Mechanical Stability of Screw-Retained Monolithic and Bi-layer Posterior Hybrid Abutment Crowns after Thermomechanical Loading: An In Vitro Study
Abstract
:1. Introduction
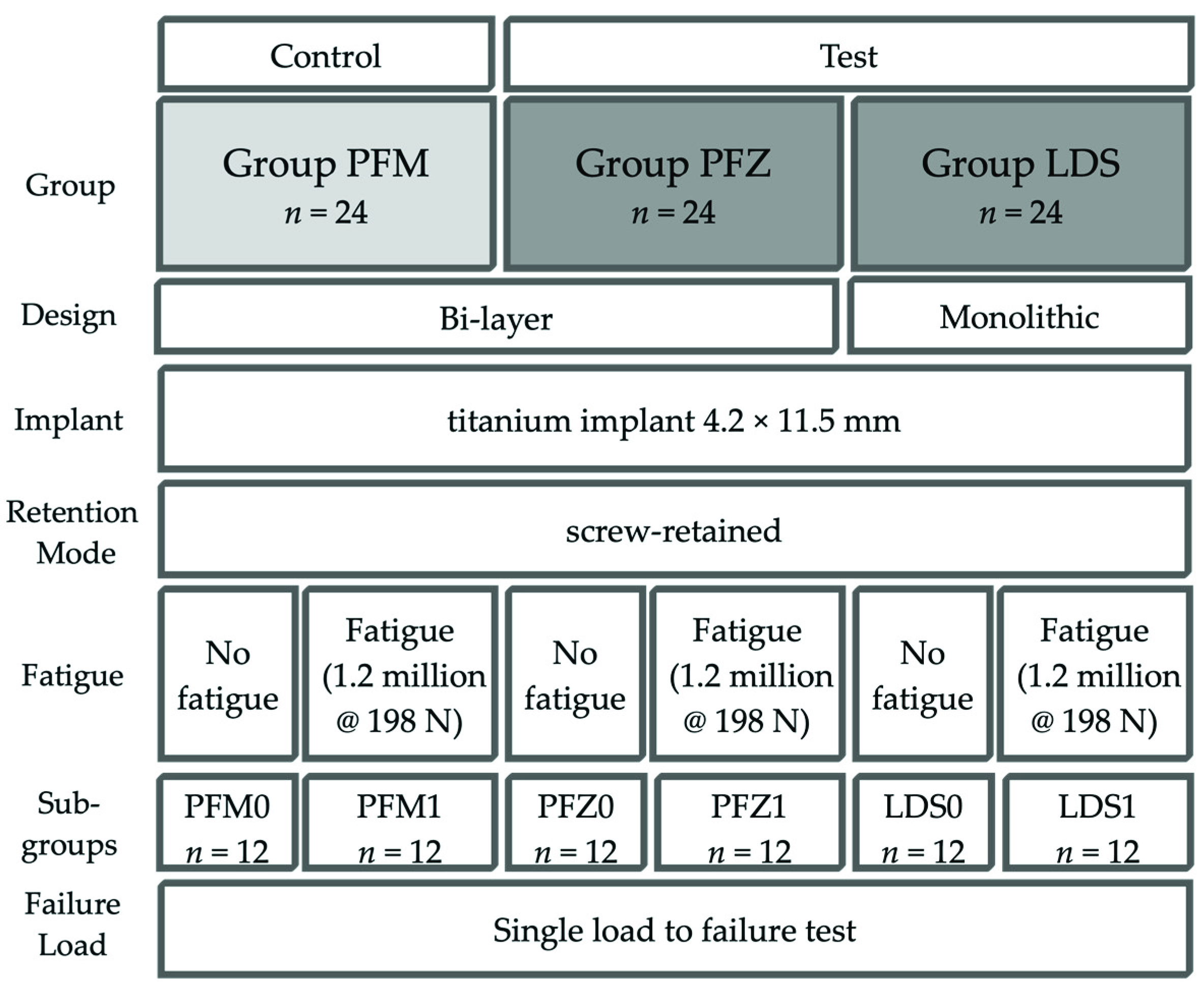
2. Materials and Methods
- Control Group PFM: bi-layer porcelain-fused-to-metal crown (Ivoclar non-precious metal 4All/IPS Inline PoM, both Ivoclar Vivadent, Schaan, Liechtenstein);
- Test Group PFZ: bi-layer porcelain-pressed-to-zirconia crown (Incoris ZI meso, Dentsply Sirona and IPS e.max ZirPress, Ivoclar Vivadent);
- Test Group LDS: monolithic LDS (IPS e.max CAD Abutment solutions, Ivoclar Vivadent).
