Corrosion Resistance Measurement of 316L Stainless Steel Manufactured by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Material Preparation
2.2. Microscopic Observation of the XY and XZ Testing Surfaces
2.3. Corrosion Testing
3. Results
3.1. Chemical Microanalysis
3.2. Characterization of the Microstructure
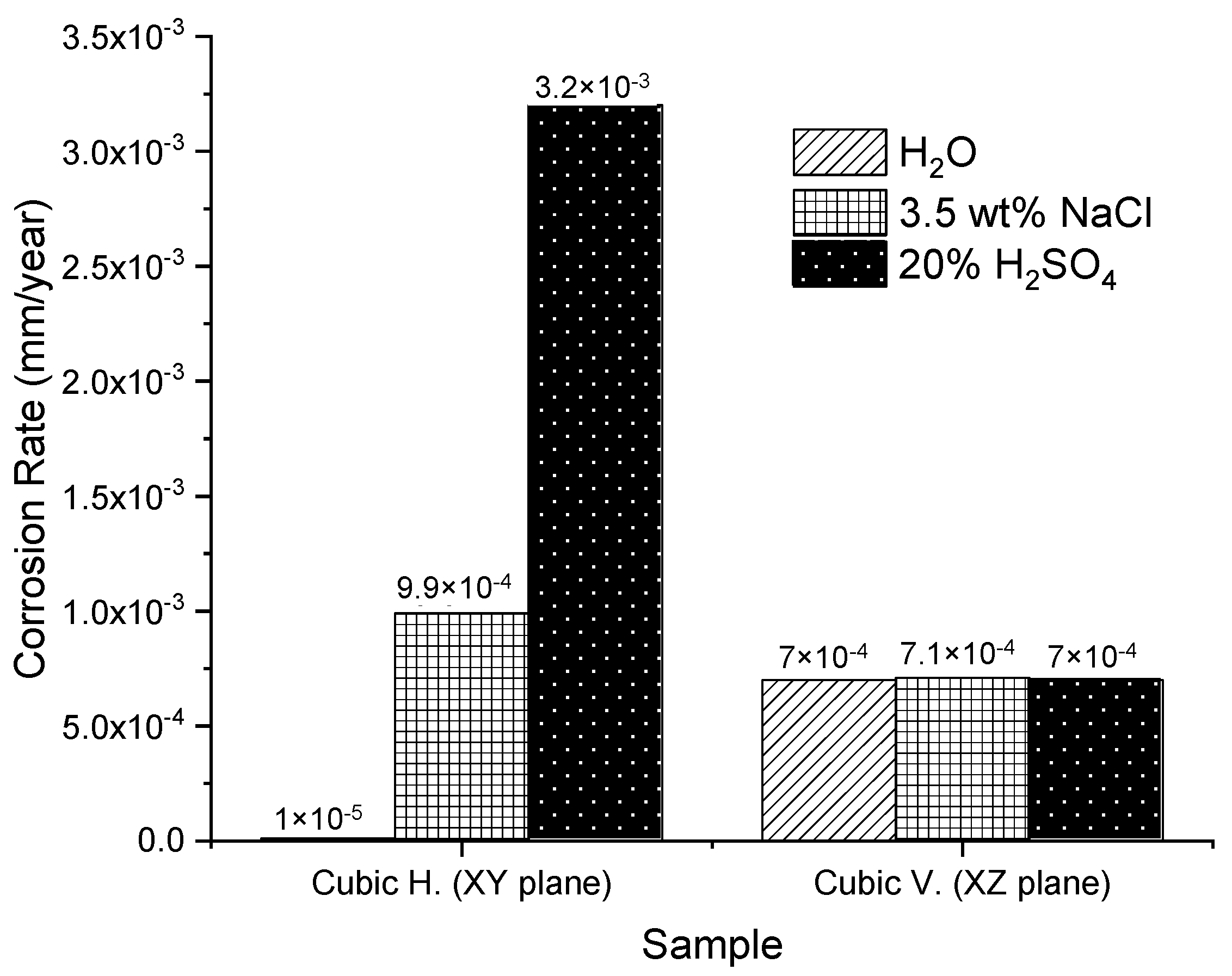
3.3. Corrosion Results
3.3.1. Linear Polarization Resistance (LPR)
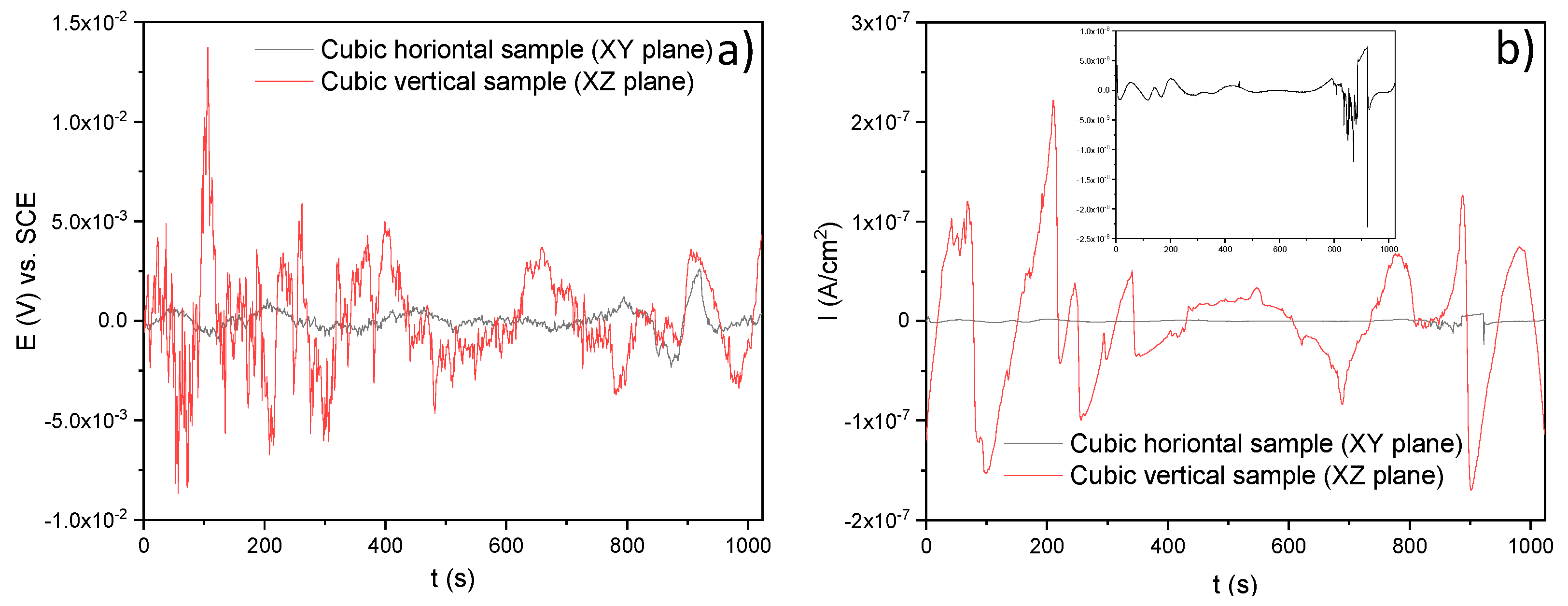
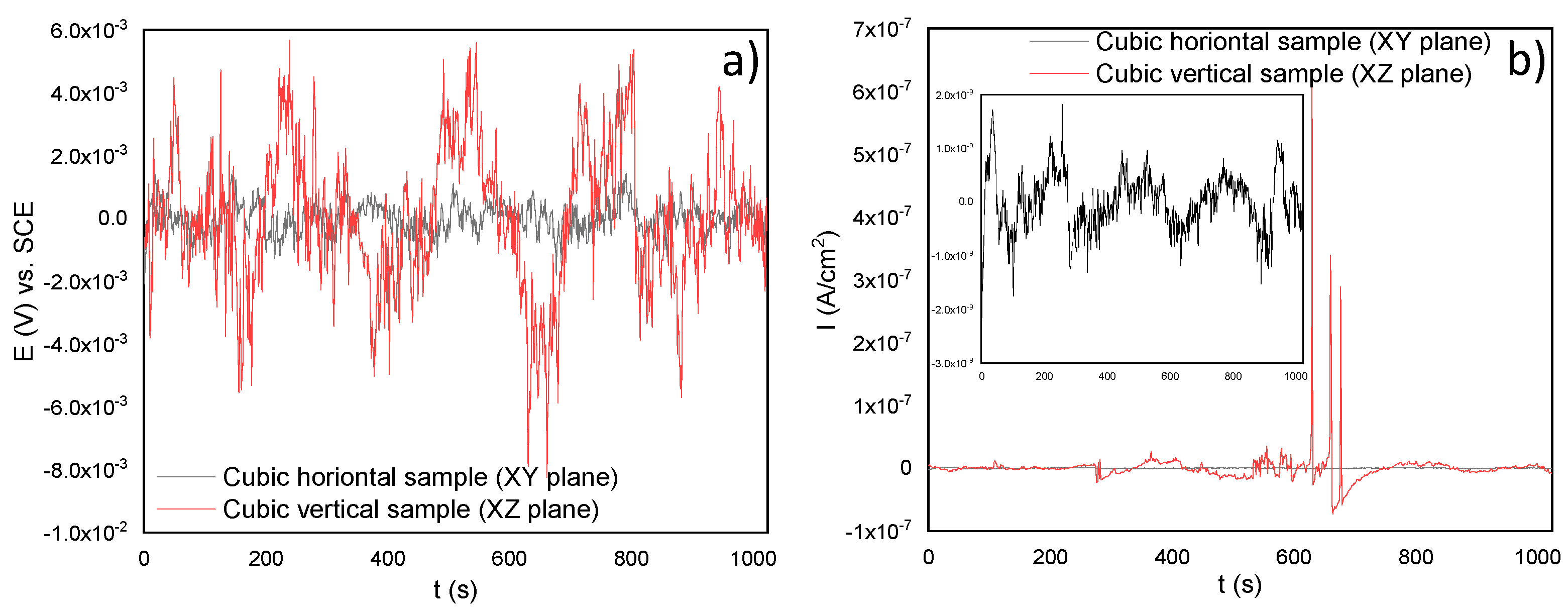
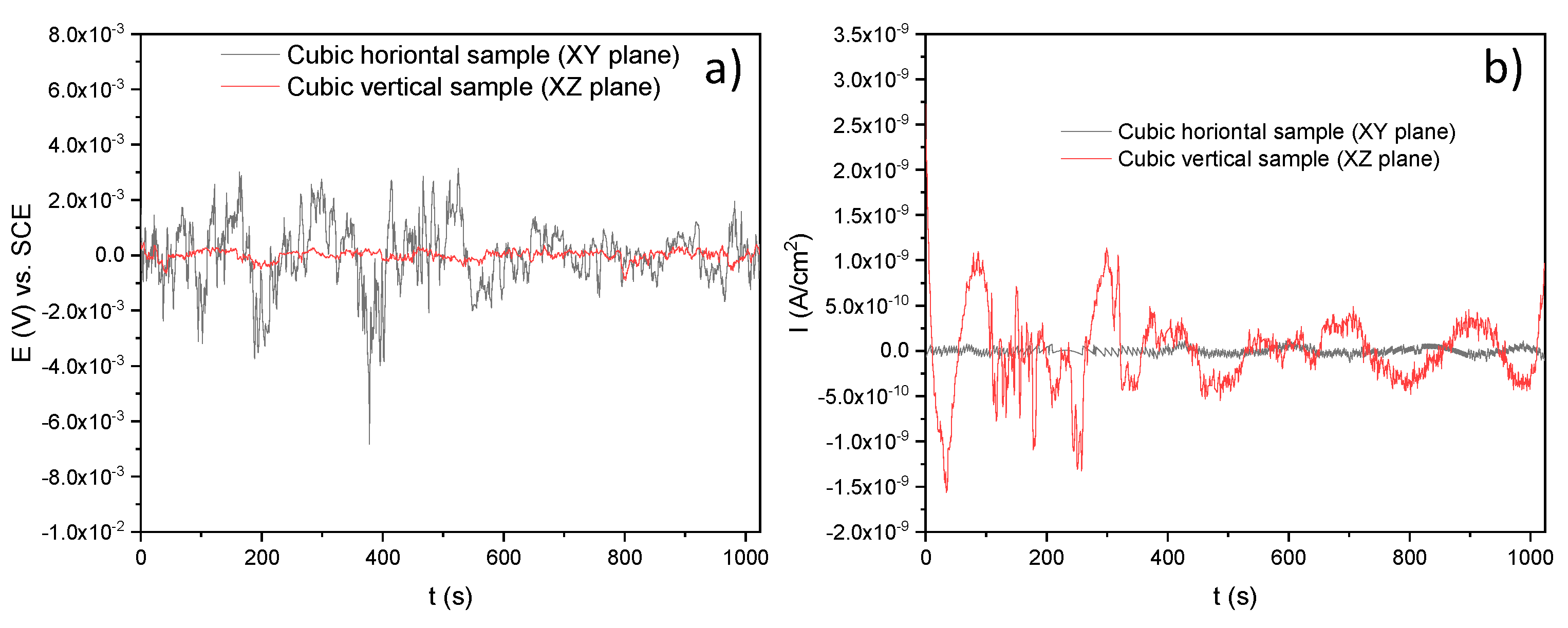
3.3.2. Electrochemical Noise (EN)
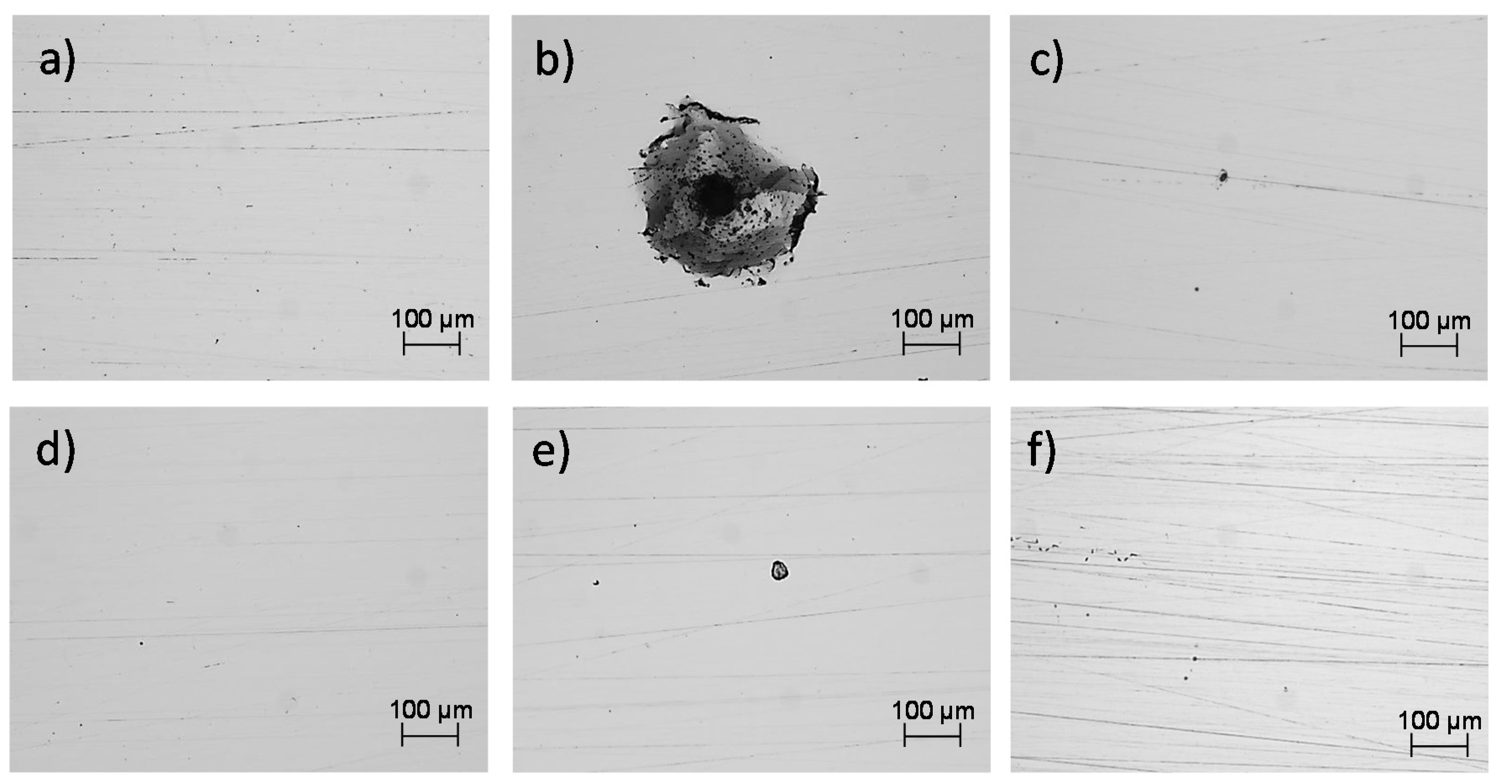
3.4. Corroded Surfaces of the SLMed Samples
4. Discussion
5. Conclusions
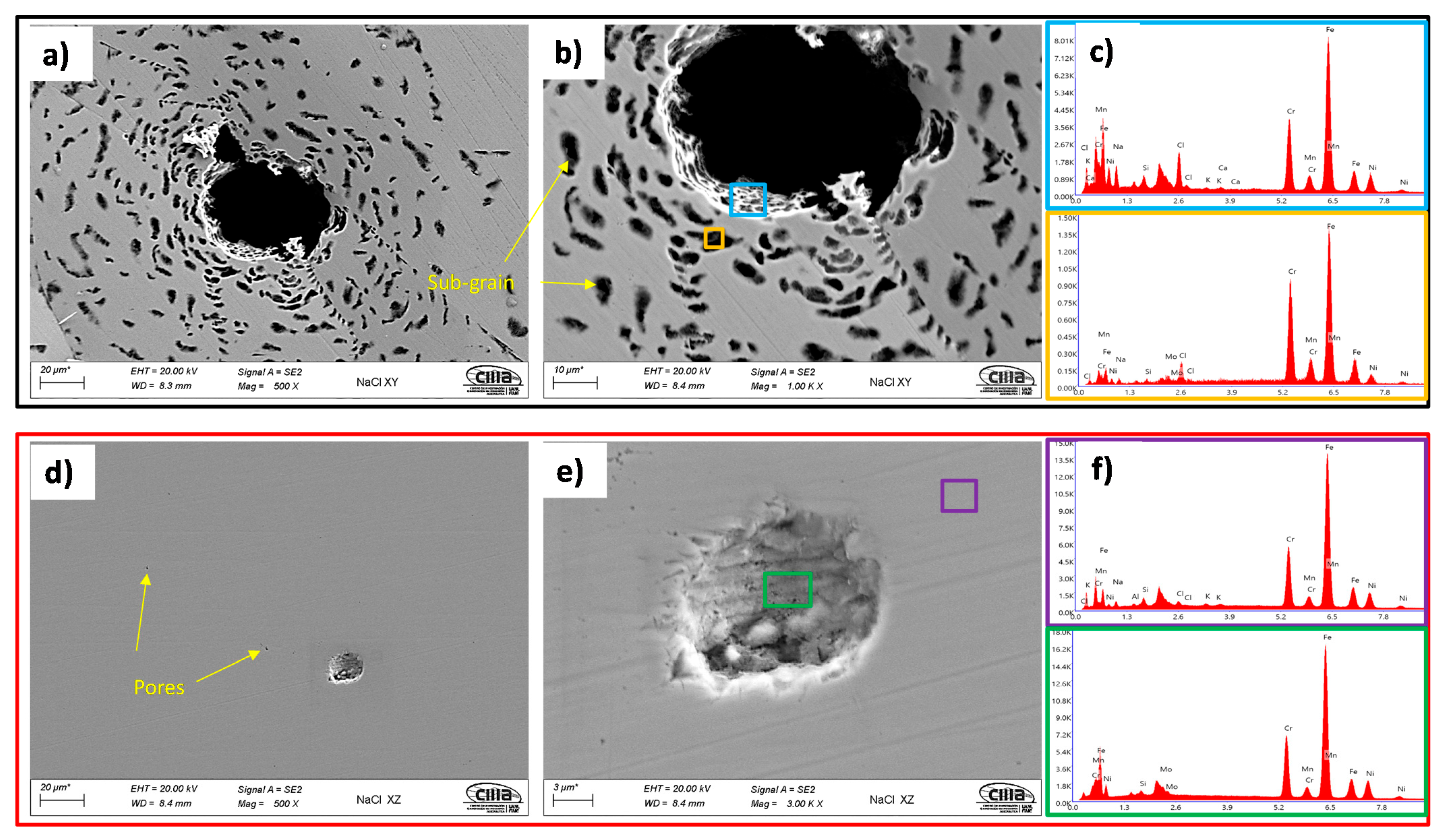
