Spectral Characteristics and Molecular Structure of (E)-1-(4-Chlorophenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-en-1-One (DAP)
Abstract
:1. Introduction
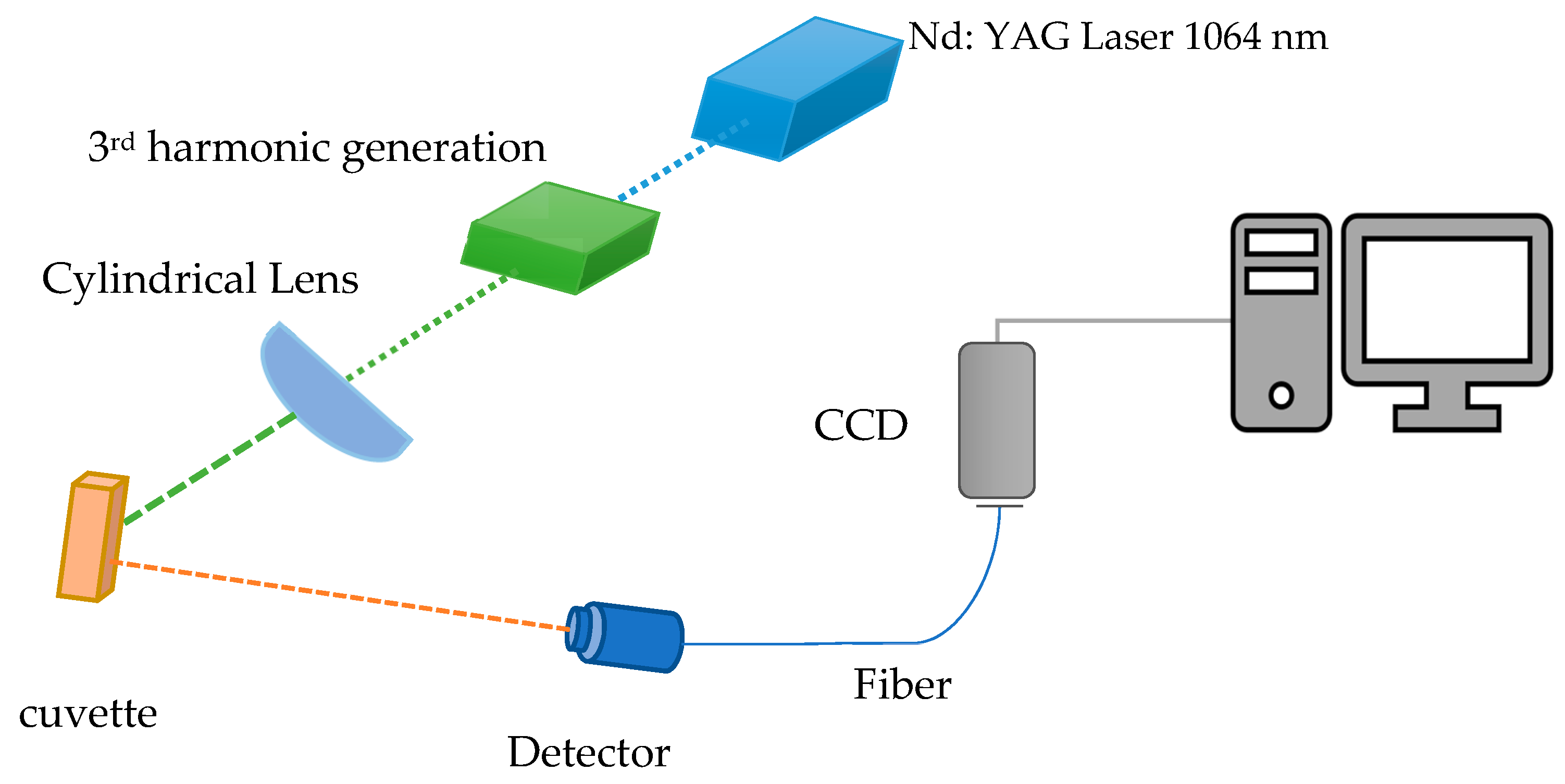
2. Materials and Methods
3. Results and Discussion
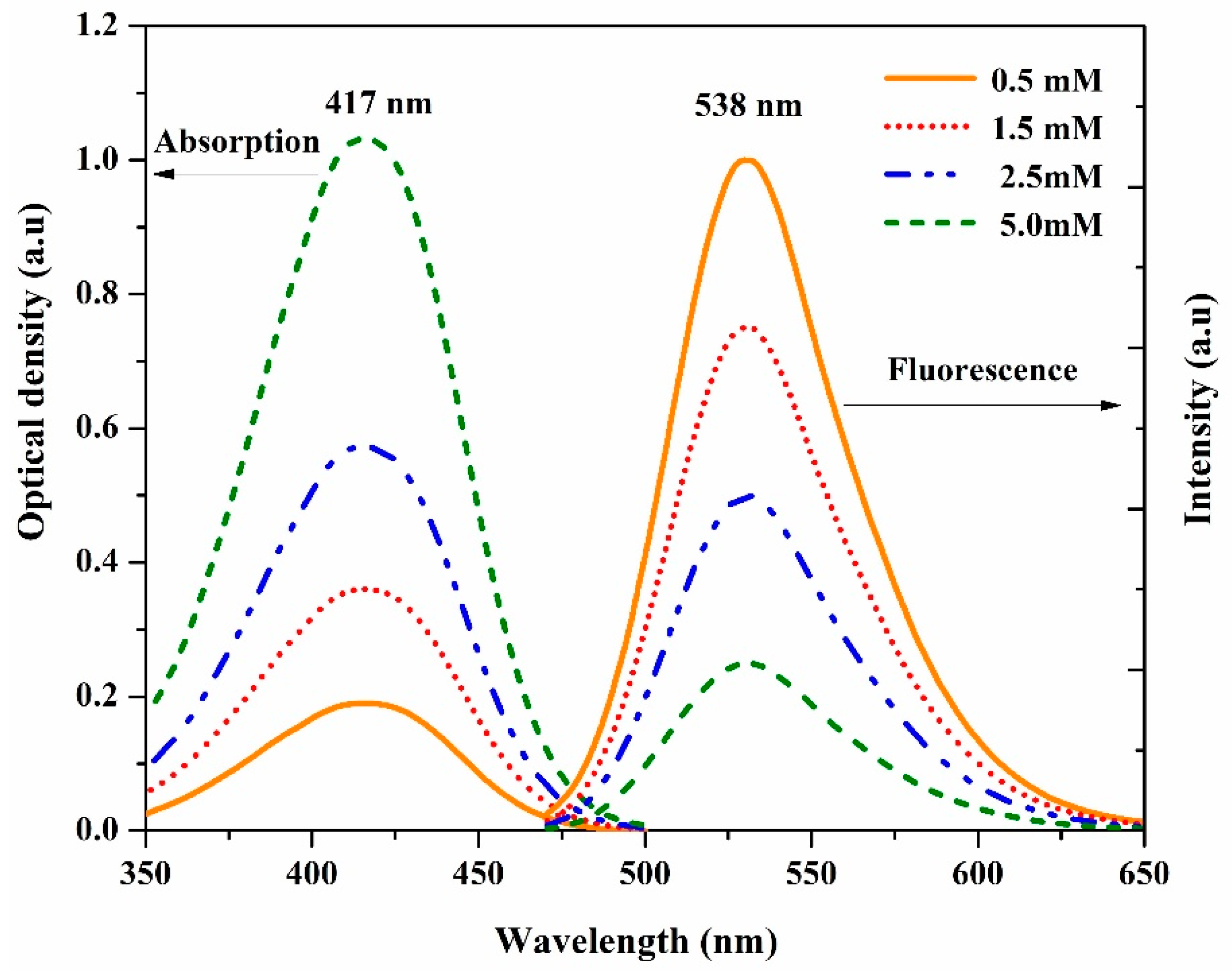