2.1. Fabrication of Crowns
2.1.1. Group LDS
2.1.2. Group PFZ
2.1.3. Group PFM
2.2. Preparation of Specimens
2.3. Cyclic Loading and Single Load to Failure (SLF)
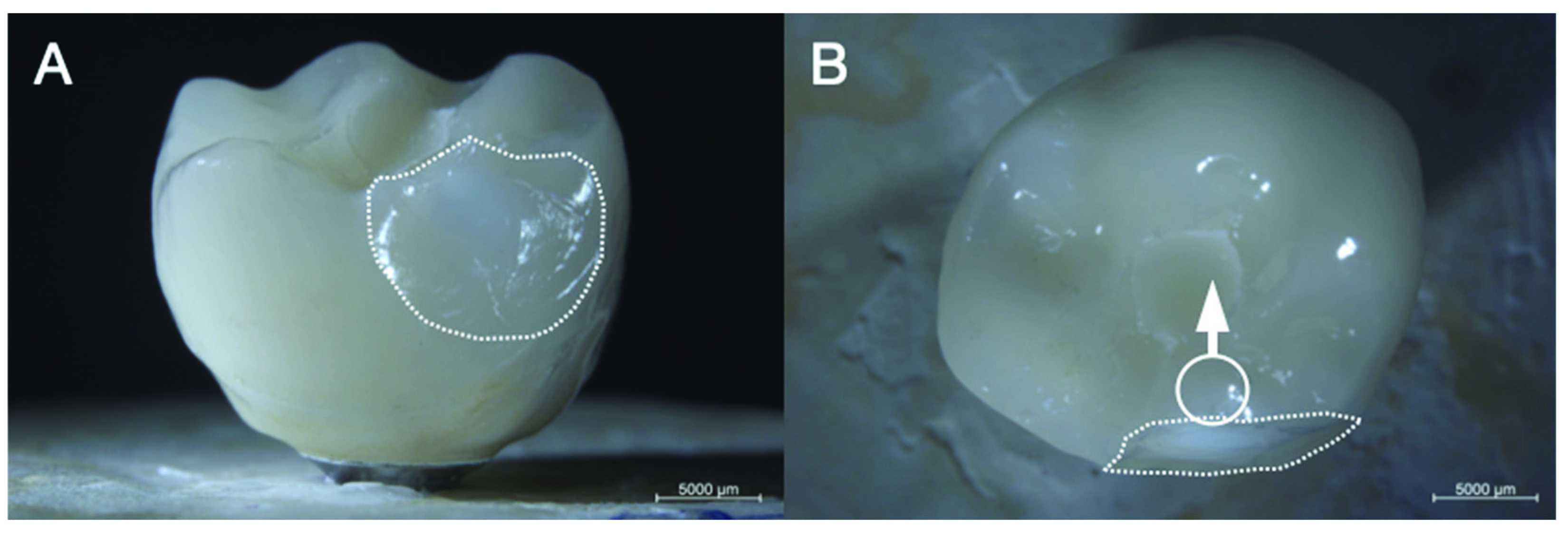
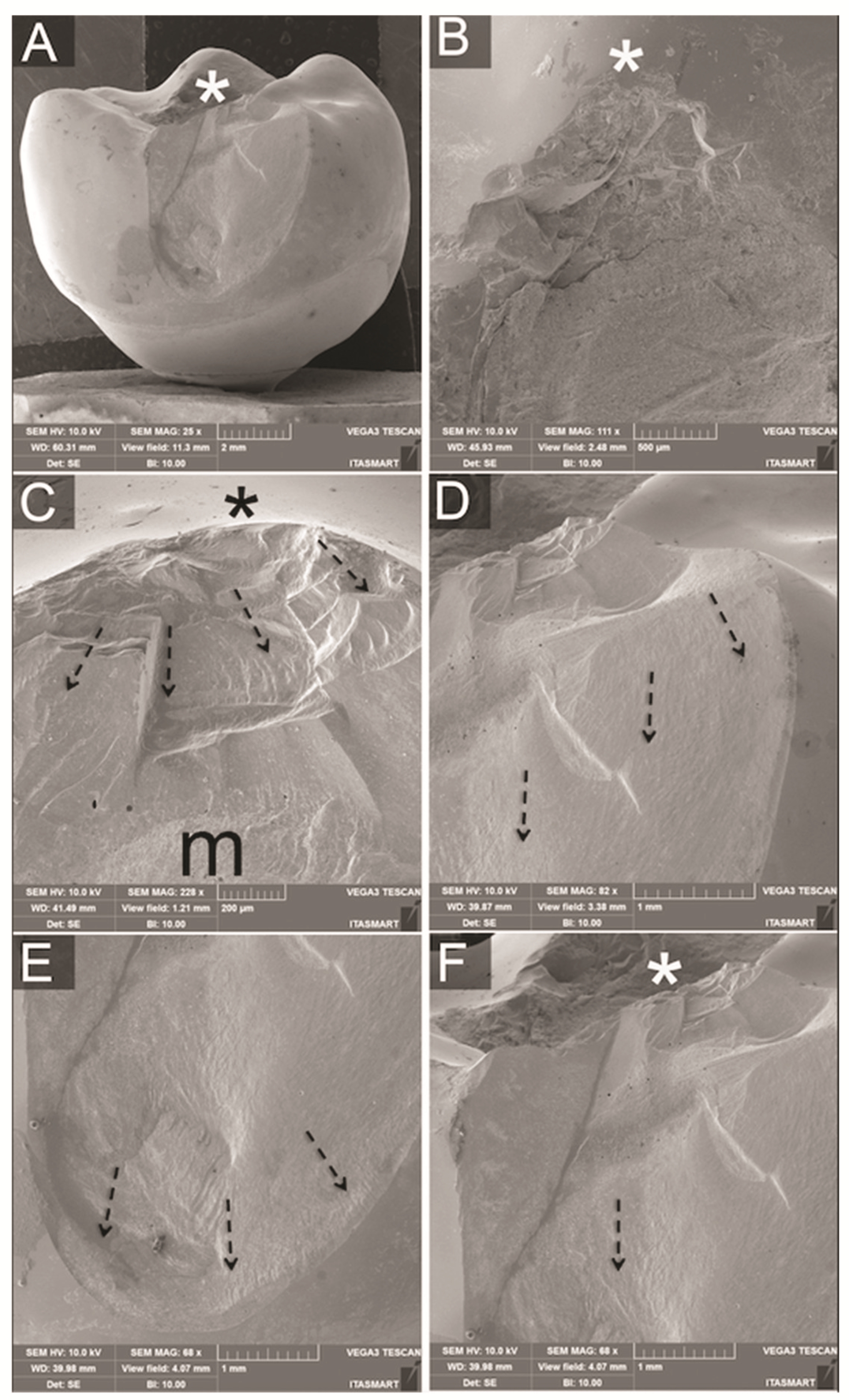
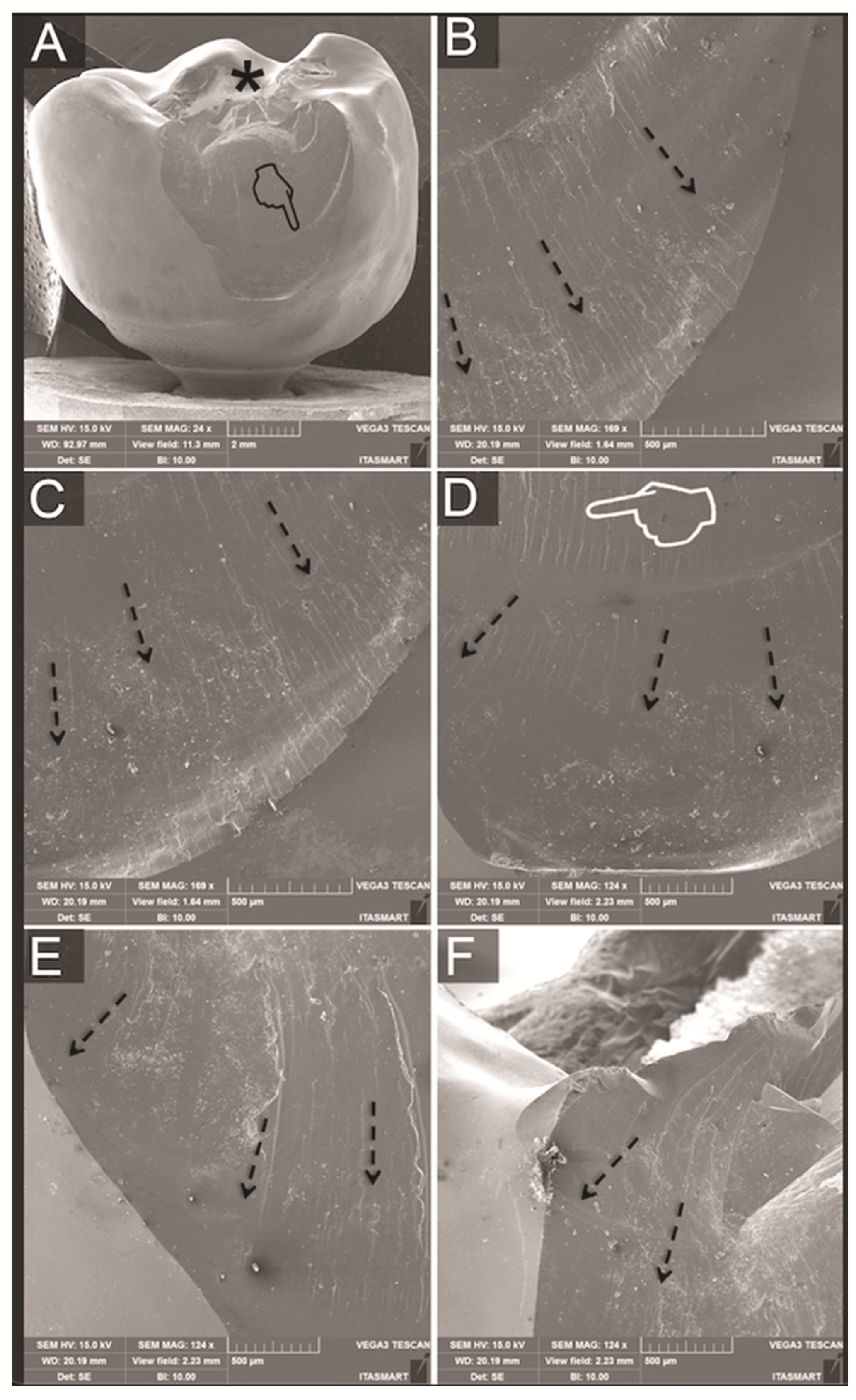
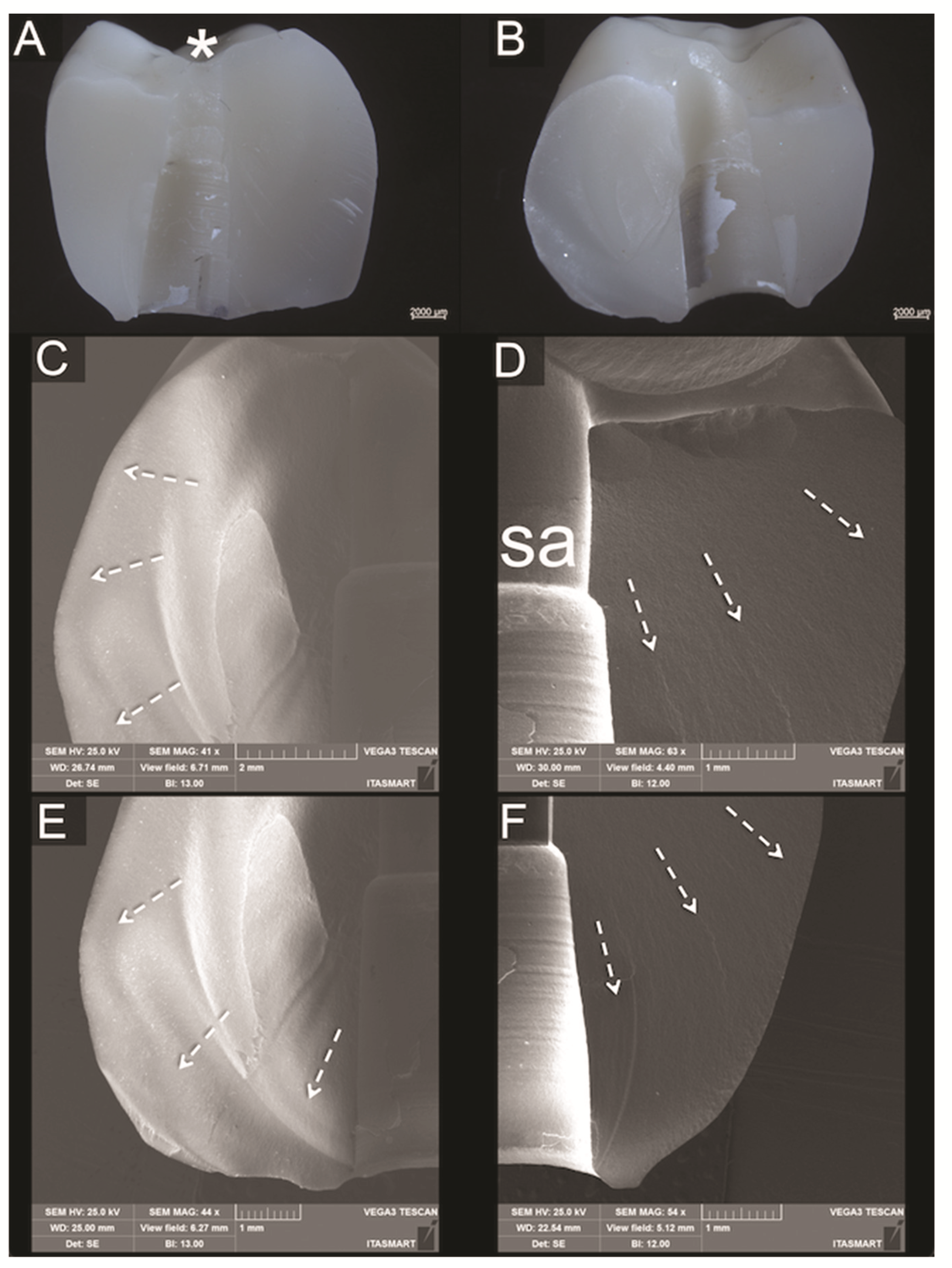
2.4. Failure Analysis
2.5. Statistical Analysis
3. Results
3.1. Cyclic Loading
3.2. Single-Load-to Failure
3.3. Fractographic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittneben, J.G.; Joda, T.; Weber, H.P.; Brägger, U. Screw retained vs. cement retained implant-supported fixed dental prosthesis. Periodontology 2000 2017, 73, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Rabel, K.; Spies, B.C.; Pieralli, S.; Vach, K.; Kohal, R.J. The clinical performance of all-ceramic implant-supported single crowns: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implant. Res. 2018, 29, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Joda, T.; Ferrari, M.; Gallucci, G.O.; Wittneben, J.G.; Brägger, U. Digital technology in fixed implant prosthodontics. Periodontology 2000 2017, 73, 178–192. [Google Scholar] [CrossRef]
- Mühlemann, S.; Kraus, R.D.; Hämmerle, C.H.F.; Thoma, D.S. Is the use of digital technologies for the fabrication of implant-supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clin. Oral. Implant. Res. 2018, 29, 184–195. [Google Scholar] [CrossRef]
- Joda, T.; Brägger, U. Time-efficiency analysis of the treatment with monolithic implant crowns in a digital workflow: A randomized controlled trial. Clin. Oral Implant. Res. 2016, 27, 1401–1406. [Google Scholar] [CrossRef]
- Derksen, W.; Tahmaseb, A.; Wismeijer, D. Randomized Clinical Trial comparing clinical adjustment times of CAD/CAM screw-retained posterior crowns on ti-base abutments created with digital or conventional impressions. One-year follow-up. Clin. Oral Implant. Res. 2021, 32, 962–970. [Google Scholar] [CrossRef]
