Novel Combinatorial Strategy Using Thermal Inkjet Bioprinting, Chemotherapy, and Radiation on Human Breast Cancer Cells; an In-Vitro Cell Viability Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug Preparation and CC50 Determination
2.2. Cell Culture
2.3. Bioprinting Process
2.4. Concurrent Treatment with Palbociclib, Letrozole, and Radiotherapy
2.5. Cytotoxicity Quantification
2.6. Statistical Analysis
3. Results
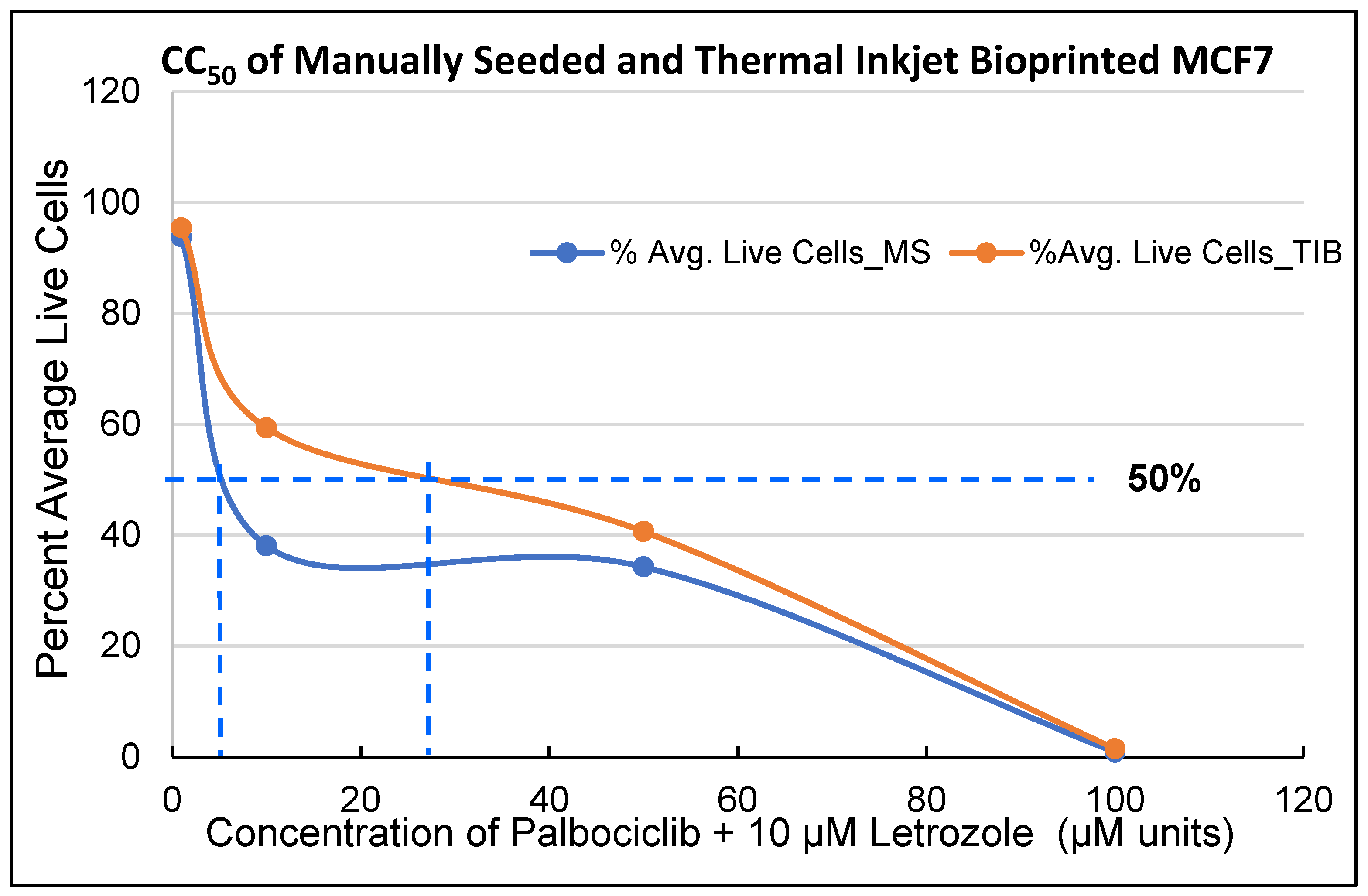
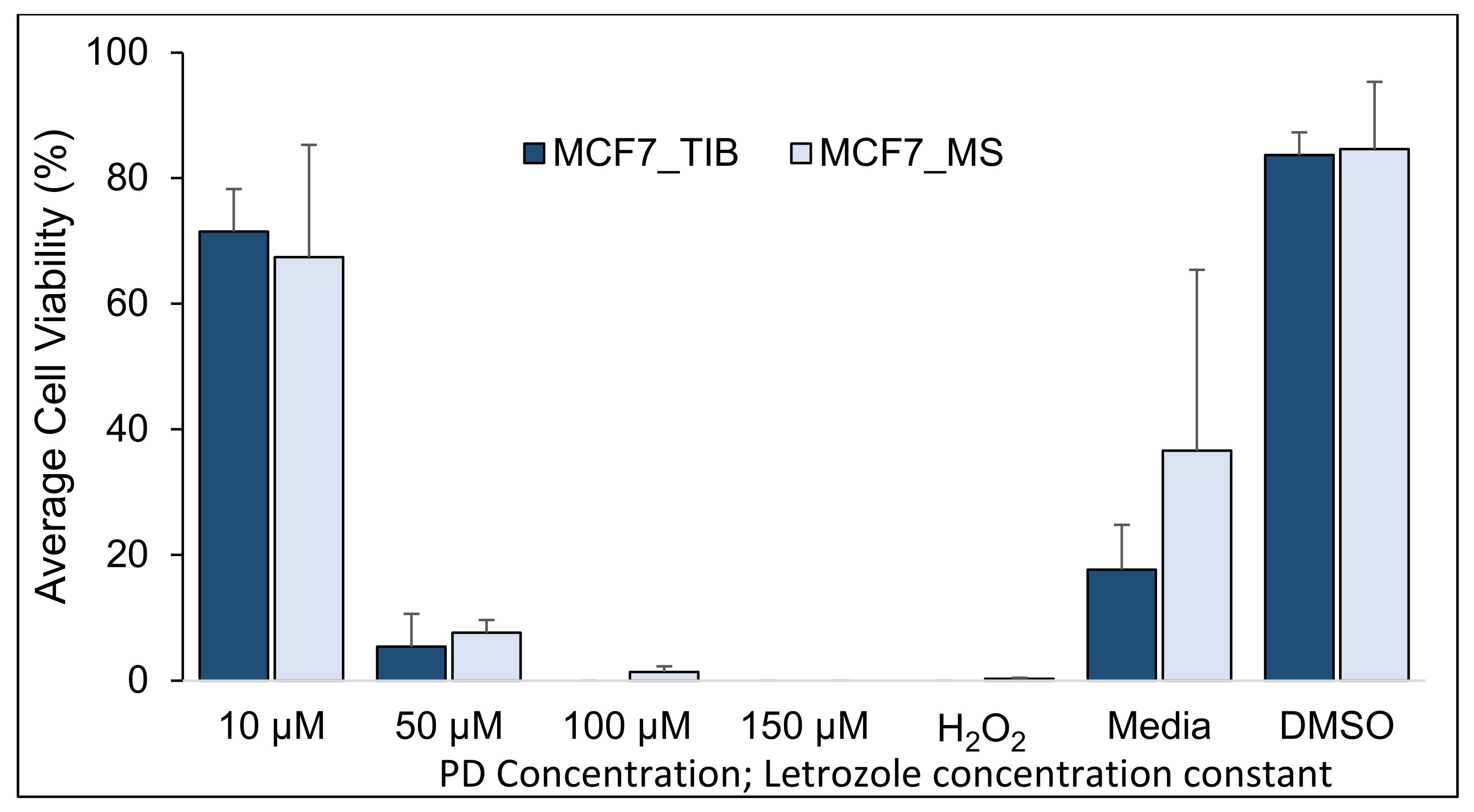
3.1. Palbociclib-Letrozole and Radiotherapy Treatment of Thermal Inkjet Bioprinted and Manually Seeded MCF7 Breast Cancer Cells (BCCs)
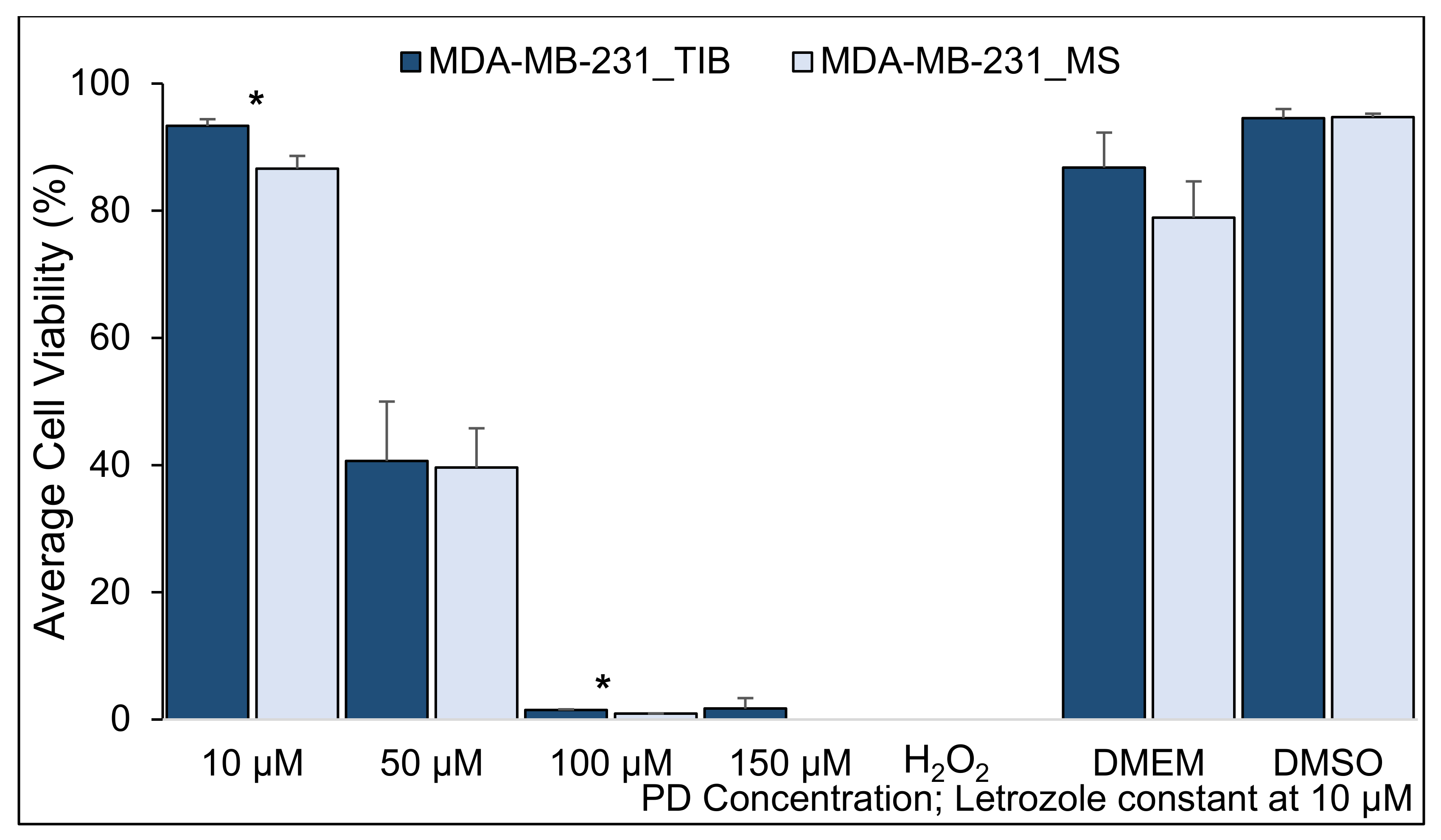
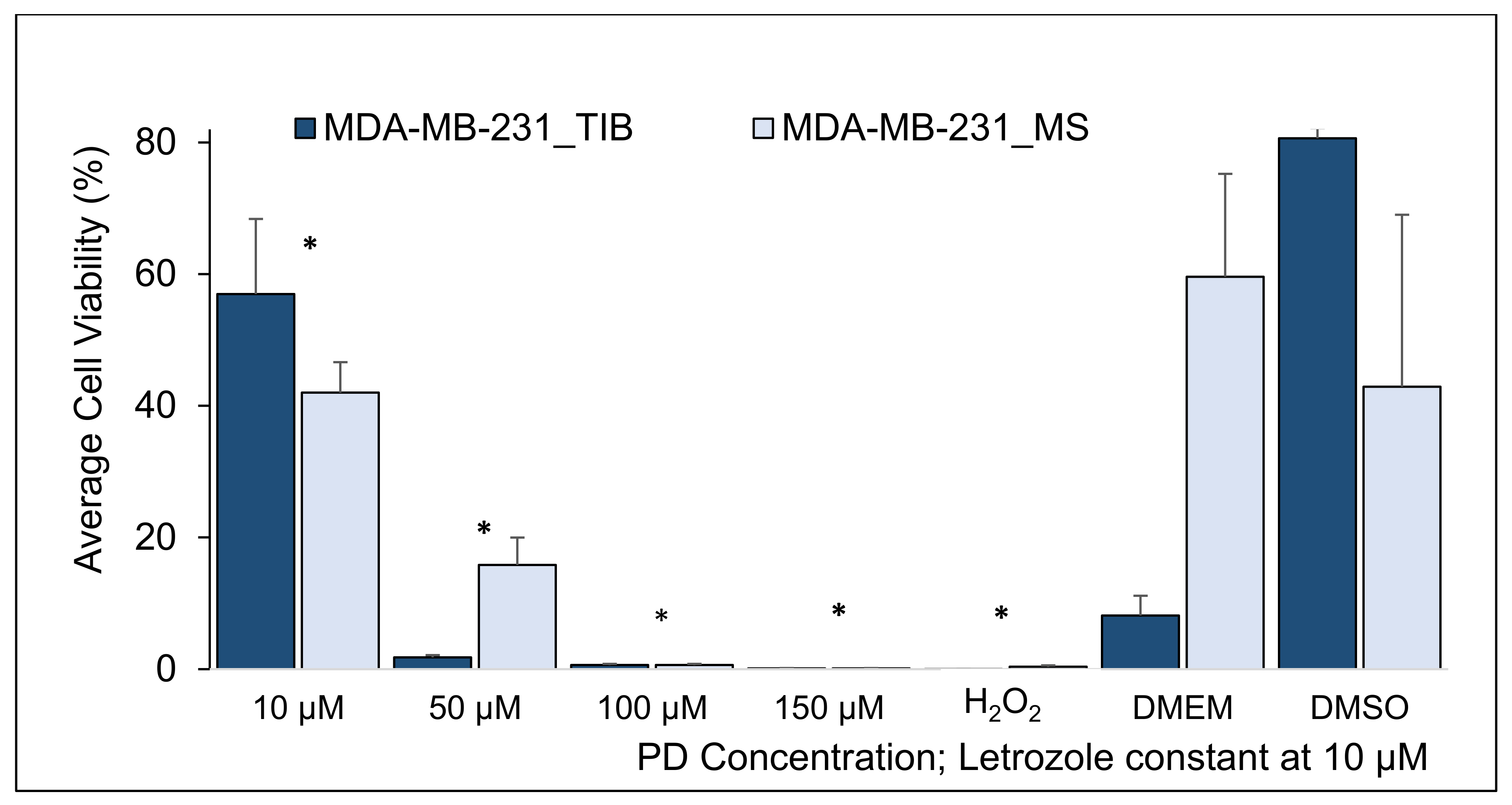
3.2. Palbociclib-Letrozole and Radiotherapy Treatment of Thermal Inkjet Bioprinted and Manually Seeded MDA-MB-231 Breast Cancer Cells
3.3. Palbociclib-Letrozole and Radiotherapy Treatment of Thermal Inkjet Bioprinted and Manually Seeded MCF-10A Breast Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2− metastatic breast cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Food & Drug Administration. Palbociclib (Ibrance). 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance (accessed on 16 December 2021).
