Production of Uniformly Sized Gallium-Based Liquid Alloy Nanodroplets via Ultrasonic Method and Their Li-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
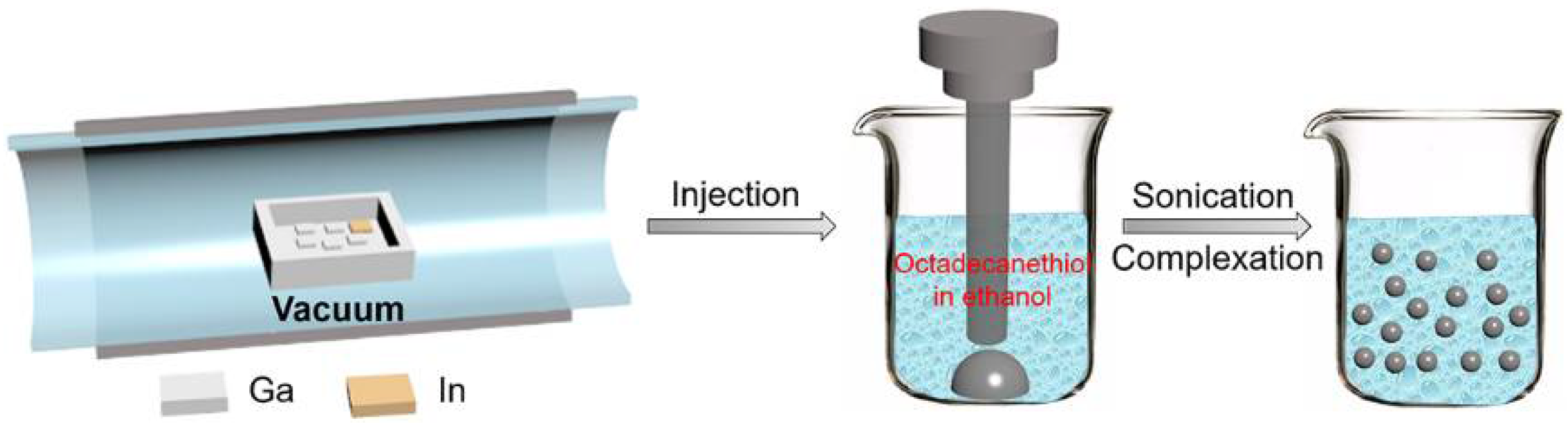
2.1. Synthesis of Eutectic GaIn Nanodroplets
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
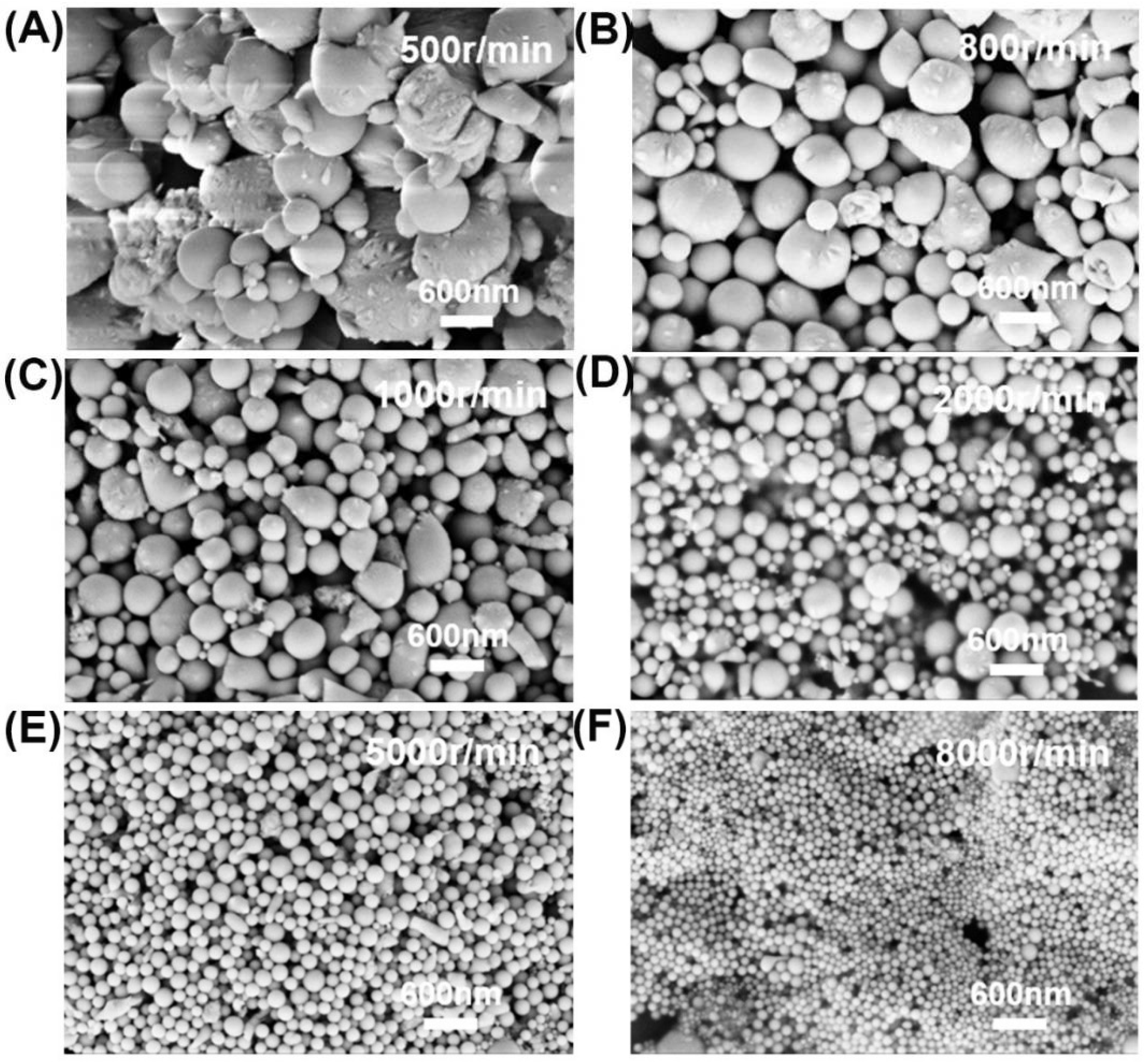
3.1. Ultrasonic Amplitude
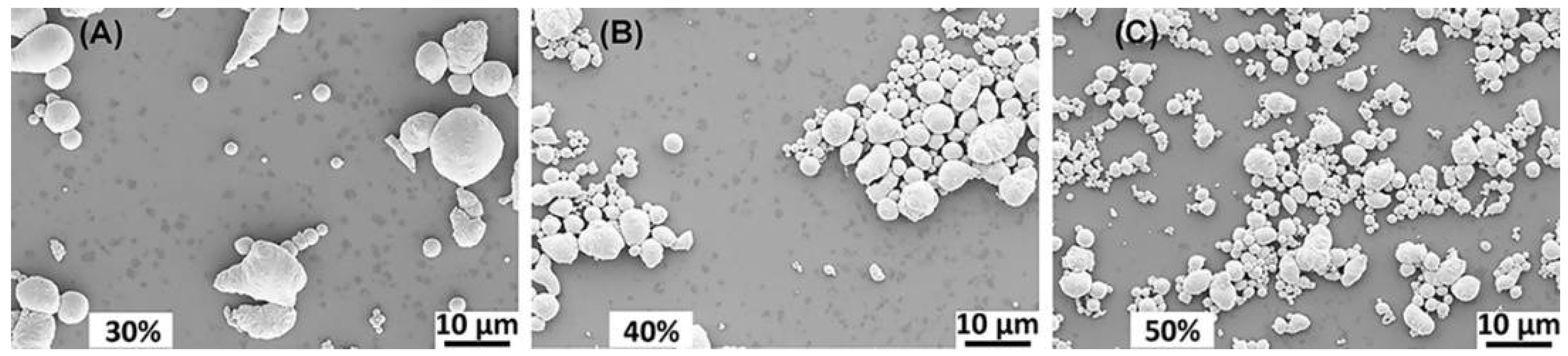
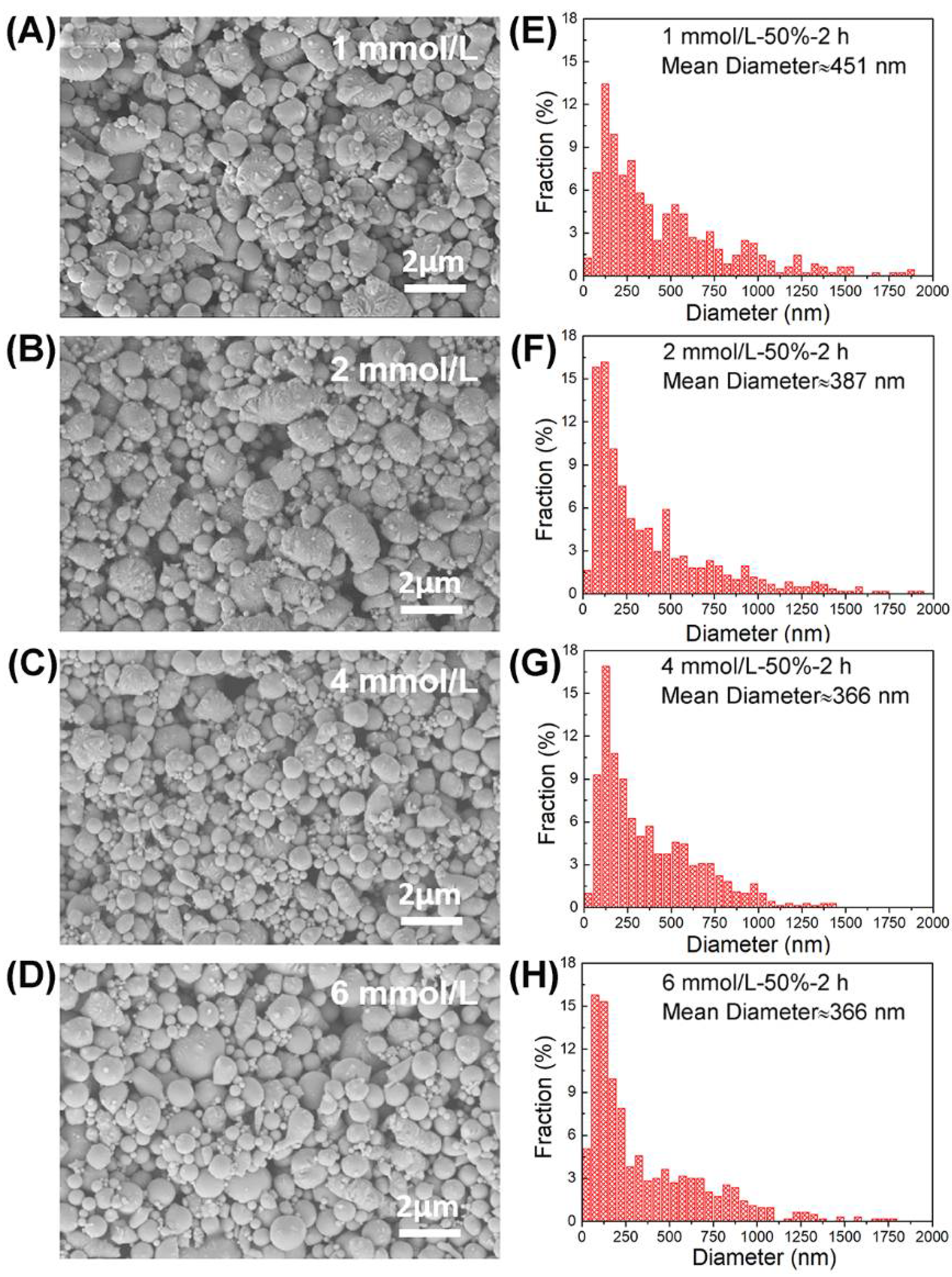
3.2. Surfactant Concentration
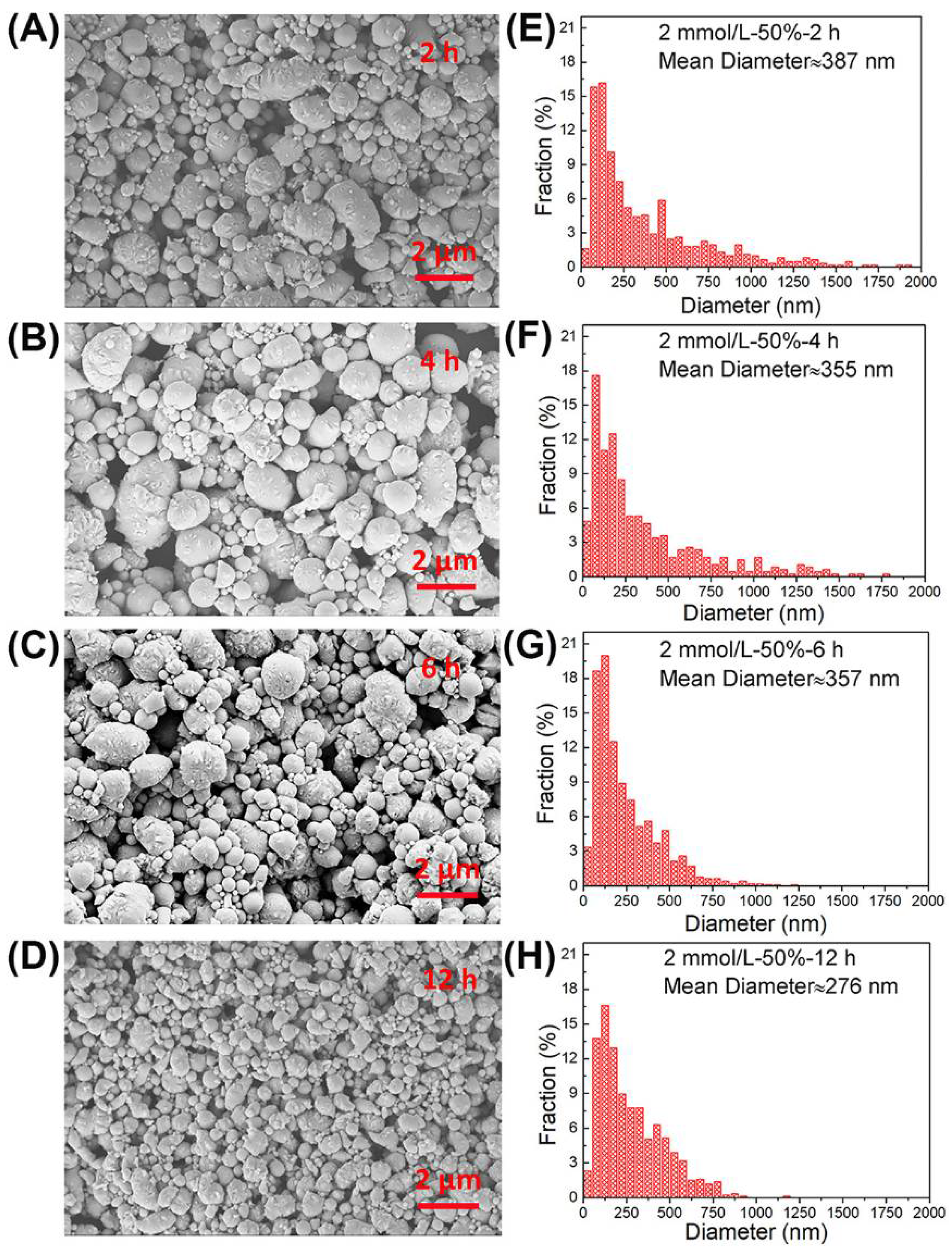
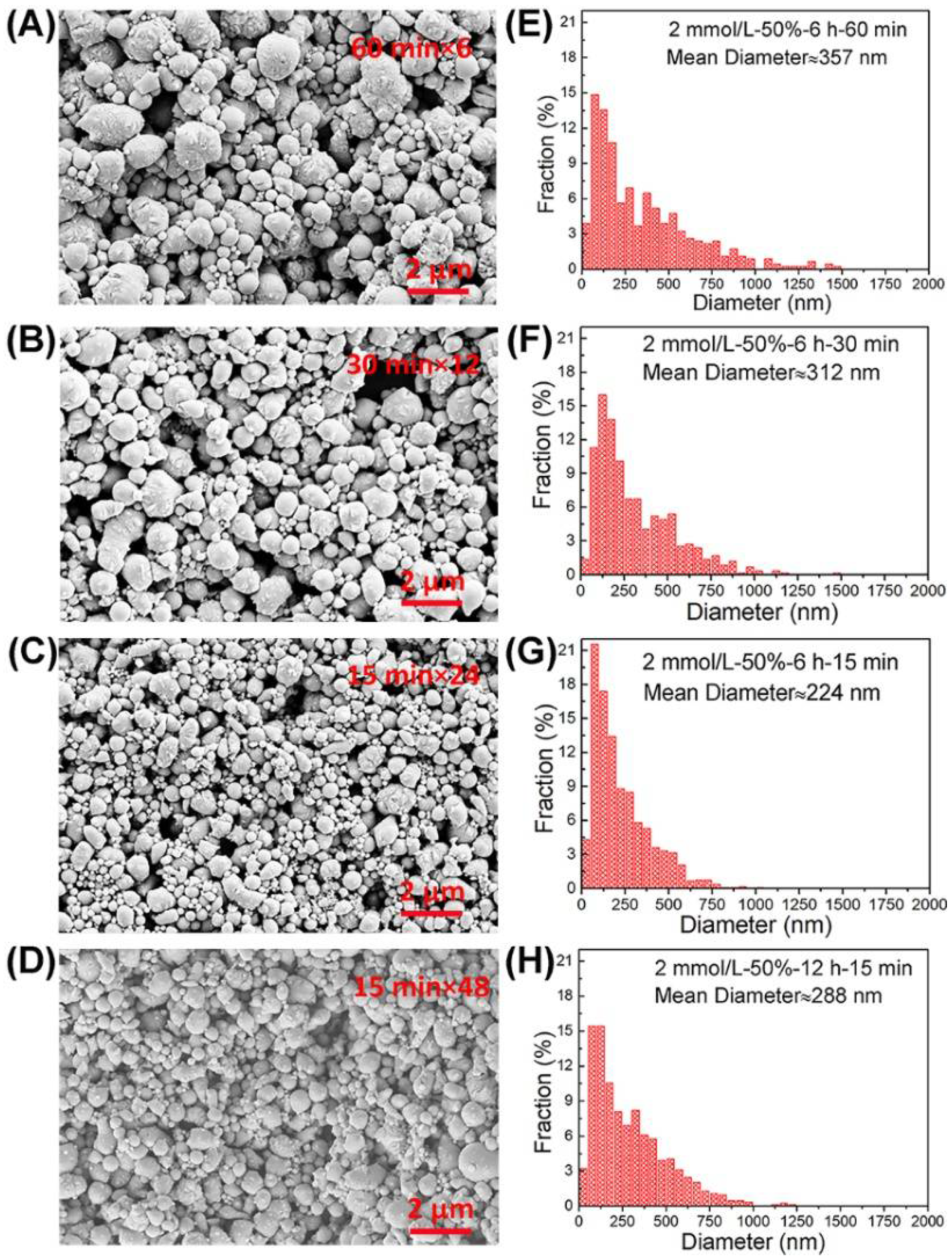
3.3. Ultrasonic Time
3.4. Temperature in Solution
3.5. Centrifugal Process
3.6. eGaIn Nanodroplets as Anode for Li-Ion Battery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duwez, P.; Willens, R.H.; Klement, W. Metastable Electron Compound in Ag-Ge Alloys. J. Appl. Phys. 1960, 31, 1137. [Google Scholar] [CrossRef] [Green