; Tian, Q.; Zhai, X.; Liu, Y. Polymer microsphere for water-soluble drug delivery via carbon dot-stabilizing W/O emulsion. J. Mater. Sci. 2019, 54, 5160–5175. [Google Scholar] [CrossRef]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiei, P.; Haddadi, A. A robust systematic design: Optimization and preparation of polymeric nanoparticles of PLGA for docetaxel intravenous delivery. Mater. Sci. Eng. C 2019, 104, 109950. [Google Scholar] [CrossRef] [PubMed]
- Ansari, R.; Sadati, S.M.; Mozafari, N.; Ashrafi, H.; Azadi, A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur. Polym. J. 2020, 128, 109607. [Google Scholar] [CrossRef]
- Nelemans, L.C.; Gurevich, L. Drug Delivery with Polymeric Nanocarriers—Cellular Uptake Mechanisms. Materials 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Cellulose-Based Hydrogels as Sustained Drug-Delivery Systems. Materials 2020, 13, 5270. [Google Scholar] [CrossRef]
- Varma, L.T.; Singh, N.; Gorain, B.; Choudhury, H.; Tambuwala, M.M.; Kesharwani, P.; Shukla, R. Recent Advances in Self-Assembled Nanoparticles for Drug Delivery. Curr. Drug Deliv. 2020, 17, 279–291. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K.; Ansari, M.T.; Sami, F.; Bera, H.; Hasnain, M.S. Chapter 11—Cashew gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 263–283. [Google Scholar]
- Cruz, M.V.; Pereira-Júnior, M.A.; Batista, K.A.; Fernandes, K.F. Use of Statistical Design Strategies to Produce Biodegradable and Eco-Friendly Films from Cashew Gum Polysaccharide and Polyvinyl Alcohol. Materials 2019, 12, 1149. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.J.; de Souza, F.R.L.; Bezerra, J.M.N.A.; Oliveira, C.; Nadvorny, D.; de La Roca Soares, M.F.; Nunes, L.C.C.; Silva-Filho, E.C.; Veiga, F.; Soares Sobrinho, J.L. Gums’ based delivery systems: Review on cashew gum and its derivatives. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef]
- Silva, F.E.; Batista, K.A.; Di-Medeiros, M.C.; Silva, C.N.; Moreira, B.R.; Fernandes, K.F. A stimuli-responsive and bioactive film based on blended polyvinyl alcohol and cashew gum polysaccharide. Mater. Sci. Eng. C 2016, 58, 927–934. [Google Scholar] [CrossRef]
- Khan, Y.S.; Gutiérrez-de-Terán, H.; Åqvist, J. Molecular Mechanisms in the Selectivity of Nonsteroidal Anti-Inflammatory Drugs. Biochemistry 2018, 57, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Al-Lawati, H.; Vakili, M.R.; Lavasanifar, A.; Ahmed, S.; Jamali, F. Delivery and Biodistribution of Traceable Polymeric Micellar Diclofenac in the Rat. J. Pharm. Sci. 2019, 108, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Proikakis, C.S.; Tarantili, P.A.; Andreopoulos, A.G. The role of polymer/drug interactions on the sustained release from poly(dl-lactic acid) tablets. Eur. Polym. J. 2006, 42, 3269–3276. [Google Scholar] [CrossRef]
- Yahia, Y.; García-Villén, F.; Djelad, A.; Belaroui, L.S.; Sanchez-Espejo, R.; Sassi, M.; López-Galindo, A.; Viseras, C. Crosslinked palygorskite-chitosan beads as diclofenac carriers. Appl. Clay Sci. 2019, 180, 105169. [Google Scholar] [CrossRef]
- Chuasuwan, B.; Binjesoh, V.; Polli, J.E.; Zhang, H.; Amidon, G.L.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; et al. Biowaiver monographs for immediate release solid oral dosage forms: Diclofenac sodium and diclofenac potassium. J. Pharm. Sci. 2009, 98, 1206–1219. [Google Scholar] [CrossRef]
- Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt Cocrystal of Diclofenac Sodium-L-Proline: Structural, Pseudopolymorphism, and Pharmaceutics Performance Study. Pharmaceutics 2020, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, D.; García-Villén, F.; Majdoub, H.; Viseras, C.; Zayani, M.B. Chitosan/beidellite nanocomposite as diclofenac carrier. Int. J. Biol. Macromol. 2019, 126, 44–53. [Google Scholar] [CrossRef]
- Duarte Junior, A.P.; Tavares, E.J.M.; Alves, T.V.G.; de Moura, M.R.; da Costa, C.E.F.; Silva Júnior, J.O.C.; Ribeiro Costa, R.M. Chitosan nanoparticles as a modified diclofenac drug release system. J. Nanoparticle Res. 2017, 19, 274. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, R.; Zhang, W.; Song, X.; Xu, L.; Zhao, Y.; Shang, L.; Zhang, J. Preparation of carboxylmethylchitosan and alginate blend membrane for diffusion-controlled release of diclofenac diethylamine. J. Mater. Sci. Technol. 2021, 63, 210–215. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Kouidri, S.; Salahinejad, E.; Tcharkhtchi, A.; Bakir, F. Controlled release from polyurethane films: Drug release mechanisms. J. Appl. Polym. Sci. 2021, 138, 50083. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R. Usage of nanocrystalline cellulose phosphate as novel sustained release system for anti-inflammatory drugs. J. Mol. Struct. 2021, 1233, 130108. [Google Scholar] [CrossRef]
- Luo, Z.; Jin, L.; Xu, L.; Zhang, Z.L.; Yu, J.; Shi, S.; Li, X.; Chen, H. Thermosensitive PEG–PCL–PEG (PECE) hydrogel as an in situ gelling system for ocular drug delivery of diclofenac sodium. Drug Deliv. 2016, 23, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Khan, A.; Rosenholm, J.M.; Minhas, M.U.; Wu, P.-C. Fabrication and Characterization of Diclofenac Sodium Loaded Hydrogels of Sodium Alginate as Sustained Release Carrier. Gels 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendyk, A.; Jachowicz, R. Unified methodology of neural analysis in decision support systems built for pharmaceutical technology. Expert Syst. Appl. 2007, 32, 1124–1131. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Velmurugan, R.; Subramanian, S. Development and optimization of ifosfamide nanostructured lipid carriers for oral delivery using response surface methodology. Appl. Nanosci. 2015, 6, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Sarangi, B.; Mishra, K.; Mohanta, G.P.; Manna, P.K. In vitro-in vivo correlation (IVIVC) of solid lipid nanoparticles loaded with poorly water-soluble drug lovastatin. Eur. Polym. J. 2020, 122, 109366. [Google Scholar] [CrossRef]
- Burchard, W. Light Scattering from Polysaccharides as Soft Materials. In Soft Matter Characterization; Borsali, R., Pecora, R., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 463–603. [Google Scholar]
- Bhadra, S.; Prajapati, A.B.; Bhadra, D. Development of pH sensitive polymeric nanoparticles of erythromycin stearate. J. Pharm. Bioallied Sci. 2016, 8, 135–140. [Google Scholar] [CrossRef]
- Burapapadh, K.; Takeuchi, H.; Sriamornsak, P. Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole. Asian J. Pharm. Sci. 2016, 11, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Niu, J.; Wu, J.-Y. Development and characterization of novel and stable silicon nanoparticles-embedded PCM-in-water emulsions for thermal energy storage. Appl. Energy 2019, 238, 1407–1416. [Google Scholar] [CrossRef]
- Mikhailov, I.V.; Amoskov, V.M.; Darinskii, A.A.; Birshtein, T.M. The Structure of Dipolar Polymer Brushes and Their Interaction in the Melt. Impact of Chain Stiffness. Polymers 2020, 12, 2887. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Jaimes-Aguirre, L.; Morales-Avila, E.; Ocampo-García, B.E.; Medina, L.A.; López-Téllez, G.; Gibbens-Bandala, B.V.; Izquierdo-Sánchez, V. Biodegradable poly(D,L-lactide-co-glycolide)/poly(L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2017, 76, 743–751. [Google Scholar] [CrossRef]