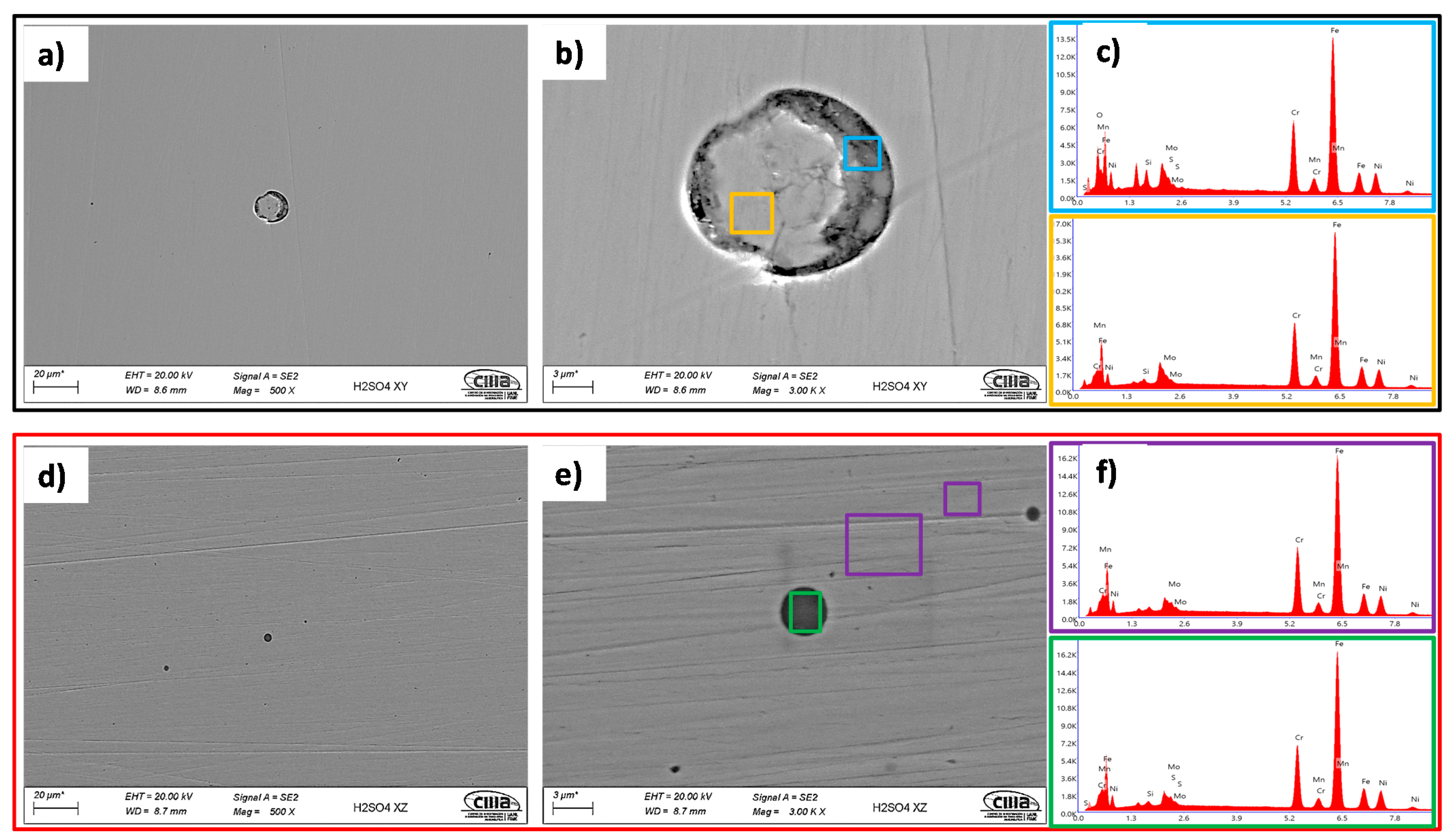
- The SEM and EDS analyses performed on the polished surface of the samples indicated the formation of irregular and spherical pores with contents of O, Al, Si, and Mn in the SLM defect. The spherical pores were attributed to the induced gas during the fast solidification process, and irregular defects with dimensions of approximately 18 were associated with the lack of metallic powder. It was confirmed by the EDS analyses of this research work that inclusions in the SLMed 316L SS were formed due to the accumulation of O, Al, Si, N, and Mn.
- Using the electrochemical technique of RPL, it was found out that the best corrosion behavior was developed in the horizontal samples than for the vertical ones. This could be because according to the tests, the SLMed 316L surfaces developed an initial passive-active behavior due to a corrosion film formed after the cutting process. At the same time, this oxide layer could form due to the interaction of the material with chloride-containing and acid solutions, similar to the oxide layers ( and ) formed through the thermal oxidation (TO) process on an SLMed 316L SS in the literature.
- Through the electrochemical noise (EN) technique, the −1.65 and −0.012 skewness factors were calculated, which indicates that the SLMed specimens developed localized corrosion in , for the horizontal and vertical samples, respectively. The localized corrosion associated with the Cl ions attacked mainly the subgrains of the microstructure and, in some cases, defects such as pores. Although the initiation of metastable pits was found due to some pre-existing SLM defects (pores) on surfaces, it could not transition to stable growing pits, thanks to the corrosion film formed in the material. For the and electrolytes, the corrosion type was uniform for the two orientations, due to it developing over the entire surface of the samples. Besides, according to the EN parameters, it was determined that the best corrosion rate in was for the horizontal samples compared with the value of for the vertical ones. However, the XY samples presented a minor corrosion resistance than for the XZ samples both tested in the and electrolytes.