Version]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markvicka, E.J.; Bartlett, M.D.; Huang, X.; Majidi, C. An autonomously electrically self-healing liquid metal-elastomer composite for robust soft-matter robotics and electronics. Nat. Mater. 2018, 17, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Ladd, C.; So, J.H.; Muth, J.; Dickey, M.D. 3D printing of free standing liquid metal microstructures. Adv. Mater. 2013, 25, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, Y.; Chen, Y.; Yang, J.; Zhu, T.; Zhu, D.; He, C.; Liu, Y.; Handschuh-Wang, S.; Zhou, X. Liquid metal sponges for mechanically durable, all-soft, electrical conductors. J. Mater. Chem. C 2017, 5, 1586–1590. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ou, J.Z.; Tang, S.Y.; Sivan, V.; David, D.Y.; Latham, K.; Khoshmanesh, K.; Arnan, M.; O’Mullane, A.P.; Kalantar-zadeh, K. Liquid metal/metal oxide frameworks. Adv. Funct. Mater. 2014, 24, 3799–3807. [Google Scholar] [CrossRef]
- Thelen, J.; Dickey, M.D.; Ward, T. A study of the production and reversible stability of EGaIn liquid metal microspheres using flow focusing. Lab Chip 2012, 12, 3961–3967. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Yi, L.T.; Liu, J. Channelless fabrication for large-scale preparation of room temperature liquid metal droplets. Adv. Eng. Mater. 2014, 16, 255–262. [Google Scholar] [CrossRef]
- Tang, S.Y.; Joshipura, I.D.; Lin, Y.; Kalantar-Zadeh, K.; Mitchell, A.; Khoshmanesh, K.; Dickey, M.D. Liquid-Metal Microdroplets Formed Dynamically with Electrical Control of Size and Rate. Adv. Mater. 2016, 28, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hutter, T.; Bauer, W.-A.C.; Elliott, S.R.; Huck, W.T.S. Formation of spherical and non-spherical eutectic gallium-indium liquid-metal microdroplets in microfluidic channels at room temperature. Adv. Funct. Mater. 2012, 22, 2624–2631. [Google Scholar] [CrossRef]