- Chowdhary, M.; Sen, N.; Chowdhary, A.; Usha, L.; Cobleigh, M.A.; Wang, D.; Patel, K.R.; Barry, P.N.; Rao, R.D. Safety and efficacy of palbociclib and radiation therapy in patients with metastatic breast cancer: Initial results of a novel combination. Adv. Radiat. Oncol. 2019, 4, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, S.; Cottu, P.; Kirova, Y.M. Preliminary results of the association of Palbociclib and radiotherapy in metastatic breast cancer patients. Radiother. Oncol. 2018, 126, 181. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Shikama, N.; Sasai, K. Severe acute radiation-induced enterocolitis after combined palbociclib and palliative radiotherapy treatment. Radiother. Oncol. 2018, 131, 240–241. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar]
- Wilson, W.C., Jr.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Rincon, J.; Dones, A.; de Maria, C.; Gonzales, R.; Boland, T. In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng. Part A 2014, 21, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Mohl, J.E.; Gutierrez, D.A.; Varela-Ramirez, A.; Boland, T. Thermal Bioprinting Causes Ample Alterations of Expression of LUCAT1, IL6, CCL26 and NRN1L Genes and Massive Phosphorylation of Critical Oncogenic Drug Resistance Pathways in Breast Cancer Cells. Front. Bioeng. Biotechnol. 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Unutmaz, D.; Ozbolat, I.T. Bioprinting towards physiologically relevant tissue models for pharmaceutics. Trends Biotechnol. 2016, 34, 722–732. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, W.; Zhu, J.-M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef]
- Hashizume, R.; Zhang, A.; Mueller, S.; Prados, M.D.; Lulla, R.R.; Goldman, S.; Saratsis, A.M.; Mazar, A.P.; Stegh, A.H.; Cheng, S.Y.; et al. Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro-Oncology 2016, 18, 1519–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lema, C.; Varela-Ramirez, A.; Aguilera, R.J. Differential nuclear staining assay for high-throughput screening to identify cytotoxic compounds. Curr. Cell. Biochem. 2011, 1, 1–14. [Google Scholar] [PubMed]
- Robles-Escajeda, E.; Das, U.; Ortega, N.M.; Parra, K.; Francia, G.; Dimmock, J.R.; Varela-Ramirez, A.; Aguilera, R.J. A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells. Cell. Oncol. 2016, 39, 265–277. [Google Scholar] [CrossRef]
- Iglesias-Figueroa, B.F.; Siqueiros-Cendón, T.S.; Gutierrez, D.A.; Aguilera, R.J.; Espinoza-Sánchez, E.A.; Arévalo-Gallegos, S.; Varela-Ramirez, A.; Rascón-Cruz, Q. Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells. Apoptosis 2019, 24, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef]
