Novel Mg-Incorporated Micro-Arc Oxidation Coatings for Orthopedic Implants Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Arc Oxidation Treatment
2.2. Surface Characterization
2.3. The Measurement of Mg2+ Concentration
2.4. Cell Culture and Viability
2.5. Initial Cell Attachment and Cell Proliferation
2.6. Alkaline Phosphatase (ALP) Activity
2.7. Extracellular Matrix (ECM) Mineralization and Collagen Secretion
2.8. Statistical Analysis
3. Results
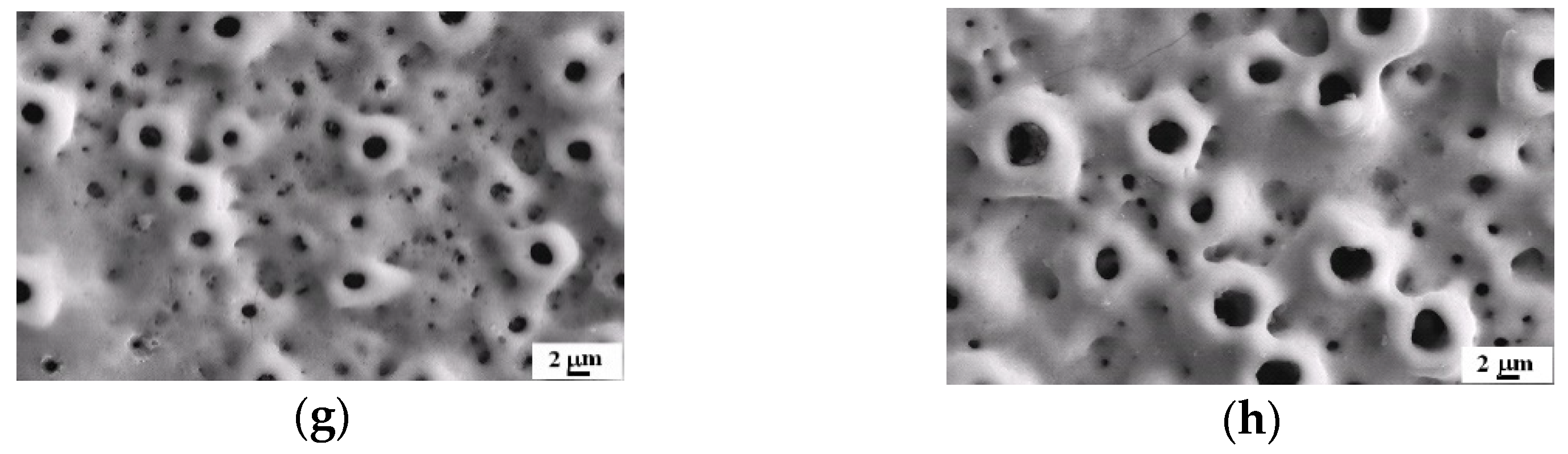
3.1. Influences of Na2MgY Concentrationon on Solution Conductivity and MAO Coatings
3.2. Influence of KOH Concentration on Solution Conductivity and MAO Coatings
3.3. Influences of H12Phy on Coating Formation
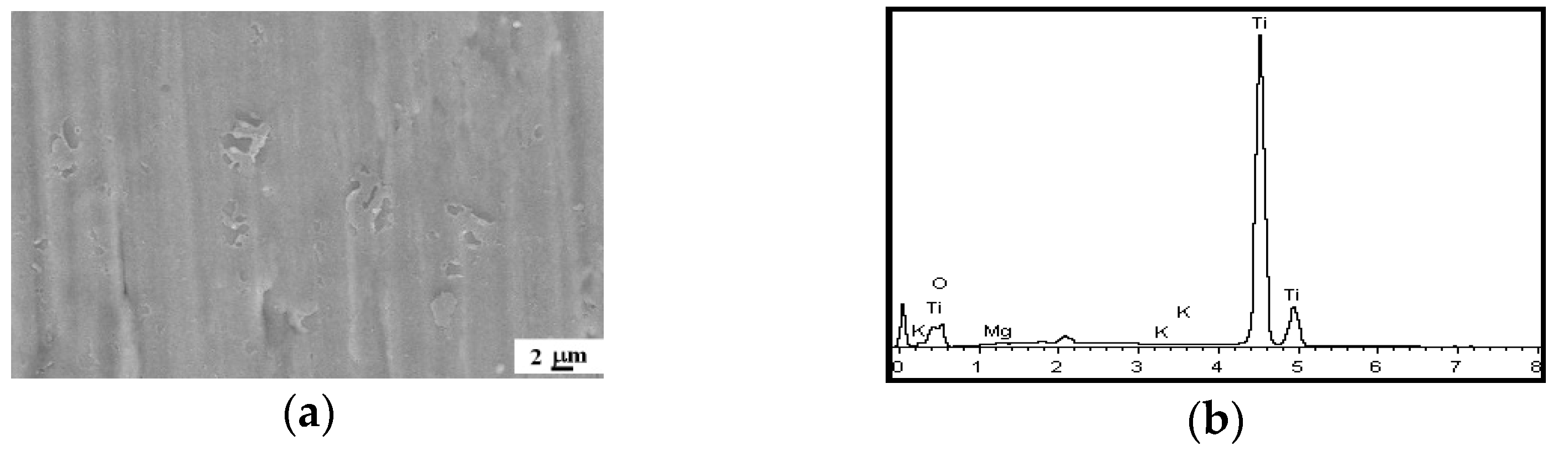
3.4. XPS Analysis
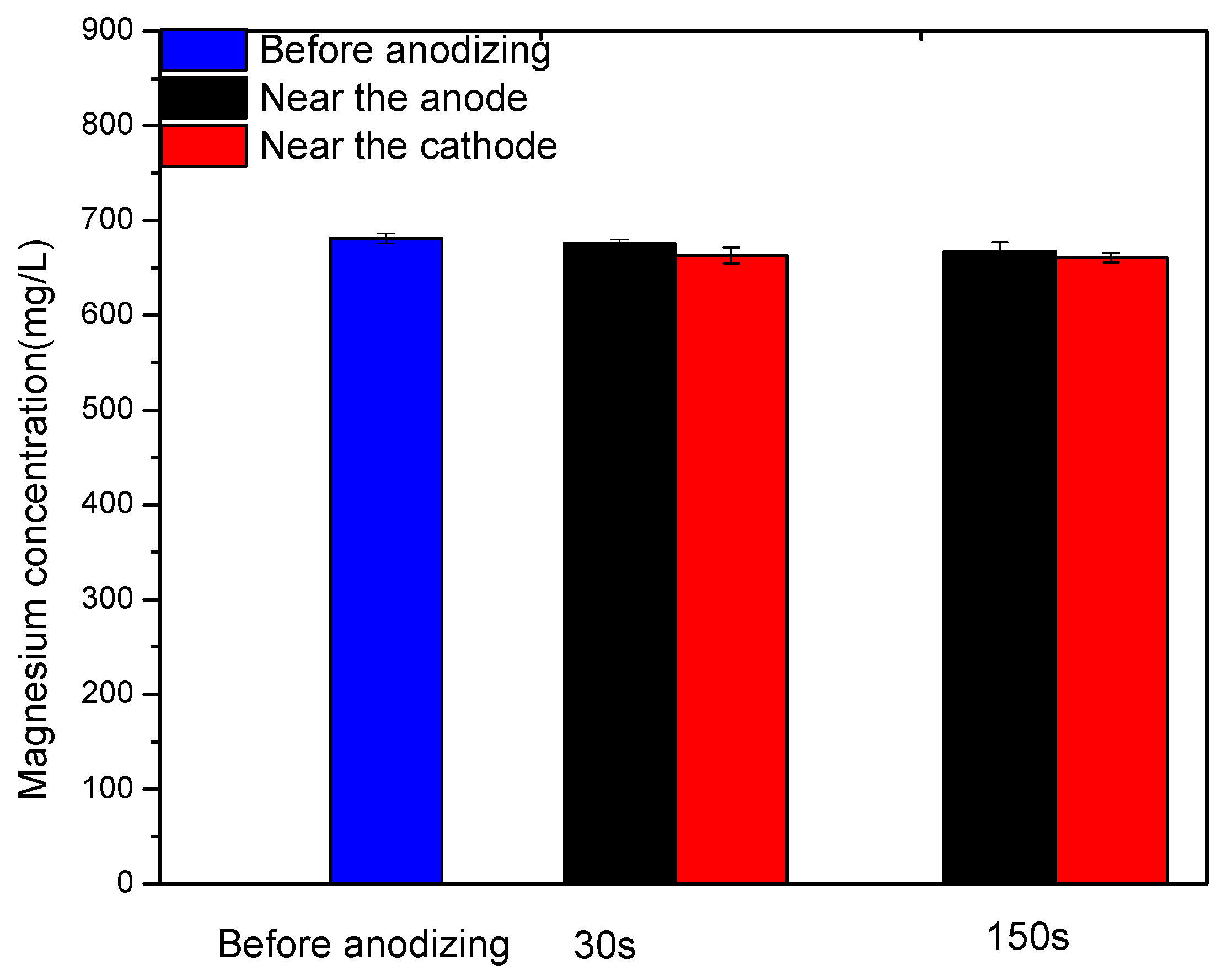
3.5. Mg Ions Concentration Analysis
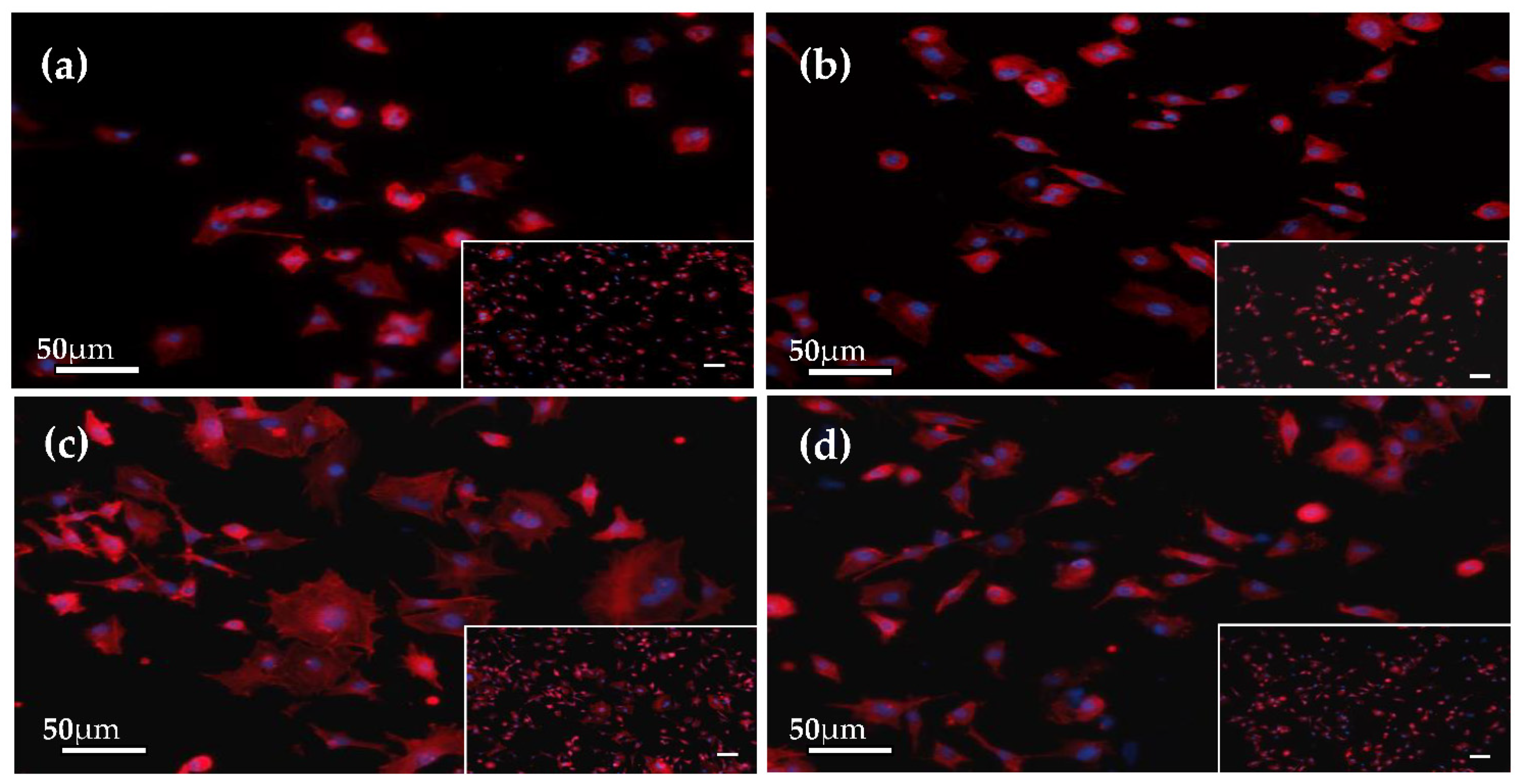
3.6. Initial Cell Attachment
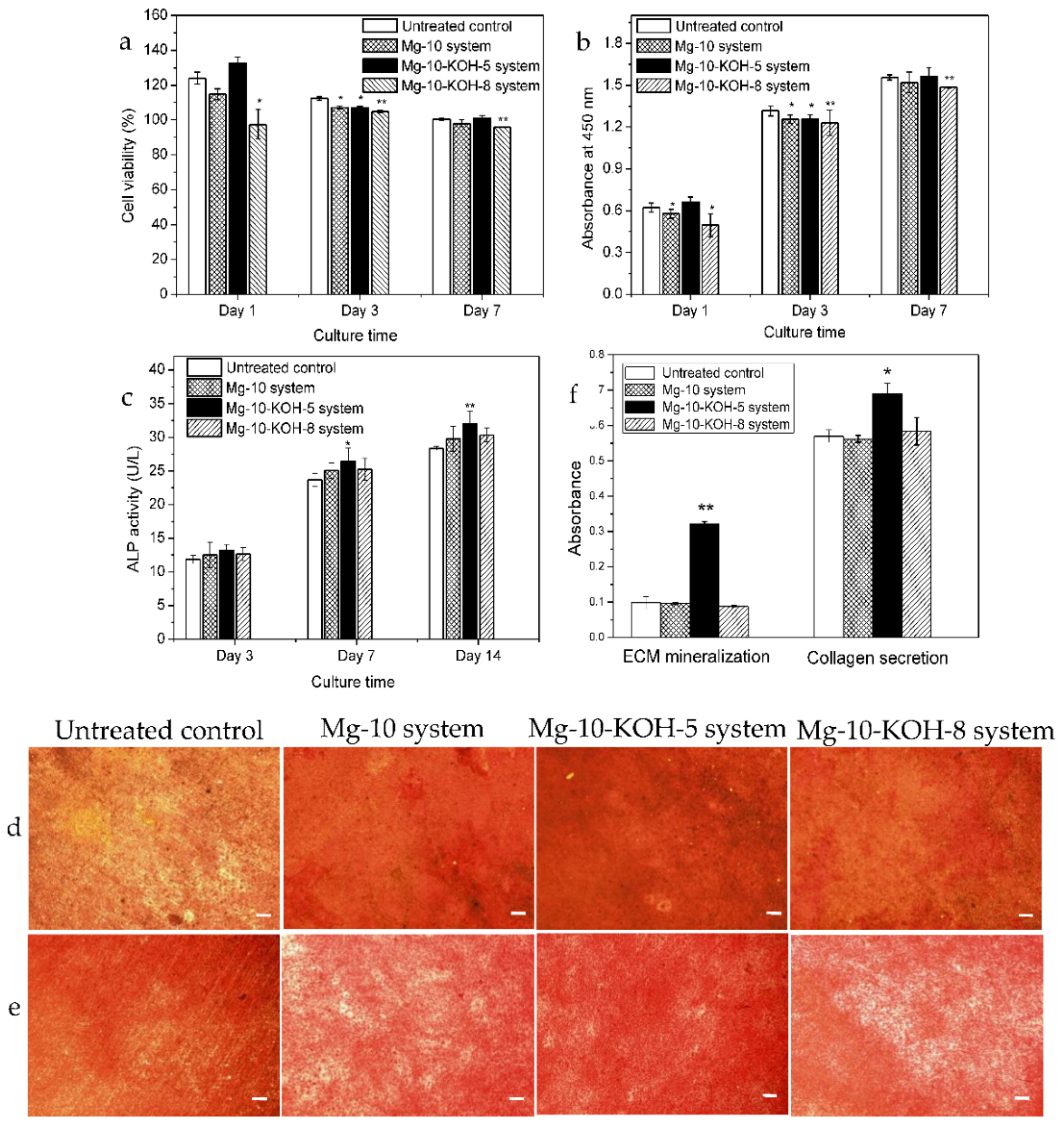
3.7. Cell Viability
3.8. Cell Proliferation
3.9. Cell Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants-A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, S.M.; Wong, H.M.; Wu, S.L.; Hu, T.; Yeung, K.W.K.; Chu, P.K. Effect of carbon and nitrogen plasma immersion ion implantation on in vitro and in vivo biocompatibility of titanium alloy. ACS Appl. Mater. Interfaces 2013, 5, 1510–1516. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Kung, K.-C.; Yang, C.-Y.; Lee, T.-M.; Lui, T.-S. Engineering three-dimensional structures using bio-inspired dopamine and strontium on titanium for biomedical application. J. Mater. Chem. B 2014, 2, 7927–7935. [Google Scholar] [CrossRef]