- Liu, T.; Sen, P.; Kim, C.-J. Characterization of nontoxic liquid-metal alloy galinstan for applications in microdevices. J. Microelectromechanical Syst. 2012, 21, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Hohman, J.N.; Kim, M.; Wadsworth, G.A.; Bednar, H.R.; Jiang, J.; LeThai, M.A.; Weiss, P.S. Directing substrate morphology via self-assembly: Ligand-mediated scission of gallium-indium microspheres to the nanoscale. Nano Lett. 2011, 11, 5104–5110. [Google Scholar] [CrossRef] [PubMed]
- Finkenauer, L.R.; Lu, Q.; Hakem, I.F.; Majidi, C.; Bockstaller, M.R. Analysis of the Efficiency of Surfactant-Mediated Stabilization Reactions of EGaIn Nanodroplets. Langmuir 2017, 33, 9703–9710. [Google Scholar] [CrossRef] [PubMed]
- Lear, T.R.; Hyun, S.-H.; Boley, J.W.; White, E.L.; Thompson, D.H.; Kramer, R.K. Liquid metal particle popping: Macroscale to nanoscale. Extreme Mech. Lett. 2017, 13, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-transformable liquid metal nanoparticles in aqueous solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.J.; Huggins, R.A. Electrochemical investigation of the lithium-gallium system. J. Electrochem. Soc. 1981, 128, 1636–1641. [Google Scholar] [CrossRef]
- Deshpande, R.D.; Li, J.; Cheng, Y.-T.; Verbrugge, M.W. Liquid metal alloys as self-healing negative electrodes for lithium ion batteries. J. Electrochem. Soc. 2011, 158, 845–849. [Google Scholar] [CrossRef]
- Yarema, M.; Worle, M.; Rossell, M.D.; Erni, R.; Caputo, R.; Protesescu, L.; Kravchyk, K.V.; Dirin, D.N.; Lienau, K.; von Rohr, F.; et al. Monodisperse colloidal gallium nanoparticles: Synthesis, low temperature crystallization, surface plasmon resonance and Li-ion storage. J. Am. Chem. Soc. 2014, 136, 12422–12430. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Fei, H.; Tian, Y.; An, Y.; Zeng, G.; Feng, J.; Qian, Y. Room-temperature liquid metal confined in MXene paper as a flexible, freestanding, and binder-free anode for next-generation lithium-ion batteries. Small 2019, 15, e1903214. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zong, J.; Wang, X.; Cao, Q.; Zhang, D.; Jiang, J.-Z. Production of Uniformly Sized Gallium-Based Liquid Alloy Nanodroplets via Ultrasonic Method and Their Li-Ion Storage. Materials 2021, 14, 1759. https://doi.org/10.3390/ma14071759
Huang C, Zong J, Wang X, Cao Q, Zhang D, Jiang J-Z. Production of Uniformly Sized Gallium-Based Liquid Alloy Nanodroplets via Ultrasonic Method and Their Li-Ion Storage. Materials. 2021; 14(7):1759. https://doi.org/10.3390/ma14071759
Chicago/Turabian StyleHuang, Chenghao, Junjie Zong, Xiaodong Wang, Qingpin Cao, Dongxian Zhang, and Jian-Zhong Jiang. 2021. "Production of Uniformly Sized Gallium-Based Liquid Alloy Nanodroplets via Ultrasonic Method and Their Li-Ion Storage" Materials 14, no. 7: 1759. https://doi.org/10.3390/ma14071759