- The morphology identified by SEM was correlated with the results obtained from the electrochemical techniques, where it was observed that the pits developed within the subgrains due to the galvanic par that developed between the Mo-enriched phases (subgrain boundaries) that were segregated during the fabrication.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arredondo, M.Z.; Boone, N.; Willmott, J.; Childs, D.T.D.; Ivanov, P.; Groom, K.M.; Mumtaz, K. Laser diode area melting for high speed additive manufacturing of metallic components. Mater. Des. 2017, 117, 305–315. [Google Scholar] [CrossRef]
- Lawrence, E.M.; Edwin, M.; Krista, N.A.; Sara, M.G.; Jennifer, H.; Diana, A.R.; Patrick, W.S.; Frank, M.; Ryan, B.W. Fabrication of metal and alloy components by additive manufacturing: Examples of 3D materials science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar]
- Omar, L.B.; Uriel, M.H.; José, R.; Christophe, P.; Kamran, M. Two-dimensional simulation of grain structure growth within selective laser melted AA-2024. Mater. Des. 2017, 113, 369–376. [Google Scholar] [CrossRef]
- Miranda, G.; Faria, S.; Bartolomeu, F.; Pinto, E.; Madeira, S.; Mateus, A.; Carreira, P.; Alves, N.; Silve, F.S.; Carvalho, O. Predictive models for physical and mechanical properties of 316L stainless steel produced by selective laser melting. Mater. Sci. Eng. A 2016, 657, 43–56. [Google Scholar] [CrossRef]
- Choi, J.P.; Shin, G.H.; Brochu, M.; Kim, Y.J.; Yang, S.S.; Kim, K.T.; Yang, D.Y.; Lee, C.W.; Yu, J.H. Densification Behavior of 316L Stainless Steel Parts Fabricated by Selective Laser Melting by Variation in Laser Energy Density. Mater. Trans. 2016, 57, 1952–1959. [Google Scholar] [CrossRef] [Green Version]
- Dai, D.; Gu, D. Tailoring surface quality through mass and momentum transfer modeling using a volume of fluid method in selective laser melting of TiC/AlSi10Mg powder. Int. J. Mach. Tools Manuf. 2015, 88, 95–107. [Google Scholar] [CrossRef]
- Agarwala, M.; Bourell, D.; Beaman, J.; Marcus, H.; Barlow, J. Direct selective laser sintering of metals. Rapid Prototyp. J. 1995, 1, 26. [Google Scholar] [CrossRef]
- Thompson, S.M.; Bian, L.; Shamsaei, N.; Yadollahi, A. An overview of Direct Laser Deposition for additive manufacturing; Part I: Transport phenomena, modeling and diagnostics. Addit. Manuf. 2015, 8, 36–62. [Google Scholar] [CrossRef]
- Cheng, B.; Price, S.; Lydon, J.; Cooper, K.; Chou, K. On Process Temperature in Powder-Bed Electron Beam Additive Manufacturing: Model Development and Validation. J. Manuf. Sci. Eng. 2014, 136, 061018. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Smurov, I. Selective laser melting technology: From the single laser melted track stability to 3D parts of complex shape. Phys. Procedia 2010, 5, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Carvalho, O.; Silva, F.S.; Miranda, G. 316L stainless steel mechanical and tribological behavior—A comparison between selective laser melting, hot pressing and conventional casting. Addit. Manuf. 2017, 16, 81–89. [Google Scholar] [CrossRef]
- Yadollahi, A.; Shamsaei, N.; Thompson, S.M.; Seely, D.W. Effects of process time interval and heat treatment on the mechanical and microstructural properties of direct laser deposited 316L stainless steel. Mater. Sci. Eng. A 2015, 644, 171–183. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mirzaeifar, R.; Moghaddam, N.S.; Turabi, A.S.; Karaca, H.E.; Elahinia, M. Effect of manufacturing parameters on mechanical properties of 316L stainless steel parts fabricated by selective laser melting: A computational framework. Mater. Des. 2016, 112, 328–338. [Google Scholar] [CrossRef]
- Saeidi, K.; Neikter, M.; Olsen, J.; Shen, Z.J.; Akhtar, F. 316L stainless steel designed to withstand intermediate temperature. Mater. Des. 2017, 135, 1–8. [Google Scholar] [CrossRef]
- Deev, A.A.; Kuznetcov, P.A.; Petrov, S.N. Anisotropy of mechanical properties and its correlation with the structure of the stainless steel 316L produced by the SLM method. Phys. Procedia 2016, 83, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, J.; Prashanth, K.G.; Ramamurty, U. Mechanical behavior of selective laser melted 316L stainless steel. Mater. Sci. Eng. A 2017, 696, 113–121. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Yusuf, S.M.; Nie, M.; Chen, Y.; Yang, S.; Gao, N. Microstructure and corrosion performance of 316L stainless steel fabricated by Selective Laser Melting and processed through high-pressure torsion. J. Alloys Compd. 2018, 763, 360–375. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Deen, K.M.; Haider, W. Corrosion behavior of additively manufactured 316L stainless steel in acidic media. Materialia 2018, 2, 111–121. [Google Scholar] [CrossRef]
- Nie, J.; Wei, L.; Jiang, Y.; Li, Q.; Luo, H. Corrosion mechanism of additively manufactured 316 L stainless steel in 3.5 wt.% NaCl solution. Mater. Today Commun. 2020, 26, 101648. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, S.; Shi, Q.; Tao, H.; Song, Y.; Zheng, J.; Xu, P.; Zhang, L. Improvement of corrosion resistance of SS316L manufactured by selective laser melting through subcritical annealing. Corros. Sci. 2019, 164, 108353. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Rao, H.; Giet, S.; Yang, K.; Wu, X.; Davies, C.H.J. The influence of processing parameters on aluminium alloy A357 manufactured by Selective Laser Melting. Mater. Des. 2016, 109, 334–346. [Google Scholar] [CrossRef]
- Serafin, D.; Bartkowiak, T.; Nowak, W.J.; Wierzba, B. Influence of microgeometry of iron surface on the oxidation process—A comparison of multiscale geometric methods and their applicability. Appl. Surf. Sci. 2020, 527, 146838. [Google Scholar] [CrossRef]
- Geenen, K.; Röttger, A.; Theisen, W. Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting. Mater. Corros. 2017, 68, 764–775. [Google Scholar] [CrossRef]
- Jinlong, L.; Meng, Y.; Tongxiang, L. The effect of surface enriched chromium and grain refinement by ball milling on corrosion resistance of 316L stainless steel. Mater. Res. Bull. 2017, 91, 91–97. [Google Scholar] [CrossRef]
- Ettefagh, A.H.; Guo, S. Electrochemical behavior of AISI316L stainless steel parts produced by laser-based powder fusion process and the effect of post annealing process. Addit. Manuf. 2018, 22, 153–156. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and Corrosion Resistance of Laser Additively Manufactured 316L Stainless Steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Sun, Y.; Moroz, A.; Alrbaey, K. Sliding wear characteristics and corrosion behaviour of selective laser melted 316L stainless steel. J. Mater. Eng. Perform. 2014, 23, 518–526. [Google Scholar] [CrossRef]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Hodgson, P.D.; Fabijanic, D. On the enhanced corrosion resistance of a selective laser melted austenitic stainless steel. Scripta Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Kwok, C.T.; Fong, S.L.; Cheng, F.T.; Man, H.C. Pitting and galvanic corrosion behavior of laser-welded stainless steels. J. Mater. Process. Technol. 2006, 176, 168–178. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Romlay, F.R.M.; Sharif, S.; Jan, N.H.M.; Tsumori, F. Surface characterisation and corrosion behaviour of oxide layer for SLMed-316L stainless steel. J. Alloys Compd. 2018, 748, 1044–1052. [Google Scholar] [CrossRef]
- Li-jun, Z.; Xu-bei, Z.; Zhao, Z.; Jian-qing, Z. Electrochemical noise characteristics in corrosion process of AZ91D magnesium alloy in neutral chloride solution. Trans. Nonferrous Met. Soc. China 2009, 19, 496–503. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Aguilar L, M.R.; Zambrano Robledo, P.; Estupiñán López, F.; Cabral Miramontes, J.A.; Nieves-Mendoza, D.; Castillo-Gonzáles, E.; Almeraya-Calderón, F. Electrochemical Noise Analysis of Nickel Based Superalloys in Acid Solutions. Int. J. Electrochem. Sci. 2014, 9, 523–533. [Google Scholar]
- ASTM International. ASTM G59-972014. Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 8 March 2021).