- Joda, T.; Brägger, U. Complete digital workflow for the production of implant-supported single-unit monolithic crowns. Clin. Oral Implant. Res. 2014, 25, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Edelhoff, D.; Schweiger, J.; Prandtner, O.; Stimmelmayr, M.; Güth, J.F. Metal-free implant-supported single-tooth restorations. Part II: Hybrid abutment crowns and material selection. Quintessence Int. 2019, 50, 260–269. [Google Scholar] [CrossRef]
- De Oliveira, N.R.C.; Pigozzo, M.N.; Sesma, N.; Lagana, D.C. Clinical efficiency and patient preference of digital and conventional workflow for single implant crowns using immediate and regular digital impression: A meta-analysis. Clin. Oral Implant. Res. 2020, 31, 669–686. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Wei, D.; Di, P.; Lin, Y. Quantitative clinical adjustment analysis of posterior single implant crown in a chairside digital workflow: A randomized controlled trial. Clin. Oral Implant. Res. 2019, 30, 1059–1066. [Google Scholar] [CrossRef]
- Gierthmuehlen, P.C.; Berger, L.; Spitznagel, F.A. Monolithic Screw-Retained Lithium Disilicate Implant Crowns: Preliminary Data of a Prospective Cohort Study. Int. J. Prosthodont. 2020, 33, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Ferrari, M.; Bragger, U. Monolithic implant-supported lithium disilicate (LS2) crowns in a complete digital workflow: A prospective clinical trial with a 2-year follow-up. Clin. Implant. Dent. Relat Res. 2017, 19, 505–511. [Google Scholar] [CrossRef]