- Fattah-alhosseinia, A.; Molaeia, M.; Attarzadehb, N.; Babaeia, K.; Attarzadehc, F. On the enhanced antibacterial activity of plasma electrolytic oxidation(PEO) coatings that incorporate particles: A review. Ceram. Int. 2000, 46, 20587–20607. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Xu, J.L.; Liu, Z.H.; Deng, L.; Sun, B.; Liu, S.D.; Wang, L.; Liu, H.Y. Preparation, corrosion resistance and hemo-compatibility of the superhydrophobic TiO2 coatings on biomedical Ti-6Al-4V alloys. Appl. Surf. Sci. 2015, 347, 591–595. [Google Scholar] [CrossRef]
- Yao, Z.; Hu, B.; Shen, Q.; Niu, A.; Jiang, Z.; Su, P.; Ju, P. Preparation of black high absorbance and high emissivity thermal control coating on Ti alloy by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 253, 166–170. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Liu, C.; Wang, D.; Liang, J. Correlations between the growth mechanism and properties of micro-arc oxida-tion coatings on titanium alloy: Effects of electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Sedelnikova, M.B.; Komarova, E.G.; Sharkeev, Y.P.; Ugodchikova, A.V.; Tolkacheva, T.V.; Rau, J.V.; Buyko, E.E.; Ivanov, V.V.; Sheikin, V.V. Modification of titanium surface via Ag-, Sr- and Si-containing micro-arc calcium phosphate coating. Bioact. Mater. 2019, 4, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Shin, K.R.; Ko, Y.G.; Shin, D.H. Surface characteristics and biological response of titanium oxide layer formed via micro-arc oxidation in K3PO4 and Na3PO4 electrolytes. J. Alloys Compd. 2014, 586, S548–S552. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Galvis, O.; Giraldo, D.Q.; Pavon, J.; Lopez-Lacomba, J.L.; Jimenez-Pique, E.; Anglada, M.; Robledo, S.M.; Castano, J.G.; Echeverria, F. Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces. J. Mater. Sci. Mater. Med. 2015, 26, 72–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.L.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Chung, C.J.; Yang, T.C.; Tang, C.H.; He, J.L. Microscopic observations of osteoblast growth on micro-arc oxidized β titanium. Appl. Surf. Sci. 2013, 266, 73–80. [Google Scholar] [CrossRef]
- Terleeva, O.P.; Slonova, A.I.; Mironov, I.V.; Rogov, A.B.; Sharkeev, Y.P. Microplasma synthesis of biocompatible coatings with additions of magnesium, silicon and silver on pure titanium from homogeneous electrolytes. Surf. Coat. Technol. 2016, 307, 1265–1273. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Yi, L.; Hu, A.N.; Jiang, L.B.; Hong, L.; Dong, J. Antibacterial and osteogenic activity of a multifunctional mi-croporous coating codoped with Mg, Cu and F on titanium. J. Mater. Chem. B 2019, 7, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, H.Z.; Li, J.F.; He, X.J.; Hang, R.Q.; Huang, X.B.; Tian, L.H.; Tang, B. Corrosion behavior of Zn-incorporated antibacterial TiO2 porous coating on titanium. Ceram. Int. 2016, 42, 17095–17100. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, J.Q.; Huang, X.Y.; Zhang, Y.N.; Hong, Y. The dual function of Cu-doped TiO2 coatings on titanium for appli-cation in percutaneous implants. J. Mater. Chem. B 2016, 4, 3788–3800. [Google Scholar]
- Li, G.Q.; Wang, Y.P.; Zhang, S.F.; Zhao, R.F.; Zhang, R.F.; Li, X.Y.; Chen, C.M. Investigation on entrance mechanism of cal-cium and magnesium into micro-arc oxidation coatings developed on Ti-6Al-4V alloys. Surf. Coat. Technol. 2019, 378, 124951. [Google Scholar] [CrossRef]