- Campbell, A.; Philipovskiy, A.; Heydarian, R.; Varela-Ramirez, A.; Gutierrez, D.A.; Solis, L.H.; Furth, M.E.; Boland, T. 2D and 3D thermally bioprinted human MCF-7 breast cancer cells: A promising model for drug discovery. J. Clin. Oncol. 2019, 37 (Suppl. S15), 2605. [Google Scholar] [CrossRef]
- Hurwitz, M.; Stauffer, P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin. Oncol. 2014, 41, 714–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henríquez, G.; Mendez, L.; Varela-Ramirez, A.; Guerrero, E.; Narayan, M. Neuroprotective Effect of Brazilin on Amyloid β (25-35)-Induced Pathology in a Human Neuroblastoma Model. ACS Omega 2020, 5, 13785–13792. [Google Scholar] [CrossRef]
- Maruko, A.; Ohtake, Y.; Kawaguchi, M.; Kobayashi, T.; Baba, T.; Kuwahara, Y.; Nakagawa, H.; Shimura, T.; Fukumoto, M.; Ohkubo, Y. X-radiation-induced down-regulation of the EGF receptor in primary cultured rat hepatocytes. Radiat. Res. 2010, 173, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Hsieh, F.-S.; Wang, C.-Y.; Chen, L.-J.; Chang, S.-S.; Tsai, M.-H.; Hung, M.-H.; Kuo, C.-W.; Shih, C.-T.; Chao, T.-I.; et al. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia–mutated kinase–mediated DNA damage response. Eur. J. Cancer 2018, 102, 10–22. [Google Scholar] [CrossRef] [PubMed]
- De Dueñas, E.M.; Gavila-Gregori, J.; Olmos-Anton, S.; Santaballa-Bertran, A.; Lluch-Hernandez, A.; Espinal-Domínguez, E.J.; Rivero-Silva, M.; Llombart-Cussac, A. Preclinical and clinical development of palbociclib and future perspectives. Clin. Transl. Oncol. 2018, 20, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Hahn, E. The enhanced killing of irradiated HeLa cells in synchronous culture by hyperthermia. Radiat. Res. 1976, 66, 337–345. [Google Scholar] [CrossRef]
- Burnet, N.G.; Nyman, J.; Turesson, I.; Wurm, R.; Yarnold, J.R.; Peacock, J.H. Prediction of normal-tissue tolerance to radiotherapy from in-vitro cellular radiation sensitivity. Lancet 1992, 339, 1570–1571. [Google Scholar] [CrossRef]
- Ehab, M.; Elbaz, M. Profile of palbociclib in the treatment of metastatic breast cancer. Breast Cancer Targets Ther. 2016, 8, 83. [Google Scholar]
- Vidula, N.; Rugo, H.S. Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: A review of preclinical and clinical data. Clin. Breast Cancer 2016, 16, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhao, F.; Zhu, D.; Han, J.; Chen, H.; Cai, Y.; Chen, Z.; Xie, W. Long non-coding RNA LUCAT1 promotes proliferation and invasion in clear cell renal cell carcinoma through AKT/GSK-3β signaling pathway. Cell. Physiol. Biochem. 2018, 48, 891–904. [Google Scholar] [CrossRef]
