Metallurgy and Mechanism of Underwater Wet Cutting Using Oxidizing and Exothermic Flux-Cored Wires
Abstract
:1. Introduction
2. Materials and Methods
3. Research Results
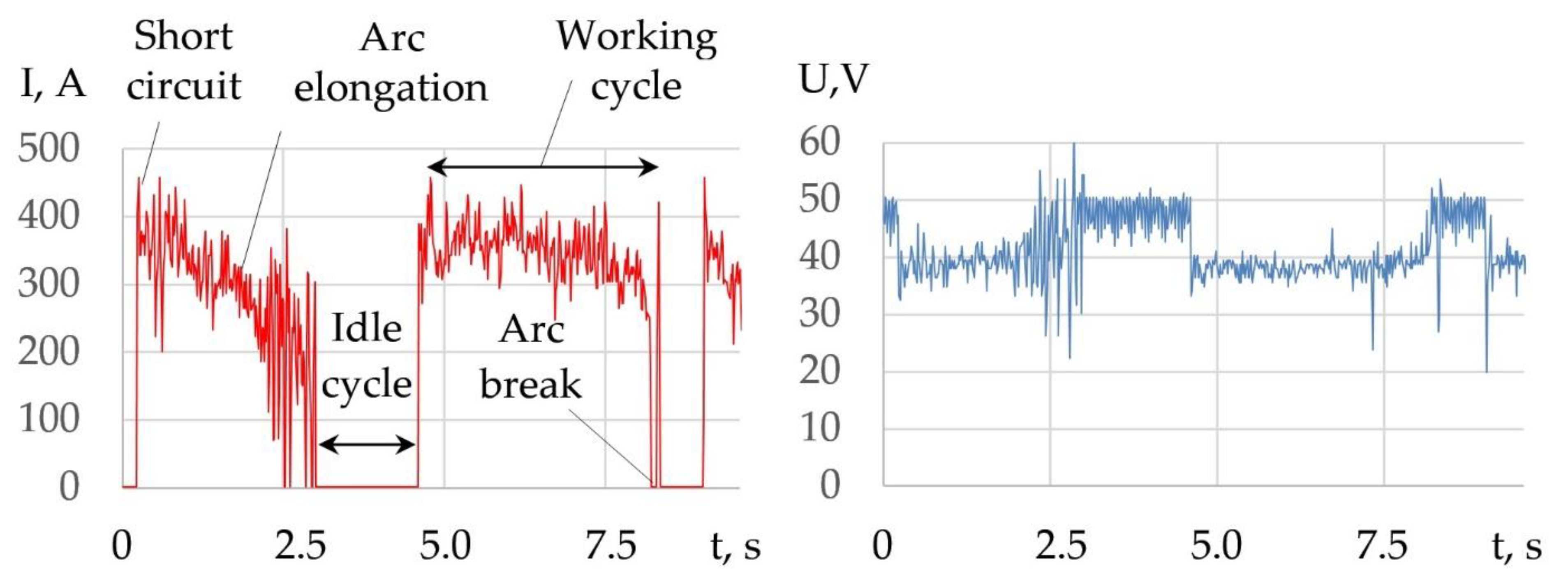
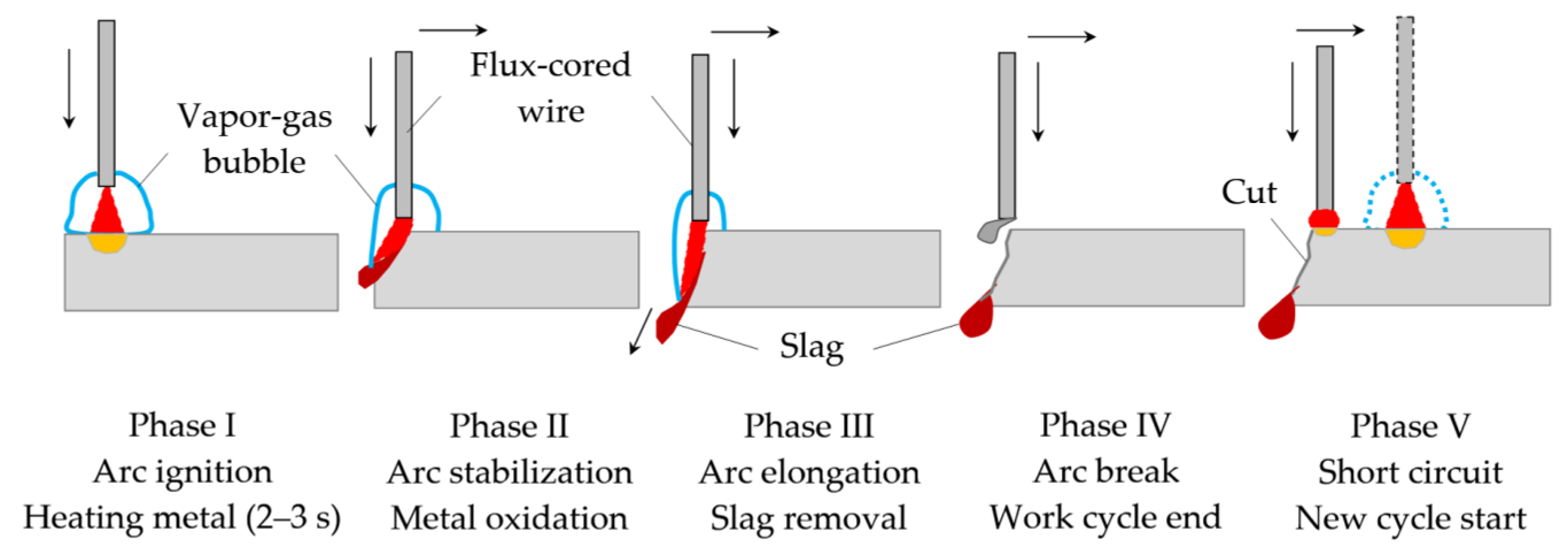
3.1. Mechanism of Underwater Wet Cutting
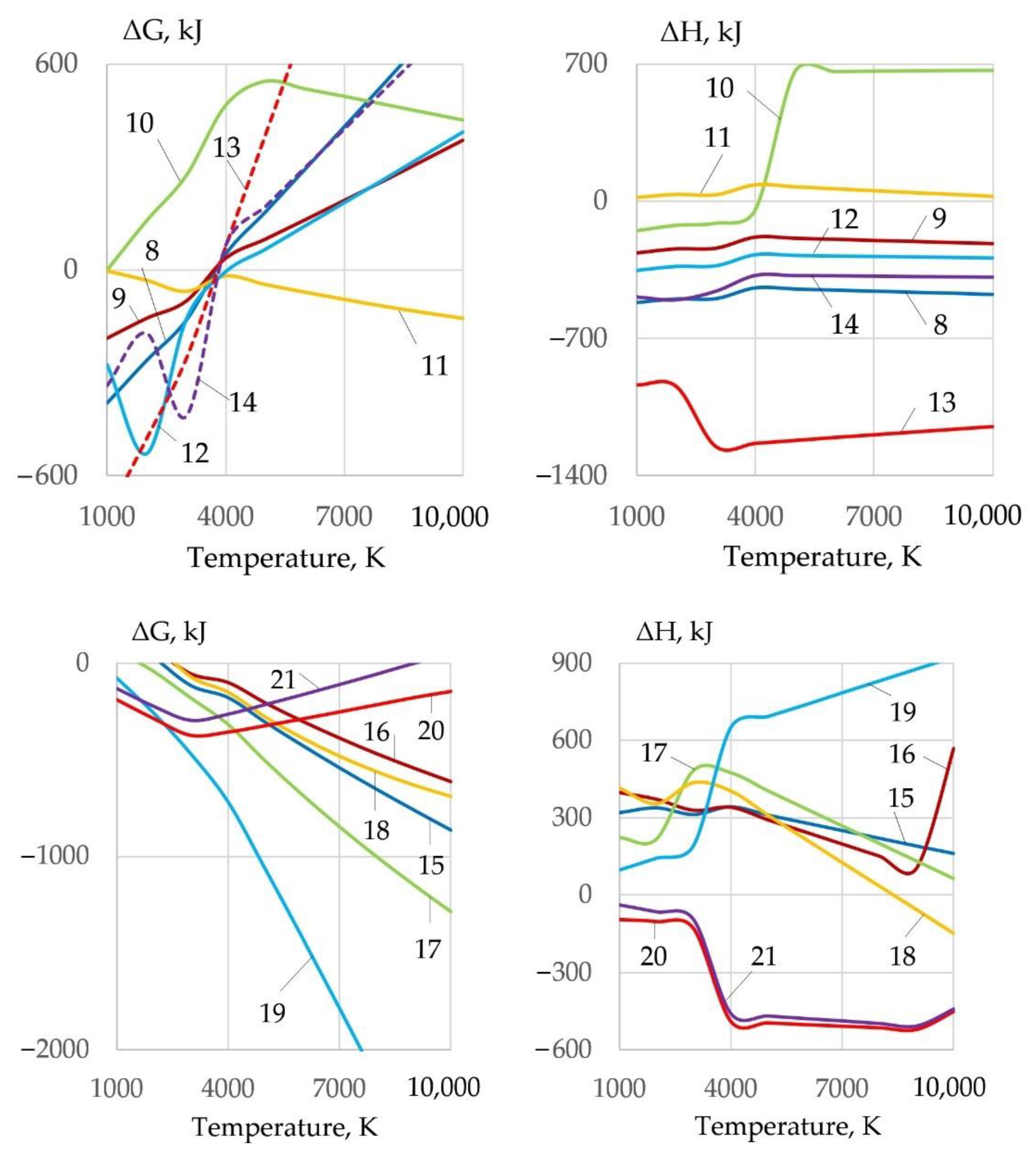
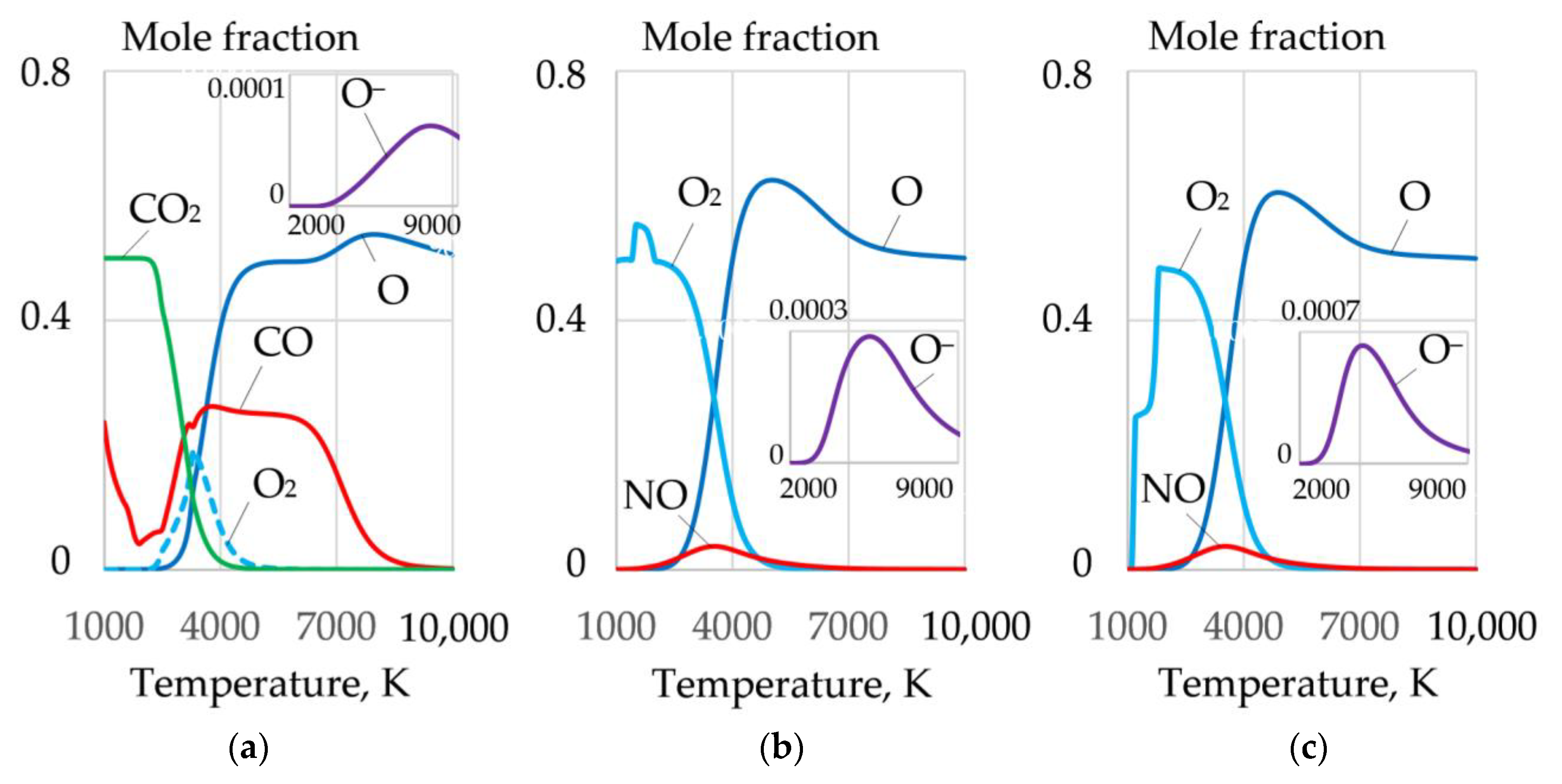
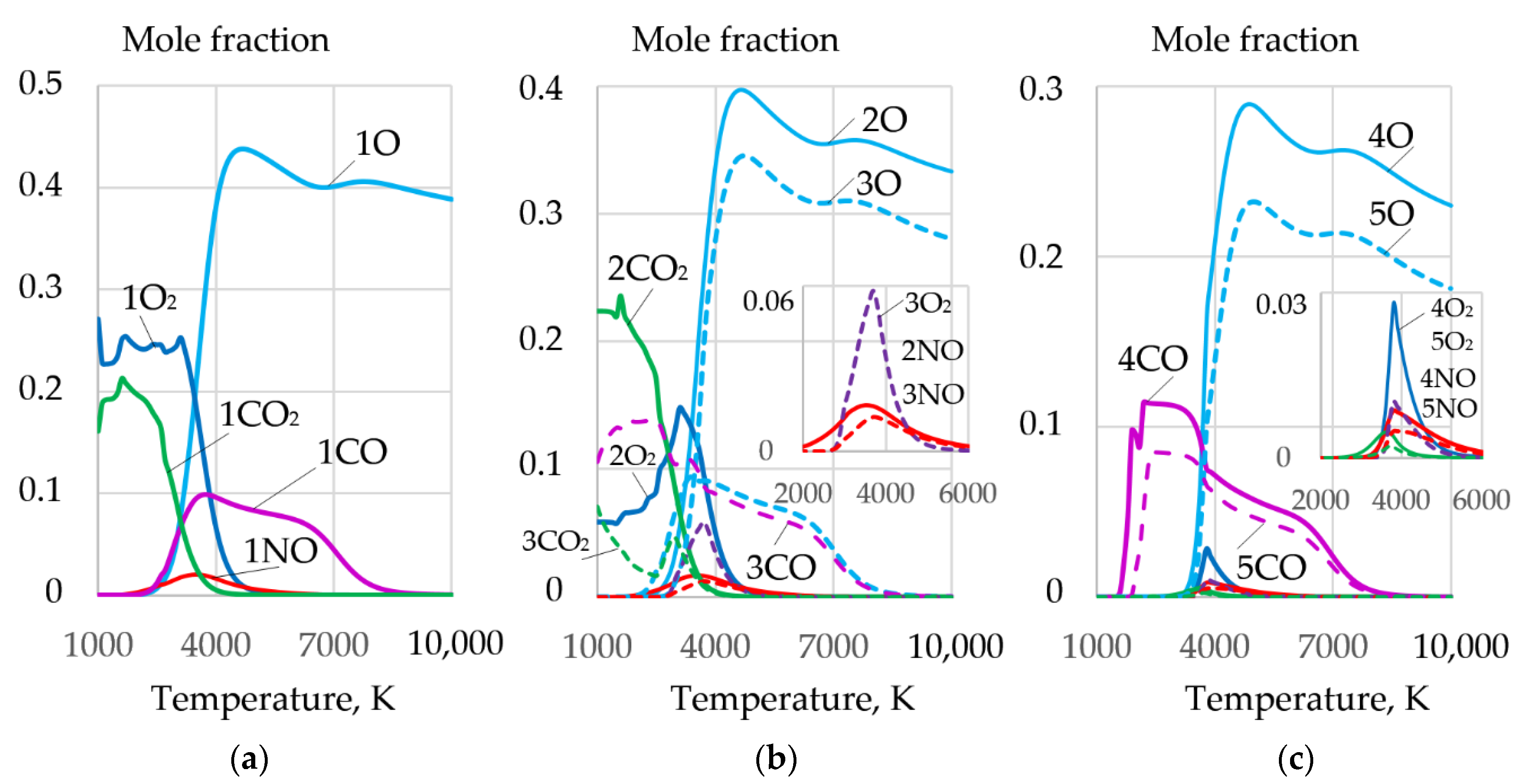
3.2. Dissociation of Components and Oxidation of Iron
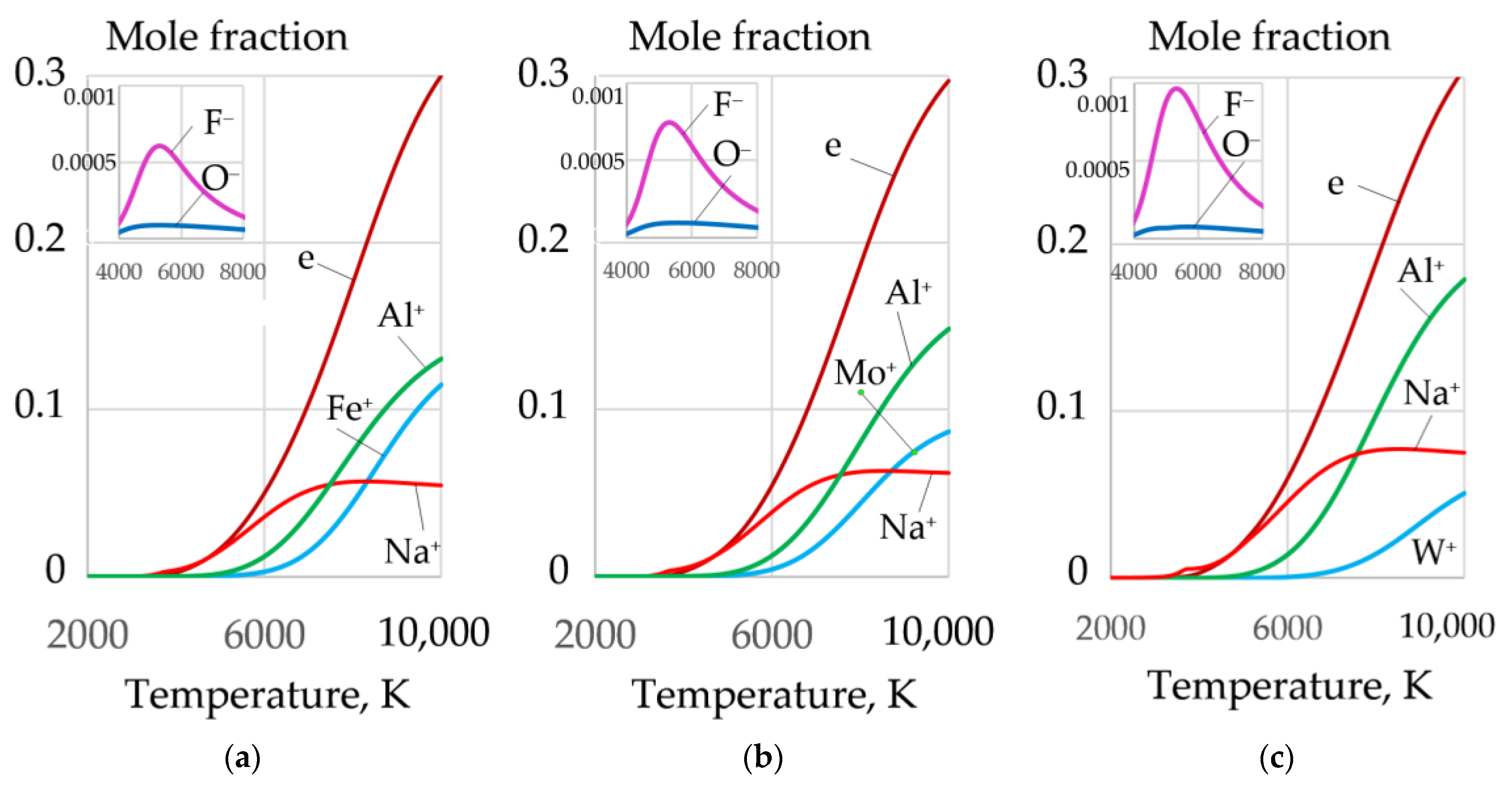