- Crea, F.; Stefano, C.D.; Milea, D.; Sammartano, S. Formation and stability of phytate complexes in solution. Coord. Chem. Rev. 2008, 252, 1108–1120. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, S.; Xu, G.; Zhao, H.; Li, Y.; Wang, X.; Huang, K.; Ren, M.; Wu, X. Crack self-healing of phytic acid conversion coating on AZ31 magnesium alloy by heat treatment and the corrosion resistance. Appl. Surf. Sci. 2014, 313, 896–904. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Tamura, Y.; Otsuki, M.; Aoki, K.; Tagami, J. Phytic Acid: An Alternative Root Canal Chelating Agent. J. Endod. 2015, 41, 242–247. [Google Scholar] [CrossRef]
- Dost, K.; Tokul, O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal. Chim. Acta 2006, 558, 22–27. [Google Scholar] [CrossRef]
- Ravichandran, R.; Seitz, V.; Venugopal, J.R.; Sridhar, R.; Sundarrajan, S.; Mukherjee, S.; Wintermantel, E.; Ramakrishna, S. Mimicking Native Extracellular Matrix with Phytic Acid-Crosslinked Protein Nanofibers for Cardiac Tissue Engineering. Macromol. Biosci. 2013, 13, 366–375. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Pérez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The role of magnesium in biomaterials related infections. Colloids Surf. B Biointerfaces 2020, 191, 110996. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.L.; Li, X.Z.; Liu, T.; Wu, H.; Liu, X.J.; Feng, Q.L.; Liu, Y. Enhanced SaOS-2 cell adhesion, proliferation and differen-tiation on Mg-incorporated micro/nano-topographical TiO2 coatings. Appl. Surf. Sci. 2018, 447, 767–776. [Google Scholar] [CrossRef]