- Zheng, A.; Song, X.; Zhang, L.; Zhao, L.; Mao, X.; Wei, M.; Jin, F. Long non-coding RNA LUCAT1/miR-5582-3p/TCF7L2 axis regulates breast cancer stemness via Wnt/β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Radiation | MS | TIB |
|---|---|---|
| PD CC50 values for MCF7 cells | ||
| 0 Gy | 8 µM | 30 µM |
| 10 Gy | 23 µM | 40 µM |
| 20 Gy | 22 µM | 23 µM |
| PD CC50 values for MDA-MB-231 cells | ||
| 0 Gy | 41 µM | 43 µM |
| 10 Gy | 36 µM | 39 µM |
| 20 Gy | 8 µM | 15 µM |
| PD CC50 values for MCF-10 A cells | ||
| 0 Gy | 34 µM | 40 µM |
| 10 Gy | 75 µM | 80 µM |
| 20 Gy | 29 µM | 31 µM |
| PD Concentration Let = 10 µM | Mean % of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI | T-Value | p-Value |
|---|---|---|---|---|---|---|
| 10 µM | 96.8 | 89.5 | 7.3 | (5.3, 9.2) | 7.7 | <0.001 |
| 50 µM | 47.0 | 34.5 | 12.5 | (4.7, 20.4) | 3.4 | 0.004 |
| 100 µM | 13.9 | 5.4 | 8.5 | (0.9, 16.1) | 2.3 | 0.029 |
| H2O2 | 0.6 | 0.4 | 0.2 | (7.8, 32.4) | 0.6 | 0.545 |
| Media | 97.7 | 92.3 | 5.4 | 0.3, 10.5) | 2.7 | 0.042 |
| DMSO | 93.5 | 98.4 | −5.0 | (−23.0, 13.0) | −1.2 | 0.355 |
| PD Concentration Let = 10 µM | Mean % of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI | T-Value | p-Value |
|---|---|---|---|---|---|---|
| 10 µM | 87.1 | 60.2 | 26.9 | (19.0, 34.7) | 8.1 | 0.0001 |
| 50 µM | 38.1 | 29.6 | 8.5 | (1.3, 15.6) | 2.9 | 0.027 |
| 100 µM | 14.2 | 18.0 | −3.8 | (−7.8, 0.2) | −2.2 | 0.061 |
| H2O2 | 12.1 | 15.4 | −3.3 | (−16.8, 10.2) | −0. 8 | 0.494 |
| Media | 81.1 | 64.6 | 16.6 | (−10.9, 44.1) | 2.6 | 0.123 |
| DMSO | 84.0 | 68.9 | 15.1 | (−10.6, 40.8) | 2.5 | 0.127 |
| PD Concentration Let = 10 µM | Mean % of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI | T-Value | p-Value |
|---|---|---|---|---|---|---|
| 10 µM | 71.5 | 67.4 | 4.1 | (−17.9, 26.1) | 0.5 | 0.654 |
| 50 µM | 5.4 | 7.6 | −2.2 | (−8.7, 4.2) | −0.9 | 0.419 |
| H2O2 | 0.0 | 0.3 | 0.08 | (−0.5, 0.01) | −3.0 | 0.057 |
| DMEM | 17.6 | 36.6 | −18.9 | (−92.6, 54.7) | −1.1 | 0.384 |
| DMSO | 83.7 | 84.6 | −1.0 | (−29.0, 27.2) | −0.1 | 0.898 |
| Two-Sample t-Tests, MDA-MB-231 Cells, RT= 0 | H0: μ1 − µ2 = 0, H1: μ1 − µ2 ≠ 0 | |||||||
|---|---|---|---|---|---|---|---|
| PD Concentration Let = 10 µM | Mean %of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI (Lower-Upper) | T-Value | p-Value (2-Tailed) | |
| 10 µM | 93.3 | 86.6 | 6.7 | 3.6 | 9.8 | 7.0 | 0.004 |
| 50 µM | 40.7 | 39.6 | 1.1 | −14.8 | 16.9 | 0.2 | 0.850 |
| 100 µM | 1.5 | 0.9 | 0.6 | 0.4 | 0.8 | 8.9 | 0.003 |
| 150 µM | 1.7 | 0.0 | 1.7 | −0.9 | 4.4 | 2.1 | 0.13 |
| Media | 86.8 | 78.9 | 7.9 | −6.7 | 22.4 | 1.7 | 0.18 |
| DMSO | 94.6 | 94.7 | −0.2 | −4.0 | 3.7 | −0.2 | 0.88 |
| PD Concentration Let = 10 µM | Two-Sample t-Tests, MDA-MB-231 Cells, RT = 20 Gy | H0: μ1 − µ2 = 0, H1: μ1 − µ2 ≠ 0 | |||||