- Batista, K.A.; Silva, C.N.S.; Fernandes, P.M.; Campos, I.T.N.; Fernandes, K.F. Development of a new bioaffinity stationary phase for lactose removal using a lactose-binding lectin immobilized onto polyaniline. Sep. Purif. Technol. 2017, 185, 54–60. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Chelopo, M.P.; Kalombo, L.; Wesley-Smith, J.; Grobler, A.; Hayeshi, R. The fabrication and characterization of a PLGA nanoparticle–Pheroid® combined drug delivery system. J. Mater. Sci. 2017, 52, 3133–3145. [Google Scholar] [CrossRef]
- Santhosh Kumar, B.; Kumar, M.; Suguna, L.; Sastry, T.; Mandal, A. Pullulan acetate nanoparticles based delivery system for Hydrophobic drug. Int. J. Pharma Bio. Sci. 2012, 3, P24–P32. [Google Scholar]
- Silva, M.C.C.; Santos, M.S.F.; Bezerra, R.D.S.; Araújo-Júnior, E.A.; Osajima, J.A.; Santos, M.R.M.C.; Fonseca, M.G.; Silva-Filho, E.C. Kaolinite/cashew gum bionanocomposite for doxazosin incorporation and its release. Int. J. Biol. Macromol. 2020, 161, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvian, J.R. Introduction to Spectroscopy; Cengage Learning: Washington, DC, USA, 2010. [Google Scholar]
- Lima, M.R.; Paula, H.C.B.; Abreu, F.O.M.S.; Silva, R.B.C.; Sombra, F.M.; Paula, R.C.M. Hydrophobization of cashew gum by acetylation mechanism and amphotecirin B encapsulation. Int. J. Biol. Macromol. 2018, 108, 523–530. [Google Scholar] [CrossRef]
- Ulu, A.; Birhanlı, E.; Köytepe, S.; Ateş, B. Chitosan/polypropylene glycol hydrogel composite film designed with TiO2 nanoparticles: A promising scaffold of biomedical applications. Int. J. Biol. Macromol. 2020, 163, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Shinzawa, H.; Uchimaru, T.; Mizukado, J.; Kazarian, S.G. Non-equilibrium behavior of polyethylene glycol (PEG)/polypropylene glycol (PPG) mixture studied by Fourier transform infrared (FTIR) spectroscopy. Vib. Spectrosc. 2017, 88, 49–55. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Alaei-Beirami, M.; Javadzadeh, Y.; Mohammadi, G.; Hamidi, A.; Andalib, S.; Adibkia, K. Comparison of physicochemical characteristics and drug release of diclofenac sodium–eudragit® RS100 nanoparticles and solid dispersions. Powder Technol. 2012, 219, 211–216. [Google Scholar] [CrossRef]
- Hasnain, S.M.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K. Isolation and characterization of Linum usitatisimum polysaccharide to prepare mucoadhesive beads of diclofenac sodium. Int. J. Biol. Macromol. 2018, 116, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Li, J.; Sun, S.; Weng, Y.; Chen, H. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale 2012, 4, 4667–4673. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, D.; García-Villén, F.; Majdoub, H.; Zayani, M.B.; Viseras, C. Complex of chitosan pectin and clay as diclofenac carrier. Appl. Clay Sci. 2019, 172, 155–164. [Google Scholar] [CrossRef]
- Dias, S.F.L.; Nogueira, S.S.; de França Dourado, F.; Guimarães, M.A.; de Oliveira Pitombeira, N.A.; Gobbo, G.G.; Primo, F.L.; de Paula, R.C.M.; Feitosa, J.P.A.; Tedesco, A.C.; et al. Acetylated cashew gum-based nanoparticles for transdermal delivery of diclofenac diethyl amine. Carbohydr. Polym. 2016, 143, 254–261. [Google Scholar] [CrossRef]