- ASTM International. ASTM G199-092014. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 8 March 2021).
- Xiong, Z.; Li, Z.; Sun, Z.; Hao, S.; Yang, Y.; Li, M.; Song, C.; Qiu, P.; Cui, L. Selective laser melting of NiTi alloy with superior tensile property and shape memory effect. J. Mater. Sci. Technol. 2019, 35, 2238–2242. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Lofaj, F.; Kvetkova, L.; Shen, Z.J. Transformation of austenite to duplex austenite-ferrite assembly in annealed stainless steel 316L consolidated by laser melting. J. Alloys Compd. 2015, 633, 463–469. [Google Scholar] [CrossRef]
- Wang, D.; Song, C.; Yang, Y.; Bai, Y. Investigation of crystal growth mechanism during selective laser melting and mechanical property characterization of 316L stainless steel parts. Mater. Des. 2016, 100, 291–299. [Google Scholar] [CrossRef]
- ASTM International. ASTM G102-892015e1. Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015; Available online: www.astm.org (accessed on 8 March 2021).
- Sanchez-Carrillo, M.; Flores-de los Rios, J.P.; Nava-Dino, C.G.; Flores-Zuñiga, H.; Narro-Garcia, R.; Maldonado-Orozco, M.C.; Estupiñan-Lopez, F.H.; Chacon-Nava, J.G. Transformation Temperatures and Electrochemical behavior of Polycrystalline Fe-Doped Ni-Mn-Ga and Co-Ni-Ga Alloys. Int. J. Electrochem. Sci. 2018, 13, 6666–6675. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Tor, S.B.; Chua, C.K. Simultaneously enhanced strength and ductility for 3D-printed stainless steel 316L by selective laser melting. NPG Asia Mater. 2018, 10, 127–136. [Google Scholar] [CrossRef]