- De Angelis, P.; Passarelli, P.C.; Gasparini, G.; Boniello, R.; D’Amato, G.; De Angelis, S. Monolithic CAD-CAM lithium disilicate versus monolithic CAD-CAM zirconia for single implant-supported posterior crowns using a digital workflow: A 3-year cross-sectional retrospective study. J. Prosthet Dent. 2020, 123, 252–256. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Sailer, I.; Latyshev, A.; Rabel, K.; Kohal, R.J.; Karasan, D. A systematic review and meta-analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all-ceramic implant-supported single crowns. Clin. Oral. Implant. Res. 2021, 32, 254–288. [Google Scholar] [CrossRef]
- Att, W.; Kurun, S.; Gerds, T.; Strub, J.R. Fracture resistance of single-tooth implant-supported all-ceramic restorations: An in vitro study. J. Prosthet. Dent. 2006, 95, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Ashman, R.B.; Rho, J.Y. Elastic modulus of trabecular bone material. J. Biomech. 1988, 21, 177–181. [Google Scholar] [CrossRef]
- Farina, A.P.; Spazzin, A.O.; Consani, R.L.; Mesquita, M.F. Screw joint stability after the application of retorque in implant-supported dentures under simulated masticatory conditions. J. Prosthet. Dent. 2014, 111, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Spazzin, A.O.; Henrique, G.E.; Nobilo, M.A.; Consani, R.L.; Correr-Sobrinho, L.; Mesquita, M.F. Effect of retorque on loosening torque of prosthetic screws under two levels of fit of implant-supported dentures. Braz. Dent. J. 2010, 21, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Kern, M.; Strub, J.R.; Lu, X.Y. Wear of composite resin veneering materials in a dual-axis chewing simulator. J. Oral. Rehabil. 1999, 26, 372–378. [Google Scholar] [CrossRef]
- Delong, R.; Sakaguchi, R.L.; Douglas, W.H.; Pintado, M.R. The wear of dental amlgam in an artifical mouth: A clinical correlation. Dent. Mater. 1985, 1, 238–242. [Google Scholar] [CrossRef]