- Ahmed, F.; Mohammed, A. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances oste-ogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Zhang, H.; Wu, Y.; Tang, C. Covalent immobilization of the phytic acid-magnesium layer on titanium improves the osteogenic and antibacterial properties. Colloids Surf. B Biointerfaces 2021, 203, 111768. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.; Byon, E.; Albrektsson, T. The bone response of oxidized bioactive and non-bioactive titanium implants. Biomaterials 2005, 26, 6720–6730. [Google Scholar] [CrossRef] [PubMed]
- Ungan, G.; Cakir, A. Investigation of MgO effect on bioactivity of coatings produced by MAO. Surf. Coat. Technol. 2015, 282, 52–60. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Zhang, W.; Bai, Y.; Liu, Y.; Meng, J.; Zhang, L. A Magnesium-Incorporated Nanoporous Titanium Coating for Rapid Osseointegration. Int. J. Nanomed. 2020, 15, 6593–6603. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, H.; Jiang, Y.; Qu, J. An X-ray photoelectron spectroscopy and electrochemical study of the self-assembled monolayer phytic acid on the 430 stainless steel in NaCl solution. Anti-Corros. Methods Mater. 2013, 60, 301–306. [Google Scholar] [CrossRef]
- Ma, C.; Nagai, A.; Yamazaki, Y.; Toyama, T.; Tsutsumi, Y.; Hanawa, T.; Wang, W.; Yamashita, K. Electrically polarized micro-arc oxidized TiO2 coatings with enhanced surface hydrophilicity. Acta Biomater. 2012, 8, 860–865. [Google Scholar] [CrossRef]