|---|---|---|---|---|---|---|
| Mean %of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI (Lower-Upper) | T-Value | p-Value | |
| 10 µM | 57.0 | 42.0 | 15 | (0.8, 29.1) | 2.7 | 0.04 |
| 50 µM | 1.8 | 15.8 | −14 | (−19.2, −8.8) | −7.5 | 0.002 |
| 100 µM | 0.6 | 1.8 | −1.3 | (−2.2, −0.4) | −3.9 | 0.018 |
| 150 µM | 0.1 | 1.4 | −1.3 | (−2.4, −0.1) | −3.0 | 0.038 |
| H2O2 | 0.0 | 0.4 | −0.4 | (−0.6, −0.1) | −3.4 | 0.042 |
| DMEM | 8.1 | 59.6 | −51.4 | (−91.0, −11.9) | −5.6 | 0.031 |
| DMSO | 80.6 | 42.9 | 37.7 | (−27.2, 100) | 2.5 | 0.13 |
| Two-Sample t-Tests, MDA-MB-231 cells RT= 10 Gy | H0: μ1 − µ2 = 0, H1: μ1 − µ2 ≠0 | |||||||
|---|---|---|---|---|---|---|---|
| PD Concentration Let = 10 µM | Mean % of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI (Lower-Upper) | T-Value | p-Value (2-Tailed) | |
| 10 µM | 91.3 | 96.9 | −5.7 | −8.4 | −2.9 | −5.8 | 0.004 |
| 50 µM | 12.5 | 23.6 | −11.2 | −13.7 | −8.7 | −12.4 | <0.0001 |
| 100 µM | 5.9 | 5.1 | 0.8 | −6.6 | 8.2 | 0.3 | 0.78 |
| 150 µM | 0.1 | 0.5 | −0.4 | −1.3 | 0.5 | −1.2 | 0.3 |
| Media | 85.6 | 93.7 | −8.1 | −16.2 | 0.1 | −4.3 | 0.05 |
| DMSO | 95.9 | 99.1 | −3.2 | −6.3 | −0.1 | −4.4 | 0.05 |
| Two-Sample t-Tests, MCF-10A, RT= 0 | H0: μ1 − µ2 = 0 | H1: μ1 − µ2 ≠ 0 | ||||
|---|---|---|---|---|---|---|
| PD Concentration Let = 10 µm | Mean % of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI | T-Value | p-Value |
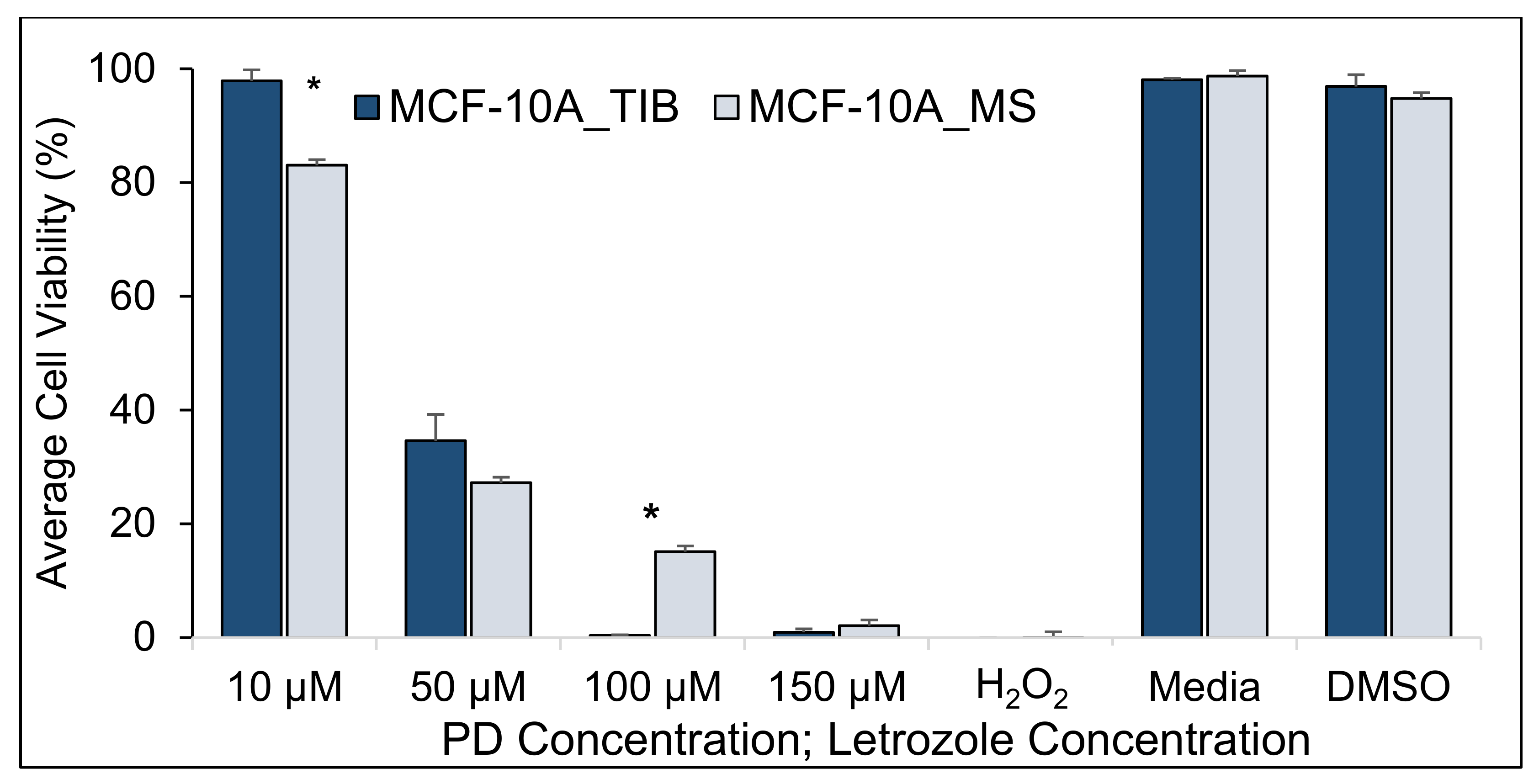
| 10 µM | 97.9 | 83.0 | 14.9 | (2.5, 27.1) | 3.8 | 0.03 |
| 50 µM | 34.6 | 27.2 | 7.4 | (−38.2, 53.1) | 0.7 | 0.55 |
| 100 µM | 0.3 | 15.1 | −14.7 | (−29.8, 0.3) | −4.2 | 0.05 |
| 150 µM | 0.9 | 2.1 | −1.2 | (−5.0, 2.7) | −1.3 | 0.32 |
| H2O2 | 0.0 | 0.00 | −0.01 | (−0.0, 0.0) | −2.0 | 0.18 |
| Media | 98.1 | 98.7 | −0.6 | (−15.1, 19.3) | −2.5 | 0.09 |
| DMSO | 96.9 | 94.8 | 2.1 | (−1.5, 0.2) | 0.5 | 0.65 |
| PD Concentration Let = 10 µM | Two-Sample t-Tests, MCF-10A, RT= 10 gray | H₀: μ₁ − µ₂ = 0 | H₁: μ₁ − µ₂ ≠ 0 | |||
|---|---|---|---|---|---|---|
| Mean % of Live Cells TIB | Mean % of Live Cells TIB | Difference | 95% CI | T-Value | p-Value | |
| 10 µM | 99.3 | 99.5 | −0.1 | (−0.8, 0.5) | −0.5 | 0.61 |
| 50 µM | 71.0 | 69.1 | 1.9 | (−0.9, 4.8) | 1.6 | 0.15 |
| 100 µM | 0.2 | 0.3 | −0.04 | (−0.2, 0.2) | −0.4 | 0.67 |
| 150 µM | 0.0 | 0.0 | −0.01 | (−0.1, 0.1) | −0.5 | 0.65 |
| H2O2 | 2.9 | 2.8 | 0.1 | (−1.8, 2.0) | 0.2 | 0.88 |
| Media | 99.6 | 99.0 | 0.6 | (−0.1, 1.3) | 3.4 | 0.07 |
| DMSO | 97.2 | 97.6 | −0.5 | (−1.1, 0.1) | −3.4 | 0.08 |
| PD Conc. Let = 10 µM | Two-Sample t-Tests, MCF-10A, RT= 20 Gray | H₀: μ₁ − µ₂ = 0 | H₁: μ₁ − µ₂ ≠ 0 | |||
|---|---|---|---|---|---|---|
| Mean % of Live Cells TIB | Mean % of Live Cells MS | Difference | 95% CI | T-Value | p-Value | |