- Delong, R.; Douglas, W.H. Development of an Artifical Oral Environment for the Testing of Dental Restoratives: Bi-Axial Force and Movement Control. J. Dent. Res. 1983, 62, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Krejci, D.; Albert, P.; Lutz, F. The influence of antagonist standarization on wear. Dent. Res. 1999, 78, 713–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, G.D. Fractography of Ceramics and Glasses; National Institute of Standards and Technology: Gaithersburg, MA, USA, 2016. [Google Scholar] [CrossRef]
- Hosseini, M.; Kleven, E.; Gotfredsen, K. Fracture mode during cyclic loading of implant-supported single-tooth restorations. J. Prosthet Dent. 2012, 108, 74–83. [Google Scholar] [CrossRef]
- Obermeier, M.; Ristow, O.; Erdelt, K.; Beuer, F. Mechanical performance of cement- and screw-retained all-ceramic single crowns on dental implants. Clin. Oral. Investig. 2018, 22, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Guess, P.C.; Bonfante, E.A.; Silva, N.R.; Coelho, P.G.; Thompson, V.P. Effect of core design and veneering technique on damage and reliability of Y-TZP-supported crowns. Dent. Mater. 2013, 29, 307–316. [Google Scholar] [CrossRef]
- Kamio, S.; Komine, F.; Taguchi, K.; Iwasaki, T.; Blatz, M.B.; Matsumura, H. Effects of framework design and layering material on fracture strength of implant-supported zirconia-based molar crowns. Clin. Oral Implant. Res. 2015, 26, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Preis, V.; Letsch, C.; Handel, G.; Behr, M.; Schneider-Feyrer, S.; Rosentritt, M. Influence of substructure design, veneer application technique, and firing regime on the in vitro performance of molar zirconia crowns. Dent. Mater. 2013, 29, e113–e121. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.B.; Quinn, G.D.; Sundar, V. Fracture Toughness of Veneering Ceramics for Fused to Metal (PFM) and Zirconia Dental Restorative Materials. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 343–352. [Google Scholar] [CrossRef]