- Zhou, R.; Wei, D.Q.; Cheng, S.; Li, B.Q.; Wang, Y.M.; Jia, D.C.; Zhou, Y.; Guo, H.F. The structure and in vitro apatite formation ability of porous titanium covered bioactive microarc oxidized TiO2-based coatings containing Si, Na and Ca. Ceram. Int. 2014, 40, 501–509. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Zhang, F.; Han, E.H. A novel phosphate conversion film on Mg-8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Mittal, V.; Bera, S.; Nithya, R.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Solid state synthesis of Mg–Ni ferrite and characterization by XRD and XPS. J. Nucl. Mater. 2004, 335, 302–310. [Google Scholar] [CrossRef]
- Alkarawi, H.H.; Zotz, G. Phytic acid in green leaves. Plant Biol. 2013, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; He, J.; Guo, H.; Zhang, J. In Vitro Corrosion and Bioactivity Study of Surface Phytic Acid Modified AZ31 Magnesium Alloy. Mater. Sci. Appl. 2014, 05, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.D.; Zhang, H.; Dong, X.D.; Ning, C.Y.; Fod, A.S.L. Physicochemical properties and in vitro cytocompatibility of modi-fied titanium surfaces prepared via micro-arc oxidation with different calcium concentrations. Appl. Surf. Sci. 2015, 329, 347–355. [Google Scholar] [CrossRef]
- Li, G.Q.; Wang, Y.P.; Qiao, L.P.; Zhao, R.F.; Zhang, S.F.; Zhang, R.F.; Chen, C.M.; Li, X.Y.; Zhao, Y. Preparation and for-mation mechanism of copper incorporated micro-arc oxidation coatings developed on Ti-6Al-4V alloys. Surf. Coat. Technol. 2019, 375, 74–85. [Google Scholar] [CrossRef]
- Burger, F.J.; Wu, J. Dielectric Breakdown in Electrolytic Capacitors. J. Electrochem. Soc. 1971, 118, 2039. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, B.U.; Yoon, S.I.; Lee, E.S.; Yoo, B.; Shin, D.H. Evaluation of plasma temperature during plasma oxidation processing of AZ91 Mg alloy through analysis of the melting behavior of incorporated particles. Electrochim. Acta 2012, 67, 6–11. [Google Scholar] [CrossRef]