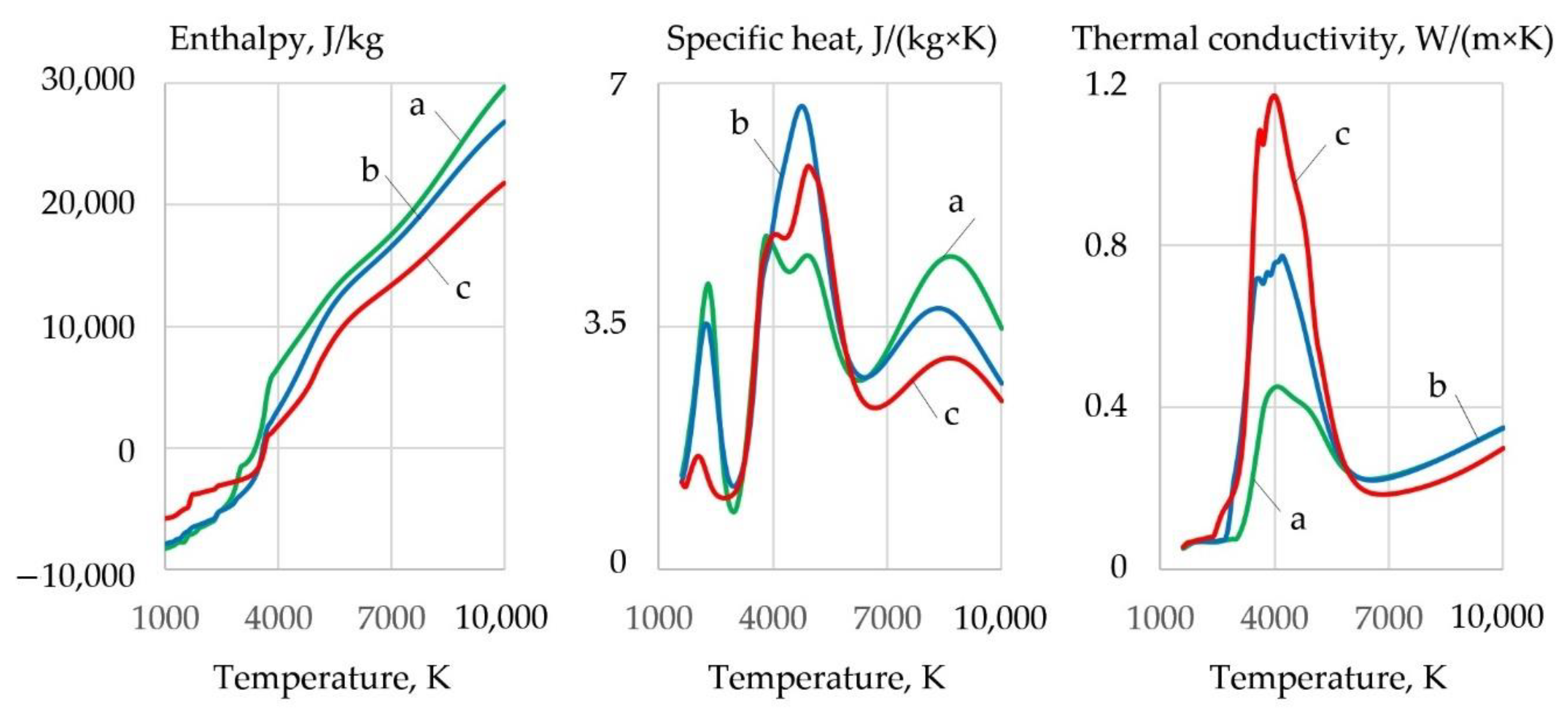
3.3. Ionization and Thermophysical Properties of Plasma
3.4. Exothermic Effect
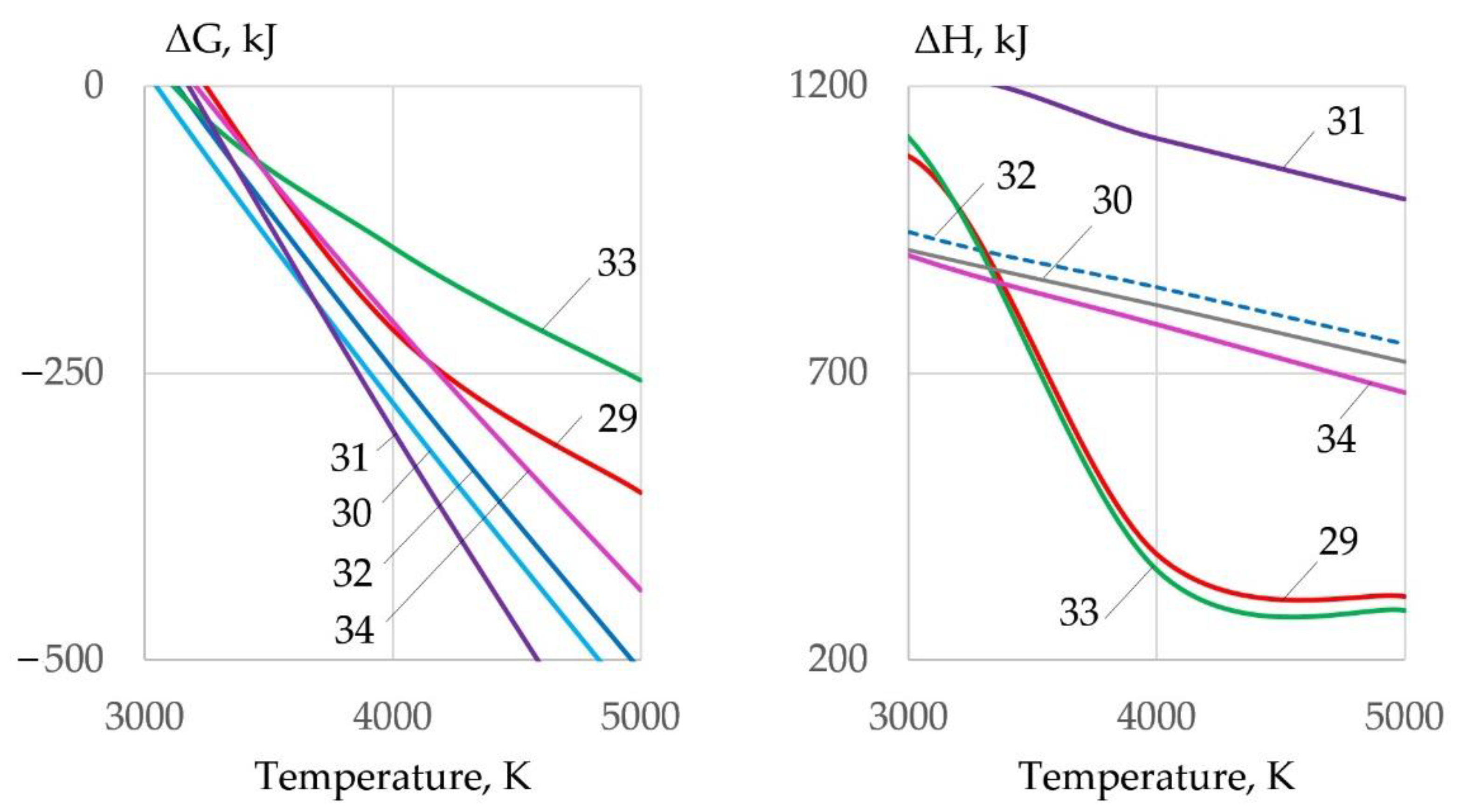
3.5. Formation of Oxides and Slag
3.6. Testing of Flux-Cored Wires
4. Conclusions
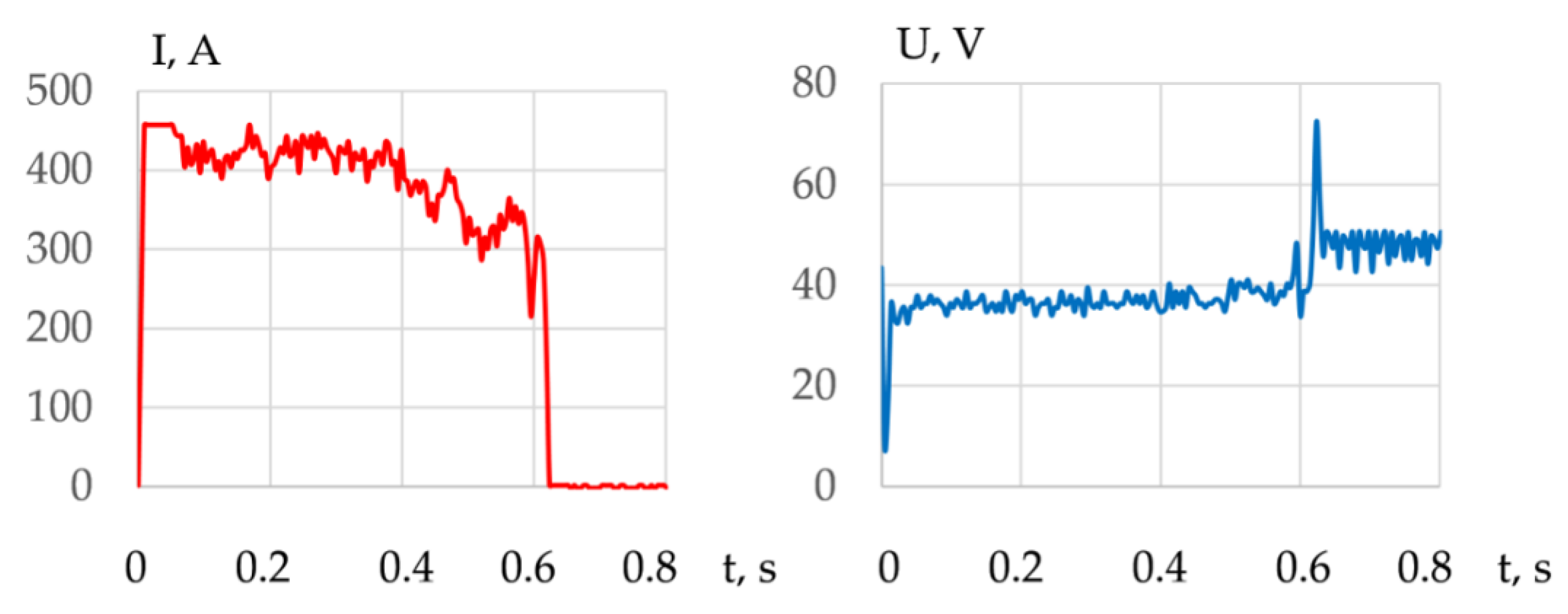
- The mechanism of underwater wet cutting with a flux-cored wire consists of the formation of a kerf in the oxygen atmosphere around the arc’s active spot. The formation of the kerf occurs within a pulsating vapor gas bubble under the influence of working