- Andreatta, F.; Lanzutti, A.; Vaglio, E.; Totis, G.; Sortino, M.; Fedrizzi, L. Corrosion behaviour of 316L stainless steel manufactured by selective laser melting. Mater. Corros. 2019, 70, 1–13. [Google Scholar] [CrossRef]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On the corrosion and metastable pitting characteristics of 316L stainless steel produced by selective laser melting. J. Electrochem. Soc. 2017, 164, 250–257. [Google Scholar] [CrossRef]
- Cottis, R.A. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Ziętala, M.; Durejko, T.; Polański, M.; ŁaKunce, I.; Płociński, T.; Zieliński, W.; Łazińska, M.; Stępniowski, W.; Czujko, T.; Kurzydłowski, K.J.; et al. The microstructure, mechanical properties and corrosion resistance of 316L stainless steel fabricated using laser engineered net shaping. Mater. Sci. Eng. A 2016, 677, 1–10. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z.J. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
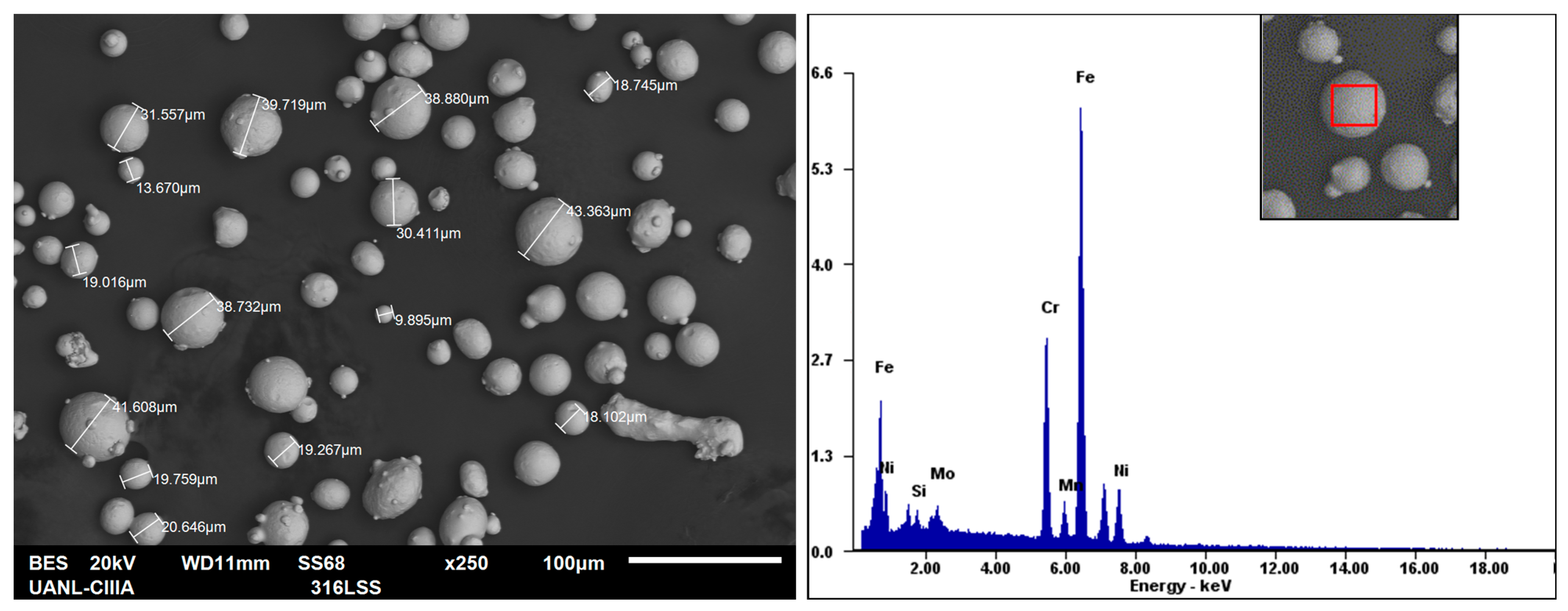
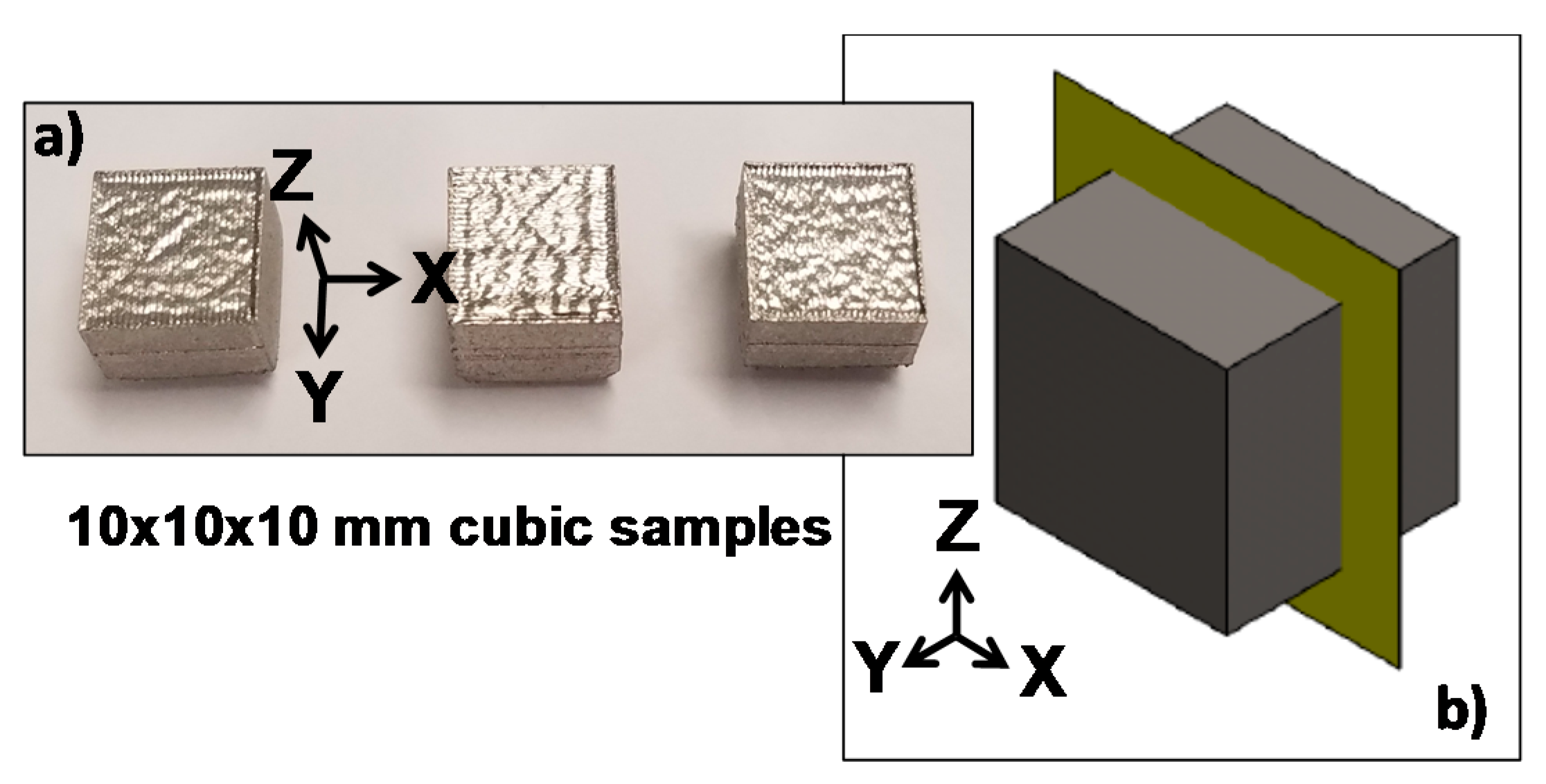
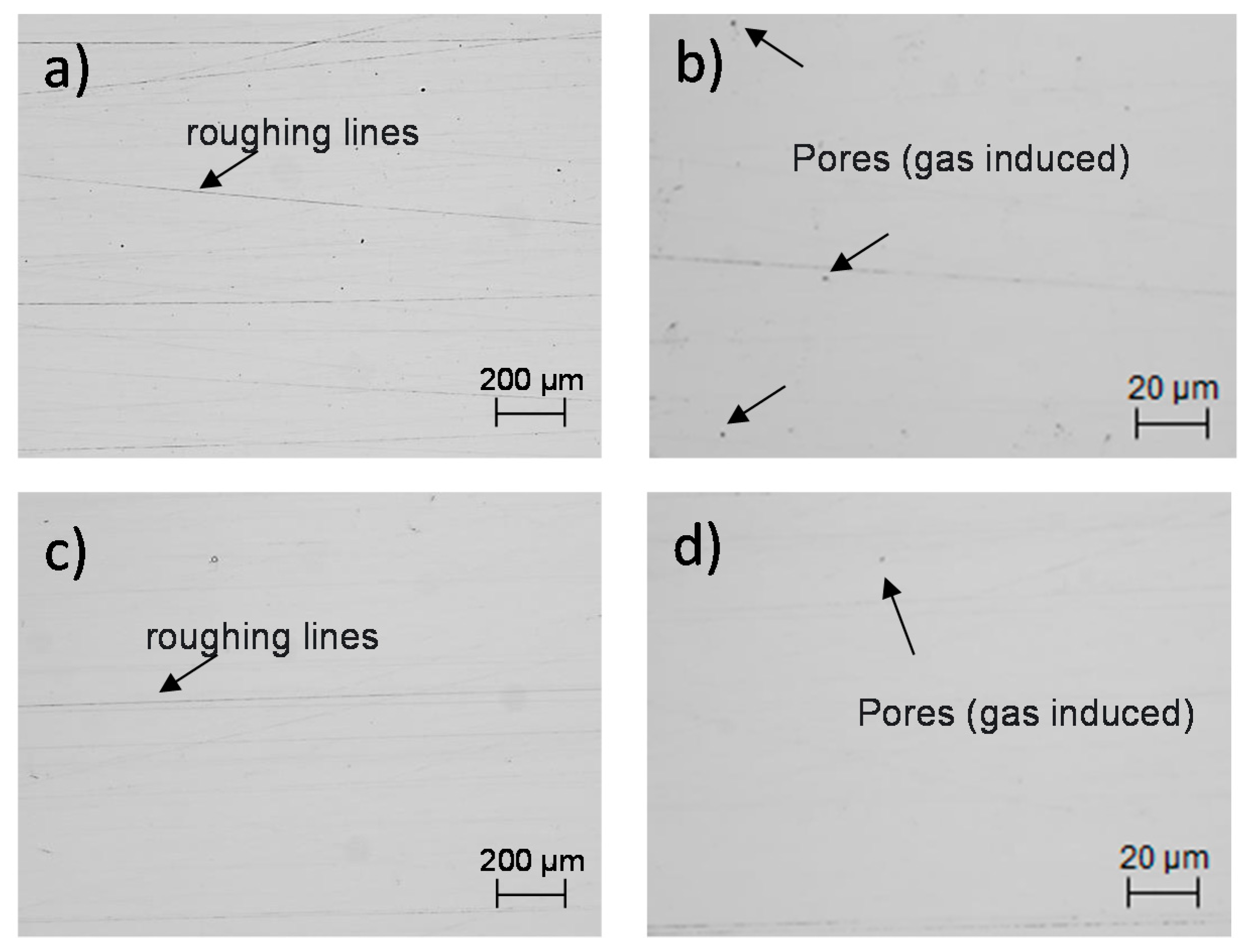

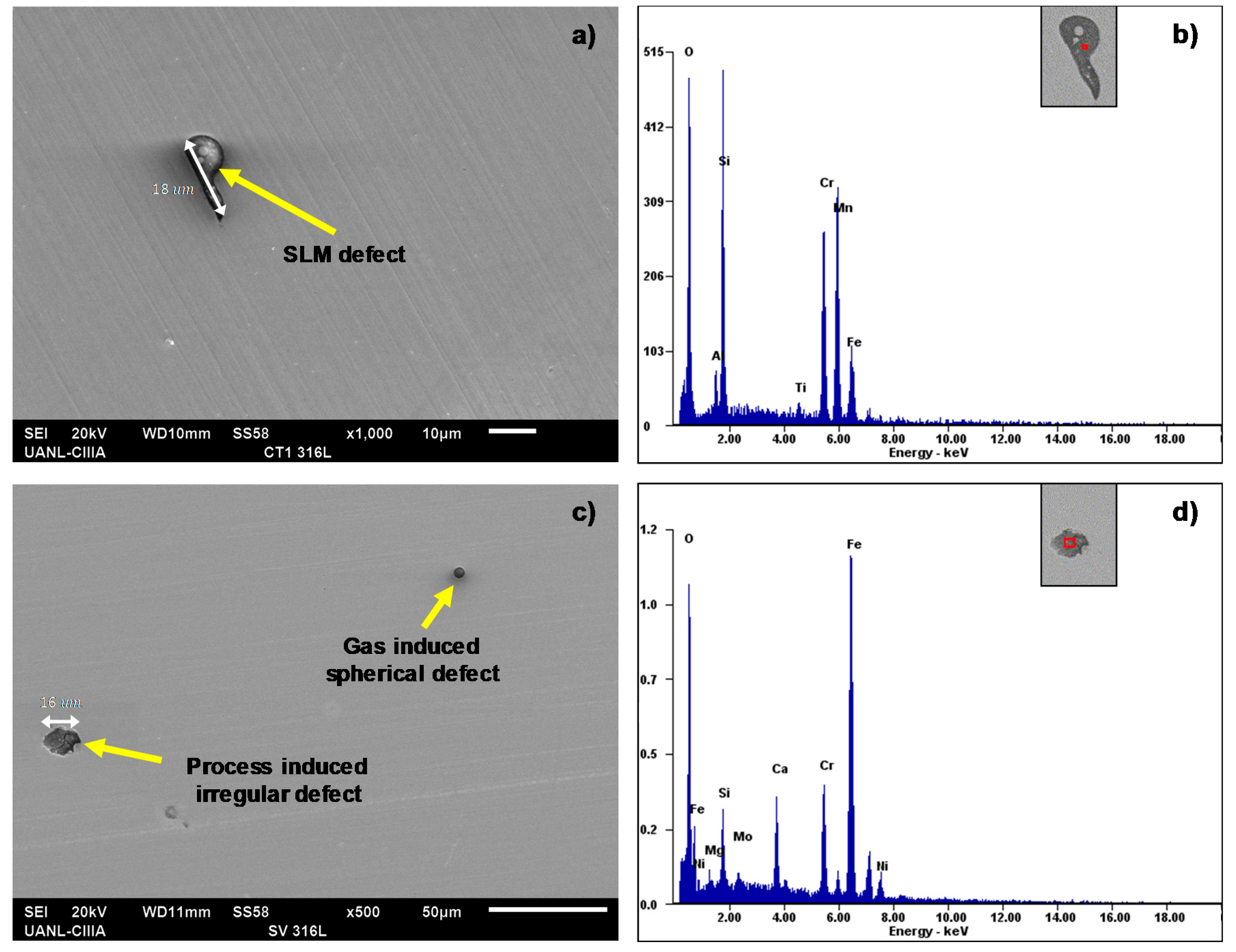


| Element | C | Cr | Cu | Mn | Mo | Ni | P | S | Si | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Powder | 0.03 | 17.5–18.0 | 0.50 | 2.0 | 2.25–2.50 | 12.5–13 | 0.025 | 0.01 | 0.75 | Bal. |
| SLM-prepared sample | - | 18.67 | 0.04 | 1.13 | 2.55 | 11.84 | - | - | - | Bal. |
| Parameter | Value |
|---|---|
| Laser power (W) | |