- Bai, Y.; Kim, K.-A.; Park, I.S.; Lee, S.J.; Bae, T.S.; Lee, M.H. In situ composite coating of titania–hydroxyapatite on titanium substrate by micro-arc oxidation coupled with electrophoretic deposition processing. Mater. Sci. Eng. B 2011, 176, 1213–1221. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.; Qu, S.; Zhang, X. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials 2003, 24, 4663–4670. [Google Scholar] [CrossRef]
- Kim, J.N.; Han, S.N.; Kim, H.K. Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3T3-L1 cells. Nutr. Res. 2014, 34, 723–731. [Google Scholar] [CrossRef]
- Lee, J.; Farag, M.M.; Park, E.K.; Lim, J.; Yun, H.-S. A simultaneous process of 3D magnesium phosphate scaffold fabrication and bioactive substance loading for hard tissue regeneration. Mater. Sci. Eng. C 2014, 36, 252–260. [Google Scholar] [CrossRef]
- Tamimi, F.; Nihouannen, D.L.; Bassett, D.C.; Ibasco, S.; Gbureck, U.; Knowles, J.; Wright, A.; Flynn, A.; Komarova, S.V.; Barralet, J.E. Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomater. 2011, 7, 2678–2685. [Google Scholar] [CrossRef]
- Li, H.; Pan, H.; Ning, C.; Tan, G.; Liao, J.; Ni, G. Magnesium with micro-arc oxidation coating and polymeric membrane: An in vitro study on microenvironment. J. Mater. Sci. Mater. Med. 2015, 26, 147. [Google Scholar] [CrossRef] [PubMed]





| Processes | Coatings | Electrolyte Concentration (g/L) | ||
|---|---|---|---|---|
| H12Phy | Na2MgY | KOH | ||
| S-Mg-5 system | Mg-5 system | 15 | 5 | |
| S-Mg-10 system | Mg-10 system | 15 | 10 | |
| S-Mg-15 system | Mg-15 system | 15 | 15 | |
| S-Mg-20 system | Mg-20 system | 15 | 20 | |