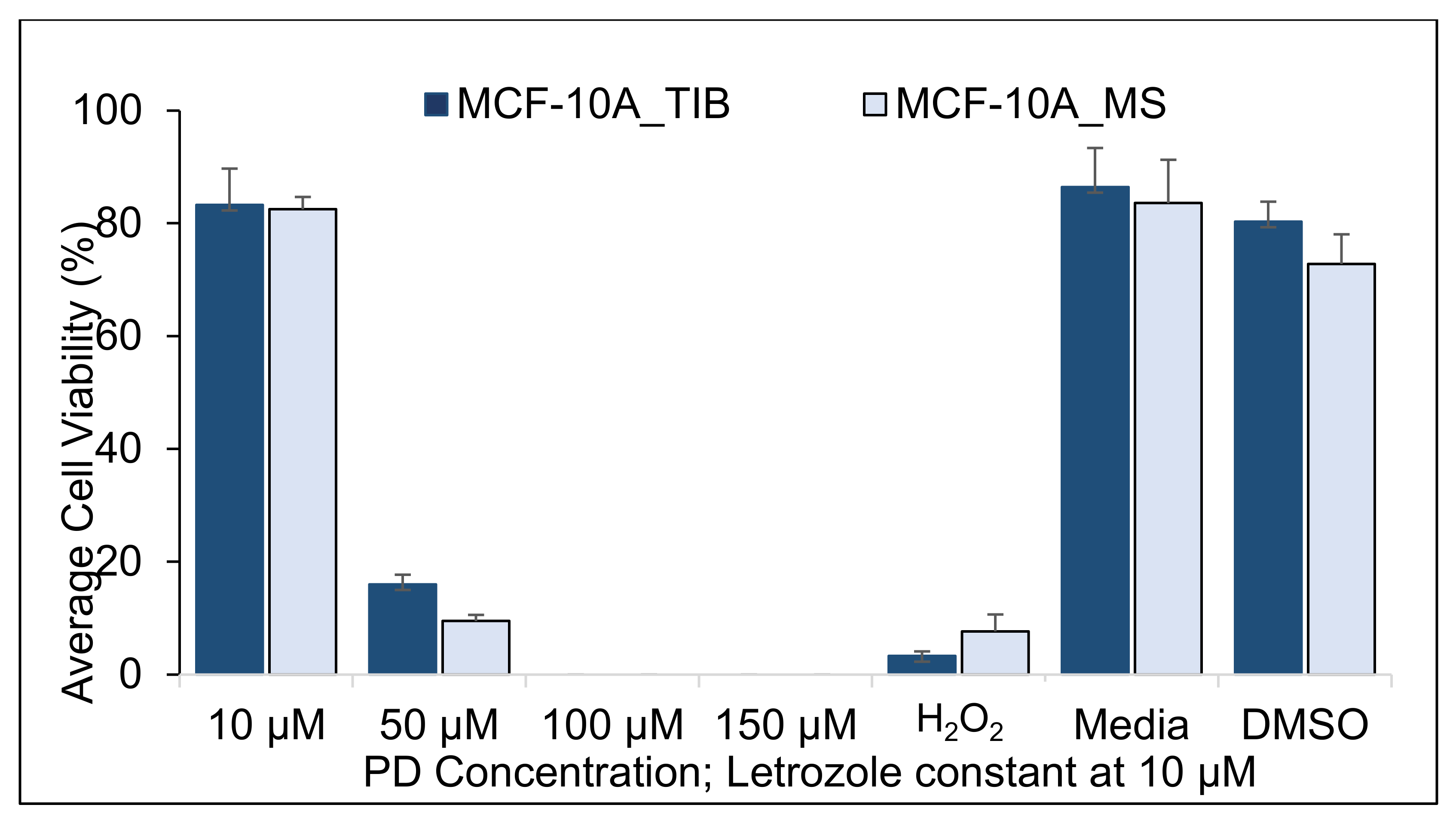
| 10 µM | 83.3 | 82.5 | 0.91 | (−7.2, 8.9) | 0.3 | 0.79 |
| 50 µM | 18.8 | 15.4 | 3.4 | (−3.8, 10.6) | 1.1 | 0.30 |
| H2O2 | 0.0 | 1.9 | −1.9 | (−7.4, 3.5) | −1.1 | 0.34 |
| Media | 86.4 | 83.6 | 2.9 | (−14.0, 23.6) | 0.5 | 0.66 |
| DMSO | 80.3 | 72.8 | 7.5 | (−9.1, 25.5) | 2.0 | 0.13 |
| Summary of TIB Compared to MS MCF7 Cell Samples | |
| With no RT | Slightly higher viability for all drug concentrations |
| With 10 Gray (Gy) | Slightly higher viability for lower drug concentrations |
| With 20 Gy | Not significant viability differences |
| Conclusion: TIB MCF7 cells at low PD-Let and RT dosages have a slightly higher viability than MS cells. Higher drug and RT dosages will kill all cells. | |
| Summary of TIB compared to MS MDA-MB-231 cell samples | |
| With no RT | Slightly higher viability at 10 μM of PD-Let concentrations |
| With 10 Gy | Less viability for lower drug concentrations |
| With 20 Gy | Higher viability for lowest drug concentration, lower viability for other drug concentrations |
| Conclusion: TIB MDA-MB-231 cell samples have higher viability than MS cells at the lowest drug concentrations. Radiation killed most of the cells at the two highest drug concentration but not at lower drug levels. | |
| Summary of TIB MCF-10A cells compared to MS samples. | |
| With no RT | Higher viability for low drug concentrations |
| With 10 Gy | Similar viability for all drug concentrations |
| With 20 Gy | Similar viability for all drug concentrations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, A.; Gutierrez, D.A.; Knight, C.; Vines, C.M.; Heydarian, R.; Philipovskiy, A.; Varela-Ramirez, A.; Boland, T. Novel Combinatorial Strategy Using Thermal Inkjet Bioprinting, Chemotherapy, and Radiation on Human Breast Cancer Cells; an In-Vitro Cell Viability Assessment. Materials 2021, 14, 7864. https://doi.org/10.3390/ma14247864
Campbell A, Gutierrez DA, Knight C, Vines CM, Heydarian R, Philipovskiy A, Varela-Ramirez A, Boland T. Novel Combinatorial Strategy Using Thermal Inkjet Bioprinting, Chemotherapy, and Radiation on Human Breast Cancer Cells; an In-Vitro Cell Viability Assessment. Materials. 2021; 14(24):7864. https://doi.org/10.3390/ma14247864
Chicago/Turabian StyleCampbell, Aleli, Denisse A. Gutierrez, Colin Knight, Charlotte M. Vines, Rosalinda Heydarian, Alexander Philipovskiy, Armando Varela-Ramirez, and Thomas Boland. 2021. "Novel Combinatorial Strategy Using Thermal Inkjet Bioprinting, Chemotherapy, and Radiation on Human Breast Cancer Cells; an In-Vitro Cell Viability Assessment" Materials 14, no. 24: 7864. https://doi.org/10.3390/ma14247864