cycles with the duration of 1.5–5 s and idle cycles with the duration of 0.6–1.7 s. In underwater cutting of 10 KhSND and 304L steel welding, the current was 344–402 A, the voltage was 36–39 V; in cutting of CuAl5 and AlMg4.5Mn0.7 alloy, the welding current was 360–406; 240 A and the voltage was 35–37; 38 V, respectively, with the optimal composition of flux-cored wire: 50–60% FeCO3 and KNO3, 20–30% aluminum, 20% Na3AlF6.
- A model of arc penetration and stabilization during underwater wet cutting using flux-cored wires is proposed. The model implies that the formation of the kerf occurs through penetration of the arc into the metal due to oxidation of the metal in a narrow zone around the arc’s active spot. The walls of the kerf mechanically compress the arc column, which stabilizes the arc and allows the arc to increase its length as it cuts. The compression of the arc by the walls of the kerf causes increasing ionization, temperature, and pressure of the arc.
- When metals oxidize, refractory molten slag from oxides of FeO, Fe2O3, Fe3O4, Cr2O3, NiO, Cu2O, and Al2O3 forms on the inner surface of the kerf at the temperature of 1000–4000 K, isolating the metal from oxygen and hindering the cutting process. The slag is forced out by the forces of gravity, arc pressure, and surface tension. To remove the slag, it was proposed to reduce the surface tension of the slag through ionic dissolution and interaction of refractory oxides in Na3AlF6 cryolite when the change of Gibbs free energy occurs from −140 kJ to −300 kJ at the temperature of 4000 K.