| S-Mg-10-KOH-2 system | Mg-10-KOH-2 system | 15 | 10 | 2 |
| S-Mg-10-KOH-5 system | Mg-10-KOH-5 system | 15 | 10 | 5 |
| S-Mg-10-KOH-8 system | Mg-10-KOH-8 system | 15 | 10 | 8 |
| S-Mg-10-KOH-11 system | Mg-10-KOH-11 system | 15 | 10 | 11 |
| Processes | Element Content (at%) | ||||||
|---|---|---|---|---|---|---|---|
| C | Na | K | O | P | Ti | Mg | |
| Mg-5 system | 2.43 | 60.37 | 4.80 | 32.40 | |||
| Mg-10 system | 3.76 | 61.76 | 9.23 | 24.68 | 0.57 | ||
| Mg-15 system | 4.94 | 59.73 | 10.69 | 22.80 | 1.84 | ||
| Mg-20 system | 3.99 | 60.97 | 10.51 | 22.19 | 2.34 | ||
| Mg-10-KOH-2 system | 5.00 | 60.97 | 10.44 | 21.71 | 1.87 | ||
| Mg-10-KOH-5 system | 4.17 | 60.47 | 9.91 | 22.50 | 2.97 | ||
| Mg-10-KOH-8 system | 8.02 | 0.21 | 59.03 | 8.57 | 17.36 | 6.82 | |
| Mg-10-KOH-11 system | 6.34 | 0.39 | 0.45 | 58.28 | 7.49 | 19.92 | 7.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhong, S.; Zeng, L.; Li, H.; Zhao, R.; Zhang, S.; Duan, X.; Huang, J.; Zhao, Y. Novel Mg-Incorporated Micro-Arc Oxidation Coatings for Orthopedic Implants Application. Materials 2021, 14, 5710. https://doi.org/10.3390/ma14195710
Zhang R, Zhong S, Zeng L, Li H, Zhao R, Zhang S, Duan X, Huang J, Zhao Y. Novel Mg-Incorporated Micro-Arc Oxidation Coatings for Orthopedic Implants Application. Materials. 2021; 14(19):5710. https://doi.org/10.3390/ma14195710
Chicago/Turabian StyleZhang, Rongfa, Sheng Zhong, Lilan Zeng, Hongyu Li, Rongfang Zhao, Shufang Zhang, Xinting Duan, Jingsong Huang, and Ying Zhao. 2021. "Novel Mg-Incorporated Micro-Arc Oxidation Coatings for Orthopedic Implants Application" Materials 14, no. 19: 5710. https://doi.org/10.3390/ma14195710