- Yousefi, V.; Tarhriz, V.; Eyvazi, S.; Dilmaghani, A. Synthesis and application of magnetic@layered double hydroxide as an anti-inflammatory drugs nanocarrier. J. Nanobiotechnology 2020, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Pitombeira, N.A.O.; Neto, J.G.V.; Silva, D.A.; Feitosa, J.P.A.; Paula, H.C.B.; Paula, R.C.M. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Araújo, A.L.; Pitombeira, N.A.; Plácido, A.; Almeida, M.P.; Veras, L.M.C.; Delerue-Matos, C.; Lima, F.C.D.A.; Neto, A.B.; Paula, R.C.M.; et al. Acetylated cashew gum-based nanoparticles for the incorporation of alkaloid epiisopiloturine. Int. J. Biol. Macromol. 2019, 128, 965–972. [Google Scholar] [CrossRef]
- Salome, C.; Onunkwo, G.; Onyishi, I. Kinetics and mechanisms of drug release from swellable and non swellable matrices: A review. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 97–103. [Google Scholar]
| Test n° | Coded Level of Variables | Actual Level of Variables | ||||
|---|---|---|---|---|---|---|
| CGP 1 | PPG | Speed | CGP (mg) | PPG (μL) | Speed (rpm) | |
| 1 | −1 | −1 | −1 | 5.0 | 5.0 | 14,000 |
| 2 | −1 | −1 | +1 | 5.0 | 5.0 | 22,000 |
| 3 | −1 | 0 | 0 | 5.0 | 7.5 | 18,000 |
| 4 | −1 | +1 | −1 | 5.0 | 10.0 | 14,000 |
| 5 | −1 | +1 | +1 | 5.0 | 10.0 | 22,000 |
| 6 | 0 | −1 | 0 | 10.0 | 5.0 | 18,000 |
| 7 | 0 | 0 | −1 | 10.0 | 7.5 | 14,000 |
| 8 (c) | 0 | 0 | 0 | 10.0 | 7.5 | 18,000 |
| 9 | 0 | 0 | +1 | 10.0 | 7.5 | 22,000 |
| 10 | 0 | +1 | 0 | 10.0 | 10.0 | 18,000 |
| 11 | +1 | −1 | −1 | 15.0 | 5.0 | 14,000 |
| 12 | +1 | −1 | +1 | 15.0 | 5.0 | 22,000 |
| 13 | +1 | 0 | 0 | 15.0 | 7.5 | 18,000 |
| 14 | +1 | +1 | −1 | 15.0 | 10.0 | 14,000 |
| 15 | +1 | +1 | +1 | 15.0 | 10.0 | 22,000 |
| 16 (c) | 0 | 0 | 0 | 10.0 | 7.5 | 18,000 |
| Test No. | Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| 1 | 896 | 0.51 | −17.33 | 93.5 |
| 2 | 1209 | 0.52 | −8.31 | 87.9 |
| 3 | 942 | 0.65 | −2.99 | 78.3 |
| 4 | 4508 | 1.00 | −11.77 | 77.6 |
| 5 | 660 | 0.52 | −10.05 | 78.2 |
| 6 | 3170 | 0.93 | −9.30 | 96.6 |
| 7 | 2097 | 0.48 | −8.88 | 91.7 |
| 8 (c) | 696 | 0.62 | −5.32 | 90.6 |
| 9 | 544 | 0.43 | −8.47 | 91.6 |
| 10 | 635 | 0.46 | −25.03 | 90.5 |
| 11 | 10080 | 0.95 | −0.08 | 92.4 |
| 12 | 1936 | 0.71 | −2.18 | 91.2 |
| 13 | 2708 | 0.63 | −3.17 | 89.8 |
| 14 | 10000 | 1.00 | −1.55 | 90.3 |
| 15 | 1052 | 0.62 | −2.05 | 89.9 |
| 16 (c) | 642 | 0.51 | −17.33 | 90.5 |
| Kinetic Model | r2 | AIC 1 |
|---|---|---|
| Zero-order | 0.9634 | 0.265 |
| First order | 0.8335 | 0.485 |
| Higuchi | 0.6963 | 0.393 |
| Korsmeyer–Peppas | 0.9975 | 0.234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.N.S.d.; Di-Medeiros, M.C.B.; Lião, L.M.; Fernandes, K.F.; Batista, K.d.A. Cashew Gum Polysaccharide Nanoparticles Grafted with Polypropylene Glycol as Carriers for Diclofenac Sodium. Materials 2021, 14, 2115. https://doi.org/10.3390/ma14092115
Silva CNSd, Di-Medeiros MCB, Lião LM, Fernandes KF, Batista KdA. Cashew Gum Polysaccharide Nanoparticles Grafted with Polypropylene Glycol as Carriers for Diclofenac Sodium. Materials. 2021; 14(9):2115. https://doi.org/10.3390/ma14092115
Chicago/Turabian StyleSilva, Cassio Nazareno Silva da, Maria Carolina Bezerra Di-Medeiros, Luciano Morais Lião, Kátia Flávia Fernandes, and Karla de Aleluia Batista. 2021. "Cashew Gum Polysaccharide Nanoparticles Grafted with Polypropylene Glycol as Carriers for Diclofenac Sodium" Materials 14, no. 9: 2115. https://doi.org/10.3390/ma14092115
APA StyleSilva, C. N. S. d., Di-Medeiros, M. C. B., Lião, L. M., Fernandes, K. F., & Batista, K. d. A. (2021). Cashew Gum Polysaccharide Nanoparticles Grafted with Polypropylene Glycol as Carriers for Diclofenac Sodium. Materials, 14(9), 2115. https://doi.org/10.3390/ma14092115