- Oxidizing cutting requires the composition of the gas mixture with high oxygen amount of up to 0.2–0.4 mole fraction at the temperature of 4000 K in the cutting zone, with electrons amounting up to 0.18–0.3 mole fraction at the temperature of 10,000 K, negative ions of oxygen and fluor less than 0.002 and 0.0018 mole fraction, enthalpy of more than 5 kJ/kg, heat capacity of more than 5.5 J/kg·K, and thermal conductivity of plasma more than 0.4 W/m·K at a temperature of 4000 K. These conditions are satisfied by the proposed oxidizing mixtures: 40–80% of KNO3, NaNO3, iron carbonate FeCO3, and 10–40% aluminum.
- The exothermic effect allows regulating the thermophysical properties of plasma and can be used in underwater wet cutting. The introduction of 60% Fe3O4, MoO2, and WO2 and 20% Al, Mg, and Ti in exothermic termite mixtures improves plasma ionization, increases electrons amount of up to a 0.3-mole fraction at the temperature of 10,000 K, reduces the enthalpy of up to 1.5 kJ/kg and heat capacity of up to 4.5 J/kg·K, and increases the thermal conductivity of plasma of up to 1.16 W/m·K at the temperature of 4000 K.
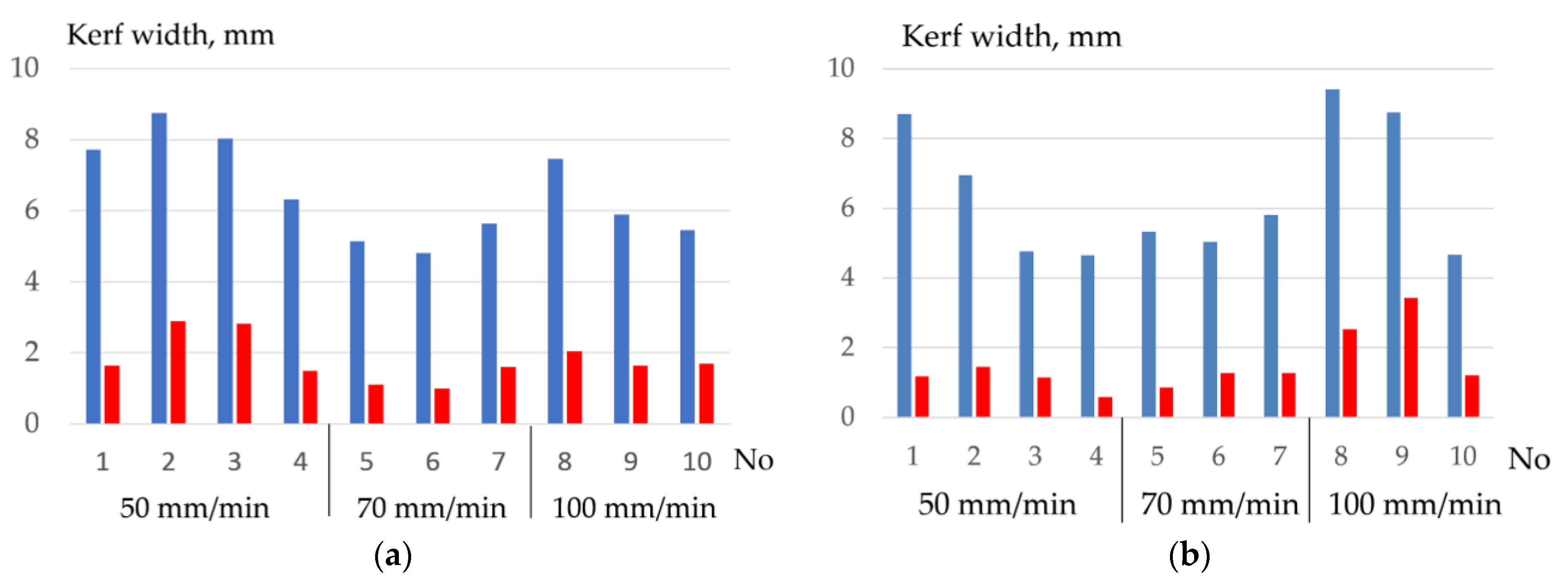
- Flux-cored wires with an oxidizing mixture are effective for cutting low-alloyed stainless steel as well as for cutting bronze and aluminum alloys. The optimum content of salts KNO3 and FeCO3 in the experimental wire was 50–60% with the introduction of 20–30% aluminum and 20% Na3AlF6. This composition allowed achieving stable cutting of 10KhSND steel with the kerf width of up to 4.7 mm, AISI 304L steel with the kerf width of up to 2.5 mm, and CuAl5 bronze with the kerf width of up to 3 mm with a deviation of ±0.3 mm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frazer, I.; Gibson, O.J.; Fyffe, L.; Lucas, B. Remotely operated underwater thermal cutting processes for the decommissioning of large North Sea platforms. In Proceedings of the OMAE’02 21st International Conference on Offshore Mechanics and Arctic Engineering, Oslo, Norway, 23–28 June 2002; pp. 1–9. [Google Scholar]
- Arias, R.; Bracarense, A.Q. Fatigue crack growth assessment in underwater wet welds. Weld. J. 2017, 8, 287–294. [Google Scholar]
- Tomków, J.; Fydrych, D.; Rogalski, G. Role of bead sequence in underwater welding. Materials 2019, 12, 3372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klett, J.; Hecht-Linowitzki, V.; Grünzel, O.; Schmidt, E.; Maier, H.J.; Hassel, T. Effect of the water depth on the hydrogen content in SMAW wet welded joints. SN Appl. Sci. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Fydrych, D.; Rogalski, G. Diffusible hydrogen management in underwater wet self-shielded flux cored arc welding. Int. J. Hydrogen Energy 2017, 42, 24532–24540. [Google Scholar] [CrossRef]
- Kononenko, V.Y. Underwater Welding and Cutting; Ukraine University: Kiev, Ukraine, 2011; pp. 200–244. (In Russian) [Google Scholar]
- Li, W.; Zhao, J.; Wang, J.; Wang, J.; Jia, H.; Li, Z.; Maksimov, S.Y. Research on arc cutting mechanism and procedure of flux-cored cutting wire in water. Int. J. Adv. Manuf. Technol. 2018, 98, 2895–2904. [Google Scholar] [CrossRef]
- Hilton, P.A.; Khan, A. Underwater cutting using a 1 μm laser source. J. Laser Appl. 2015, 27, 032013. [Google Scholar] [CrossRef]
- Wang, J.Y.; He, C.H.; Li, W.H.; Yang, F. Characteristics of underwater swirling plasma arc cut quality. Adv. Mater. Res. 2010, 97–101, 3974–3977. [Google Scholar] [CrossRef]
- Bach, F.-W.; Lindemaier, J.; Philipp, E.; Versemann, R. Contact arc metal cutting—Introduction of an extraordinary underwater cutting technology. Weld. World 1998, 41, 132–137. [Google Scholar]
- Gretskiy, Y.Y.; Nefedov, Y.N. Study of peculiarities of underwater flux-cored wire arc cutting without additional supply of oxygen. In Underwater Wet Welding and Cutting; Woodhead Publishing: Cambridge, UK, 1998; pp. 87–95. [Google Scholar]
- Nefedov, Y.N.; Danchenko, M.E. Technology and experience of application of underwater flux-cored wire arc semi-automatic cutting. In Underwater Wet Welding and Cutting; Woodhead Publishing: Cambridge, UK, 1998; pp. 96–104. [Google Scholar]
- Danchenko, M.E.; Gretskiy, Y.Y.; Savich, I.M.; Golovko, N.V. Flux-Cored Wire for Underwater Cutting; Certificate for Invention of the USSR No. 1718501; USSR State Committee for Inventions and Discoveries: Moscow, Russia, 1989. (In Russian) [Google Scholar]
