Optically Controlled Supercapacitors: Functional Active Carbon Electrodes with Semiconductor Particles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Characterizations
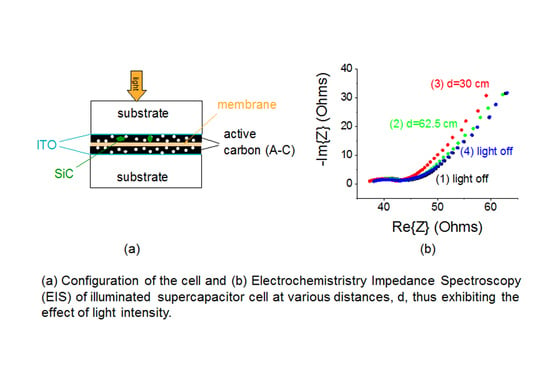
3.2. Optical Related Effects
3.3. Thermal Considerations
3.4. Model
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grebel, H. Asymmetric Supercapacitors: Optical and Thermal Effects When Active Carbon Electrodes Are Embedded with Nano-Scale Semiconductor Dots. C 2021, 7, 7. [Google Scholar] [CrossRef]
- Tsay, K.-C.; Zhang, L.; Zhang, J. Effects of electrode layer composition/thickness and electrolyte concentration on both specific capacitance and energy density of supercapacitor. Electrochim. Acta 2012, 60, 428–436. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, X.; He, Y.; Jiang, Y.; Lin, K. Nitrogen Doped Macroporous Carbon as Electrode Materials for High Capacity of Supercapacitor. Polymers 2017, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaempgen, M.; Chan, C.K.; Ma, J.; Cui, Y.; Gruner, G. Printable Thin Film Supercapacitors Using Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Long, J.W.; Bélanger, D.; Brousse, T.; Sugimoto, W.; Sassin, M.B.; Crosnier, O. Asymmetric electrochemical capacitors—Stretching the limits of aqueous electrolytes. MRS Bull. 2011, 36, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Rahmanifar, M.; Hemmati, M.; Noori, A.; El-Kady, M.F.; Mousavi, M.F.; Kaner, R.B. Asymmetric supercapacitors: An alternative to activated carbon negative electrodes based on earth abundant elements. Mater. Today Energy 2019, 12, 26–36. [Google Scholar] [CrossRef]
- Miao, X.; Rojas-Cessa, R.; Mohamed, A.; Grebel, H. The Digital Power Networks: Energy Dissemination Through a Micro-Grid. In Proceedings of the 2010 IEEE/ACM International Conference on Green Computing and Communications & International Conference on Cyber, Physical and Social Computing, Washington, DC, USA, 18–20 December 2010. [Google Scholar]
- Rojas-Cessa, R.; Grebel, H.; Jiang, Z.; Fukuda, C.; Pita, H.; Chowdhury, T.S.; Dong, Z.; Wan, Y. Integration of alternative energy sources into digital micro-grids. Environ. Prog. Sustain. Energy 2018, 37, 155–164. [Google Scholar] [CrossRef]
- Polat, E.O.; Kocabas, C. Broadband Optical Modulators Based on Graphene Supercapacitors. Nano Lett. 2013, 13, 5851–5857. [Google Scholar] [CrossRef]
- Palacin, M.R. Battery Materials Design Essentials. Accounts Mater. Res. 2021, 2, 319–326. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Saha, S.; Samanta, P.; Murmu, N.C.; Kuila, T. A review on the heterostructure nanomaterials for supercapacitor application. J. Energy Storage 2018, 17, 181–202. [Google Scholar] [CrossRef]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does The Shape Win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, W.; Li, R.; Zhang, Y.; Xu, G.; Cheng, H. Fabrication of Highly Transparent and Conductive Indium–Tin Oxide Thin Films with a High Figure of Merit via Solution Processing. Langmuir 2013, 29, 13836–13842. [Google Scholar] [CrossRef]
- Kyrish, M.; Utzinger, U.; Descour, M.R.; Baggett, B.K.; Tkaczyk, T.S. Ultra-slim plastic endomicroscope objective for non-linear microscopy. Opt. Express 2011, 19, 7603–7615. [Google Scholar] [CrossRef]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380–700 nm) of pure water. II. Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef]
- Han, H.; Chen, J.; Diamant, Y.; Etienne, M.; Walser, A.; Dorsinville, R.; Grebel, H. Nonlinear transmission properties of nanostructures with single-wall carbon nanotubes and conductive polymers. Appl. Phys. Lett. 2005, 86, 053113. [Google Scholar] [CrossRef]
- Chen, J.; Han, H.; Diamant, Y.; Grebel, H.; Etienne, M.; Dorsinville, R. Optical and optoelectronic properties of inverse photonic crystals composed of single-wall carbon nanotubes and conductive polymers. In Proceedings of the Conference on Lasers and Electro-Optics, San Francisco, CA, USA, 16–21 May 2004. Paper CWA38. [Google Scholar]
- Srivastava, I.; Khamo, J.S.; Pandit, S.; Fathi, P.; Huang, X.; Cao, A.; Haasch, R.T.; Nie, S.; Zhang, K.; Pan, D. Influence of Electron Acceptor and Electron Donor on the Photophysical Properties of Carbon Dots: A Comparative Investigation at the Bulk-State and Single-Particle Level. Adv. Funct. Mater. 2019, 29, 1902466. [Google Scholar] [CrossRef]
- Mei, B.-A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- SiC-Silicon Carbide. Thermal Properties. Available online: http://www.ioffe.ru/SVA/NSM/Semicond/SiC/thermal.html (accessed on 27 July 2021).
- Wang, Z.; Alaniz, J.E.; Jang, W.; Garay, J.E.; Dames, C. Thermal Conductivity of Nanocrystalline Silicon: Importance of Grain Size and Frequency-Dependent Mean Free Paths. Nano Lett. 2011, 11, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Brown, J. The design of metallic delay dielectrics. Proc. IEE Part III Radio Comm. Eng. 1950, 97, 45–48. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Grebel, H.; Iqbal, Z.; White, C.W. Artificial dielectrics: Nonlinear properties of Si nanoclusters formed by ion implantation in SiO2 glassy matrix. J. App. Phys. 1998, 84, 6502–6506. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebel, H. Optically Controlled Supercapacitors: Functional Active Carbon Electrodes with Semiconductor Particles. Materials 2021, 14, 4183. https://doi.org/10.3390/ma14154183
Grebel H. Optically Controlled Supercapacitors: Functional Active Carbon Electrodes with Semiconductor Particles. Materials. 2021; 14(15):4183. https://doi.org/10.3390/ma14154183
Chicago/Turabian StyleGrebel, Haim. 2021. "Optically Controlled Supercapacitors: Functional Active Carbon Electrodes with Semiconductor Particles" Materials 14, no. 15: 4183. https://doi.org/10.3390/ma14154183