- Waltimo, A.; Kemppainen, P.; Kononen, M. Maximal contraction force and endurance of human jaw-closing muscles in isometric clenching. Scand. J. Dent. Res. 1993, 101, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Varga, S.; Spalj, S.; Lapter Varga, M.; Anic Milosevic, S.; Mestrovic, S.; Slaj, M. Maximum voluntary molar bite force in subjects with normal occlusion. Eur. J. Orthod. 2011, 33, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Bankoglu Gungor, M.; Karakoca Nemli, S.; Yilmaz, H.; Aydin, C. Fracture resistance of different implant supported ceramic abutment/crown systems. Eur. Oral. Res. 2019, 53, 80–87. [Google Scholar] [CrossRef]
- Sailer, I.; Muhlemann, S.; Zwahlen, M.; Hammerle, C.H.; Schneider, D. Cemented and screw-retained implant reconstructions: A systematic review of the survival and complication rates. Clin. Oral Implant. Res. 2012, 23, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Weigl, P.; Saarepera, K.; Hinrikus, K.; Wu, Y.; Trimpou, G.; Lorenz, J. Screw-retained monolithic zirconia vs. cemented porcelain-fused-to-metal implant crowns: A prospective randomized clinical trial in split-mouth design. Clin. Oral Investig. 2019, 23, 1067–1075. [Google Scholar] [CrossRef]
- Wolfart, S.; Rittich, A.; Gross, K.; Hartkamp, O.; von der Stuck, A.; Raith, S.; Reich, S. Cemented versus screw-retained posterior implant-supported single crowns: A 24-month randomized controlled clinical trial. Clin. Oral Implant. Res. 2021. [Google Scholar] [CrossRef]
- Staubli, N.; Walter, C.; Schmidt, J.C.; Weiger, R.; Zitzmann, N.U. Excess cement and the risk of peri-implant disease—A systematic review. Clin. Oral Implant. Res. 2017, 28, 1278–1290. [Google Scholar] [CrossRef]