- Danchenko, M.E.; Savich, I.M.; Golovko, N.V. Flux-Cored Wire for Underwater Cutting; Certificate for Invention of the USSR No. 1358254; USSR State Committee for Inventions and Discoveries: Moscow, Russia, 1995. (In Russian) [Google Scholar]
- Grishanov, A.A.; Pankov, V.I. Flux-Cored Wire for Underwater Cutting; Certificate for Invention of the USSR No. 2113960; USSR State Committee for Inventions and Discoveries: Moscow, Russia, 1998. (In Russian) [Google Scholar]
- Liu, D.; Li, H.; Feng, J.; Guo, N.; Liu, J. Chemical Core Cutting Wire for Underwater Wet Method Electric Arc Cutting. China Patent No. CN104858565A, 26 August 2015. [Google Scholar]
- Wang, J.; Wang, J.; Li, W.; Zhu, J. Flux-Cored Cutting Wire for Underwater Wet-Type Arc Cutting and Preparation Method Thereof. China Patent No. CN102554520A, 11 July 2012. [Google Scholar]
- Liu, D.; Lia, H.; Yan, Y.; Guo, N.; Song, X.; Feng, J. Effects of processing parameters on arc stability and cutting quality in underwater wet flux-cored arc cutting at shallow water. J. Manuf. Process. 2018, 33, 24–34. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Yu, R.; Wang, J.; Wang, J.; Wu, M.; Maksimov, S.Y. High-speed photography analysis for underwater flux-cored wire arc cutting process. In Transactions on Intelligent Welding Manufacturing; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 141–150. [Google Scholar]
- Li, W.; Zhao, J.; Wang, Y.; Wang, J.; Wang, J.; Jia, H.; Li, Z.; Wu, J.; Li, W. Research on underwater flux cored arc cutting mechanism based on simulation of kerf formation. J. Manuf. Process. 2019, 40, 169–177. [Google Scholar] [CrossRef]
- Wang, J.; Shi, J.; Wang, J.; Li, W.; Liu, C.; Xu, G.; Maksimov, S.Y.; Zhu, Q. Numerical study on the temperature field of underwater flux-cored wire arc cutting process. Int. J. Adv. Manuf. Technol. 2017, 91, 2777–2786. [Google Scholar] [CrossRef]
- Parshin, S.G.; Levchenko, A.M.; Maystro, A.S. Metallurgical model of diffusible hydrogen and non-metallic slag inclusions in underwater wet welding of high-strength steel. Metals 2020, 10, 1498. [Google Scholar] [CrossRef]
- Parshin, S.; Levchenko, A.; Wang, P.; Maystro, A. Mathematical analysis of the influence of the flux-cored wire chemical composition on the electrical parameters and quality in the underwater wet cutting. Adv. Mater. Sci. 2021, 21, 77–89. [Google Scholar] [CrossRef]
- Parshin, S.; Levchenko, A. Technology and equipment for underwater wet welding and cutting of high strength steel arctic structures using flux-cored wires. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2020. [Google Scholar]
- Li, H.L.; Liu, D.; Guo, N.; Chen, H.; Du, Y.P.; Feng, J.C. The effect of alumino-thermic addition on underwater wet welding process stability. J. Mater. Process. Technol. 2017, 245, 149–156. [Google Scholar] [CrossRef]
- Simko, F.; Rakhmatullin, A.; Boca, M.; Danek, V.; Bessada, C. A high-temperature multinuclear NMR study of Na3AlF6–FeO and Na3AlF6-Fe2O3 melts. Eur. J. Inorg. Chem. 2006, 22, 4528–4532. [Google Scholar] [CrossRef]
- Dewing, E.W.; Thonstad, J. Solutions of iron oxides in molten cryolite. Metall. Mater. Trans. B 2000, 31, 609–613. [Google Scholar] [CrossRef]
- Šuleková, D.; Danielik, V.; Fellner, P.; Thonstad, J. Solubility of chromium(III) oxide in the molten cryolite system. Metall. Mater. Trans. B 2013, 44, 328–331. [Google Scholar] [CrossRef]
- Danielik, V.; Fellner, P.; Sulekova, D.; Thonstad, J. Solubility of Cr species in low temperature cryolite-based electrolyte at 900 °C. J. Electrochem. Soc. 2013, 160, C142–C145. [Google Scholar] [CrossRef]
- Jentoftsen, T.E.; Lorentsen, O.-A.; Dewing, E.W.; Haarberg, G.M.; Thonstad, J. Solubility of some transition metal oxides in cryolite–alumina melts: Part I. Solubility of FeO, FeAl2O4, NiO, and NiAl2O4. Metall. Mater. Trans. B 2002, 33, 901–908. [Google Scholar] [CrossRef]
- Lorentsen, O.-A.; Jentoftsen, T.E.; Dewing, E.W.; Thonstad, J. The Solubility of some transition metal oxides in cryolite-alumina melts: Part III. Solubility of CuO and Cu2O. Metall. Mater. Trans. B 2007, 38, 833–839. [Google Scholar] [CrossRef]
- Zhang, Y.; Rapp, R.A. The solubility of titanium dioxide in cryolite-alumina melts at 1300 K. Metall. Mater. Trans. B 2004, 35, 182–186. [Google Scholar] [CrossRef]