| Scanning speed (mm/s) | |
| Hatch spacing (μm) | |
| Layer thickness (μm) | |
| Scan rotation between successive layers | |
| Energy density (J/mm3) |
| Sample | Electrolyte | Electrochemical Parameters | ||
|---|---|---|---|---|
| Horizontal (XY plane) | ||||
| Sample | Electrolyte | Electrochemical Parameters | ||
|---|---|---|---|---|
| Vertical (XZ plane) | ||||
| Electrolyte | Sample | ||
|---|---|---|---|
| Horizontal | Vertical | ||
| (XY Plane) | (XZ Plane) | ||
| Localized | Localized | ||
| Uniform | Uniform | ||
| Uniform | Uniform | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Nogales, R.; Estupiñán-López, F.; Gaona-Tiburcio, C.; Lopez-Botello, O.E.; Ramírez-Rodríguez, J.G.; Zambrano-Robledo, P.C. Corrosion Resistance Measurement of 316L Stainless Steel Manufactured by Selective Laser Melting. Materials 2021, 14, 4509. https://doi.org/10.3390/ma14164509
Guzmán-Nogales R, Estupiñán-López F, Gaona-Tiburcio C, Lopez-Botello OE, Ramírez-Rodríguez JG, Zambrano-Robledo PC. Corrosion Resistance Measurement of 316L Stainless Steel Manufactured by Selective Laser Melting. Materials. 2021; 14(16):4509. https://doi.org/10.3390/ma14164509
Chicago/Turabian StyleGuzmán-Nogales, Rigoberto, Francisco Estupiñán-López, Citlalli Gaona-Tiburcio, Omar E. Lopez-Botello, Juan G. Ramírez-Rodríguez, and Patricia C. Zambrano-Robledo. 2021. "Corrosion Resistance Measurement of 316L Stainless Steel Manufactured by Selective Laser Melting" Materials 14, no. 16: 4509. https://doi.org/10.3390/ma14164509