- Thoma, D.S.; Sailer, I.; Muhlemann, S.; Gil, A.; Jung, R.E.; Hammerle, C.H.F. Randomized controlled clinical study of veneered zirconia abutments for single implant crowns: Clinical, histological, and microbiological outcomes. Clin. Implant. Dent. Relat Res. 2018, 20, 988–996. [Google Scholar] [CrossRef]
- Rosentritt, M.; Hahnel, S.; Engelhardt, F.; Behr, M.; Preis, V. In vitro performance and fracture resistance of CAD/CAM-fabricated implant supported molar crowns. Clin. Oral Investig. 2017, 21, 1213–1219. [Google Scholar] [CrossRef]
- Honda, J.; Komine, F.; Kamio, S.; Taguchi, K.; Blatz, M.B.; Matsumura, H. Fracture resistance of implant-supported screw-retained zirconia-based molar restorations. Clin. Oral Implant. Res. 2017, 28, 1119–1126. [Google Scholar] [CrossRef]
- Honda, J.; Komine, F.; Kusaba, K.; Kitani, J.; Matsushima, K.; Matsumura, H. Fracture loads of screw-retained implant-supported zirconia prostheses after thermal and mechanical stress. J. Prosthodont. Res. 2020, 64, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Freifrau von Maltzahn, N.; Bernard, S.; Kohorst, P. Two-part implant abutments with titanium and ceramic components: Surface modification affects retention forces-An in-vitro study. Clin. Oral Implant. Res. 2019, 30, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Kemarly, K.; Arnason, S.C.; Parke, A.; Lien, W.; Vandewalle, K.S. Effect of Various Surface Treatments on Ti-Base Coping Retention. Oper. Dent. 2020, 45, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.; Lawson, N.C.; Liu, P.R.; Lin, C.P.; Givan, D.A. Retentive Force of Zirconia Implant Crowns on Titanium Bases Following Different Surface Treatments. Int. J. Oral Maxillofac. Implant. 2018, 33, 530–535. [Google Scholar] [CrossRef]
- Linkevicius, T.; Caplikas, A.; Dumbryte, I.; Linkeviciene, L.; Svediene, O. Retention of zirconia copings over smooth and airborne-particle-abraded titanium bases with different resin cements. J. Prosthet. Dent. 2019, 121, 949–954. [Google Scholar] [CrossRef]