- Khalaj, G.; Yoozbashizadeh, H.; Khodabandeh, A.; Tamizifar, M. Austenite grain growth modelling in weld heat affected zone of Nb/Ti microalloyed linepipe steel. Mater. Sci. Technol. 2014, 30, 424–433. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Khalaj, M.; Nazerfakhari, M.; Khalaj, G. Artificial neural networks for hardness prediction of HAZ with chemical composition and tensile test of X70 pipeline steels. J. Iron Steel Res. Int. 2015, 22, 446–450. [Google Scholar] [CrossRef]









| Method | Maximum Thickness of Steel, mm | Current, A | Voltage, V | Oxygen Consumption, m3/h | Cutting Speed for Steel, mm/min |
|---|---|---|---|---|---|
| Manual oxy-arc cutting using tubular electrodes of 8 mm | 100 | 180–350 | 25–45 | 0.8–1 | 30–40 (for 10 mm) |
| Manual oxy-arc cutting using exothermic electrodes of 9.5 mm | 35 | 150–300 | 25–45 | 12–60 | 400–500 (for 10 mm) |
| Manual arc cutting using coated electrodes of 4 mm | 16 | 200–280 | 30–45 | – | 60–80 (for 10 mm) |
| Automatic plasma cutting | 80 | 300–800 | 200–300 | Air consumption of 0.06–0.12 | 300–400 (for 15 mm) |
| Automatic laser cutting | 100 | Power of 2–10 kW | – | Air consumption of 0.1–0.3 m3/h | 300–1000 (for 15 mm) |
| Semi-automatic/automatic cutting using flux-cored wires of 2 mm | 20 | 300–500 | 32–42 | – | 70–250 (for 10 mm) |
| Wires | Flux-Core Composition, wt.% | |||
|---|---|---|---|---|
| KNO3 | FeCO3 | Na3AlF6 | Al | |
| PPR-APL1–1 | 30 | 30 | 20 | 20 |
| PPR-APL1–2 | 25 | 25 | 20 | 30 |
| PPR-APL1–3 | 35 | 35 | 20 | 10 |
| Materials | Travel Speed, mm/min | Wire Feed Rate, m/min |
|---|---|---|
| 10KhSND of 10 mm (3 plates) | 50 (1–4 tests); 70 (5–7 tests); 100 (8–10 tests) | 7; 8; 9; 10 (1–4 tests); 8; 9; 10 (5–7 tests); 8; 9; 10 (8–10 tests) |
| AISI 304L of 16 mm (1 plate) | 70 (1–3 tests); 100 (4–6 tests) | 8; 10; 12 (1–3 tests); 8; 10; 12 (4–6 tests) |
| CuAl5 of 10 mm (1 plate) | 100 (1–3 tests) | 10; 12; 14 (1–3 tests) |
| AlMg4.5Mn0.7 of 6 mm (1 plate) | 200 | 5.5 |
| AlMg4.5Mn0.7 of 12 mm (1 plate) | 150 | 5.7 |
| Cut Number | I, A | U, V | Maximal Working Cycle Time, s | Maximal Idle Cycle Time, s | Short-Circuit Frequency, Hz |
|---|---|---|---|---|---|
| 1 | 344 ± 80 | 38.7 ± 3.6 | 3.6 | 1.7 | 0.41 |
| 2 | 371 ± 67 | 38 ± 5.9 | 4.1 | 1.6 | 0.54 |
| 3 | 417 ± 43 | 36.5 ± 3.9 | 5 | 1.4 | 0.46 |
| 4 | 411 ± 35 | 36.7 ± 3 | 2 | 1.2 | 0.78 |
| 5 | 381 ± 43 | 38 ± 3.6 | 1.5 | 1.1 | 0.81 |
| 6 | 400 ± 50 | 36.7 ± 3 | 1.8 | 0.6 | 1.23 |
| 7 | 420 ± 26 | 36.3 ± 2.8 | 1.5 | 1.1 | 1.04 |
| 8 | 377 ± 35 | 38.1 ± 2.6 | 2.2 | 1.2 | 0.59 |
| 9 | 391 ± 34 | 37.5 ± 3.3 | 2.9 | 1.2 | 0.49 |
| 10 | 400 ± 31 | 37 ± 1.57 | 2.2 | 0.7 | 0.89 |
| Cut Number | I, A | U, V | Maximal Working Cycle Time, s | Maximal Idle Cycle Time, s | Short-Circuit Frequency, Hz |
|---|---|---|---|---|---|
| 1 | 350 ± 68 | 39.4 ± 5.4 | 3 | 1.2 | 1.3 |
| 2 | 377 ± 66 | 37.6 ± 6.3 | 0.6 | 0.3 | 2.4 |
| 3 | 389 ± 68 | 36.9 ± 6.1 | 0.5 | 0.28 | 3.1 |
| 4 | 368 ± 75 | 38.4 ± 5.9 | 2.5 | 0.8 | 0.8 |
| 5 | 391 ± 66 | 37.4 ± 5.5 | 1 | 0.53 | 1.8 |
| 6 | 402 ± 56 | 36.9 ± 3.1 | 0.5 | 0.5 | 2.2 |
| Cut Number | I, A | U, V | Maximal Working Cycle Time, s | Maximal Idle Cycle Time, s | Short-Circuit Frequency, Hz |
|---|---|---|---|---|---|
| 1 | 360 ± 90 | 37.2 ± 4.5 | 2.3 | 0.24 | 1.53 |
| 2 | 375 ± 85 | 36.5 ± 3.5 | 0.4 | 0.28 | 2.27 |
| 3 | 406 ± 54 | 35.4 ± 1,4 | 0.6 | 0.35 | 2.93 |
| Steel | Travel Speed, mm/min | Wire Feed Rate, m/min | Hardness, HV | HAZ Width, mm | |
|---|---|---|---|---|---|
| Base Metal | HAZ | ||||
| 10KhSND of 10 mm with PPR-APL1-1 | 100 | 10 | 132–157 | 116–164 | 4–5.2 |
| 10KhSND of 10 mm with PPR-APL1-2 | 100 | 10 | 130–163 | 107–177 | 4–4.5 |
| 10KhSND of 10 mm with PPR-APL1-3 | 100 | 10 | 132–158 | 106–168 | 6.2–7 |
| AISI 304 of 16 mm with PPR-APL1-1 | 100 | 10 | 208–238 | 249–398 | 2–2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parshin, S.G.; Levchenko, A.M.; Wang, P. Metallurgy and Mechanism of Underwater Wet Cutting Using Oxidizing and Exothermic Flux-Cored Wires. Materials 2021, 14, 4655. https://doi.org/10.3390/ma14164655
Parshin SG, Levchenko AM, Wang P. Metallurgy and Mechanism of Underwater Wet Cutting Using Oxidizing and Exothermic Flux-Cored Wires. Materials. 2021; 14(16):4655. https://doi.org/10.3390/ma14164655
Chicago/Turabian StyleParshin, Sergey G., Alexey M. Levchenko, and Pengfei Wang. 2021. "Metallurgy and Mechanism of Underwater Wet Cutting Using Oxidizing and Exothermic Flux-Cored Wires" Materials 14, no. 16: 4655. https://doi.org/10.3390/ma14164655