- IvoclarVivadent. Available online: https://www.ivoclarvivadent.com/en/p/all/ips-emax-system-technicians/ips-emax-system-new-products/ips-emax-press-abutment-solutions (accessed on 19 June 2020).
- Nouh, I.; Kern, M.; Sabet, A.E.; Aboelfadl, A.K.; Hamdy, A.M.; Chaar, M.S. Mechanical behavior of posterior all-ceramic hybrid-abutment-crowns versus hybrid-abutments with separate crowns-A laboratory study. Clin. Oral Implant. Res. 2019, 30, 90–98. [Google Scholar] [CrossRef] [Green Version]


| Group | Without Fatigue | With Fatigue | Influence of Fatigue |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p-Value | |
| LDS | 2981 ± 798 a,b | 2722 ± 497 A | p = 0.349 |
| PFZ | 2152 ± 572 c | 1686 ± 691 B | p = 0.099 |
| PFM | 2633 ± 389 b,c | 2349 ± 578 A | p = 0.099 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitznagel, F.A.; Bonfante, E.A.; Campos, T.M.B.; Vollmer, M.A.; Boldt, J.; Doerken, S.; Gierthmuehlen, P.C. Mechanical Stability of Screw-Retained Monolithic and Bi-layer Posterior Hybrid Abutment Crowns after Thermomechanical Loading: An In Vitro Study. Materials 2021, 14, 7539. https://doi.org/10.3390/ma14247539
Spitznagel FA, Bonfante EA, Campos TMB, Vollmer MA, Boldt J, Doerken S, Gierthmuehlen PC. Mechanical Stability of Screw-Retained Monolithic and Bi-layer Posterior Hybrid Abutment Crowns after Thermomechanical Loading: An In Vitro Study. Materials. 2021; 14(24):7539. https://doi.org/10.3390/ma14247539
Chicago/Turabian StyleSpitznagel, Frank A., Estevam A. Bonfante, Tiago M. B. Campos, Maximilian A. Vollmer, Johannes Boldt, Sam Doerken, and Petra C. Gierthmuehlen. 2021. "Mechanical Stability of Screw-Retained Monolithic and Bi-layer Posterior Hybrid Abutment Crowns after Thermomechanical Loading: An In Vitro Study" Materials 14, no. 24: 7539. https://doi.org/10.3390/ma14247539