3.1. The Steady-State of DAP
3.2. Stokes Shift
3.3. Quantum Yield of Fluorescence
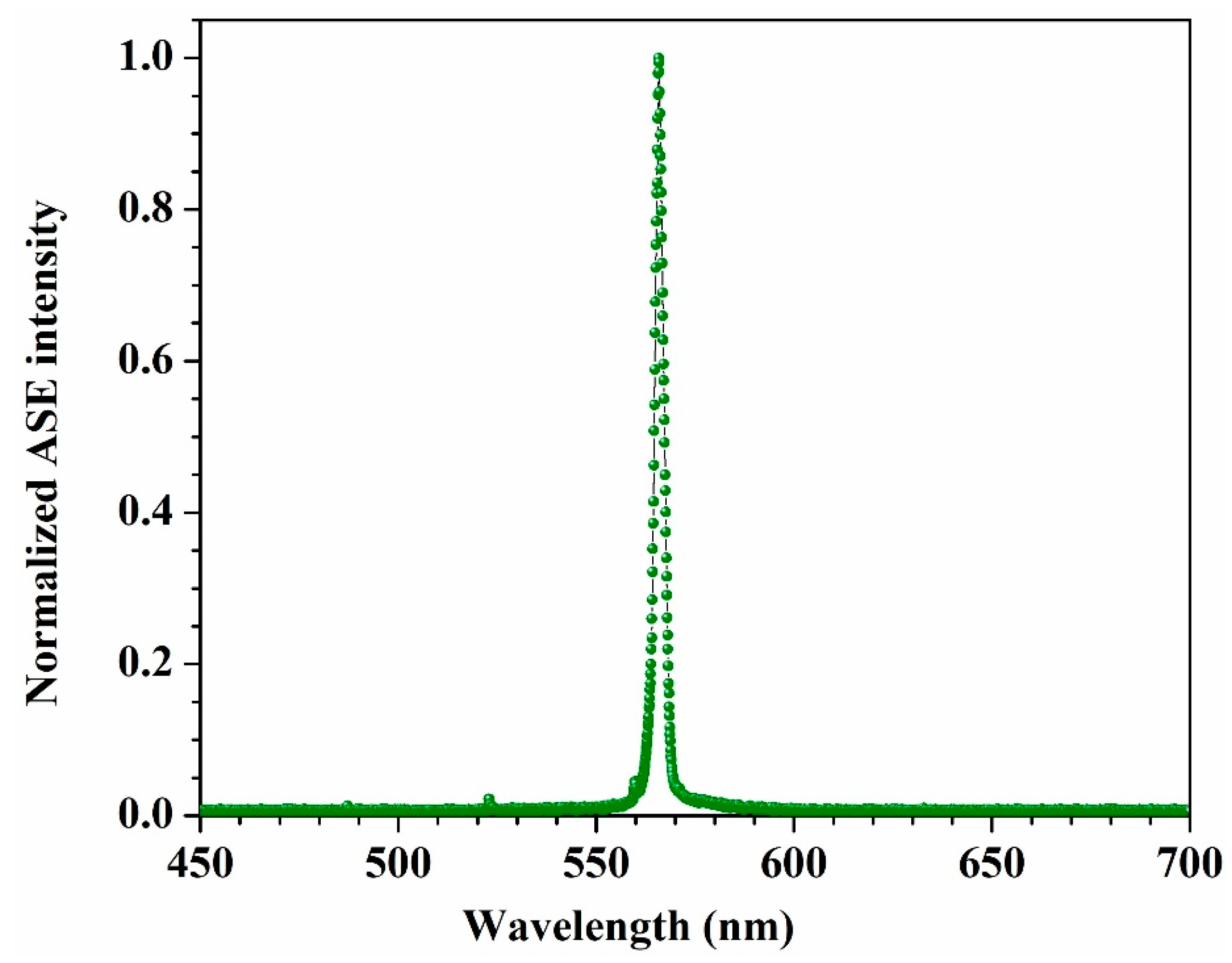
3.4. Amplified Spontaneous Emission (ASE)
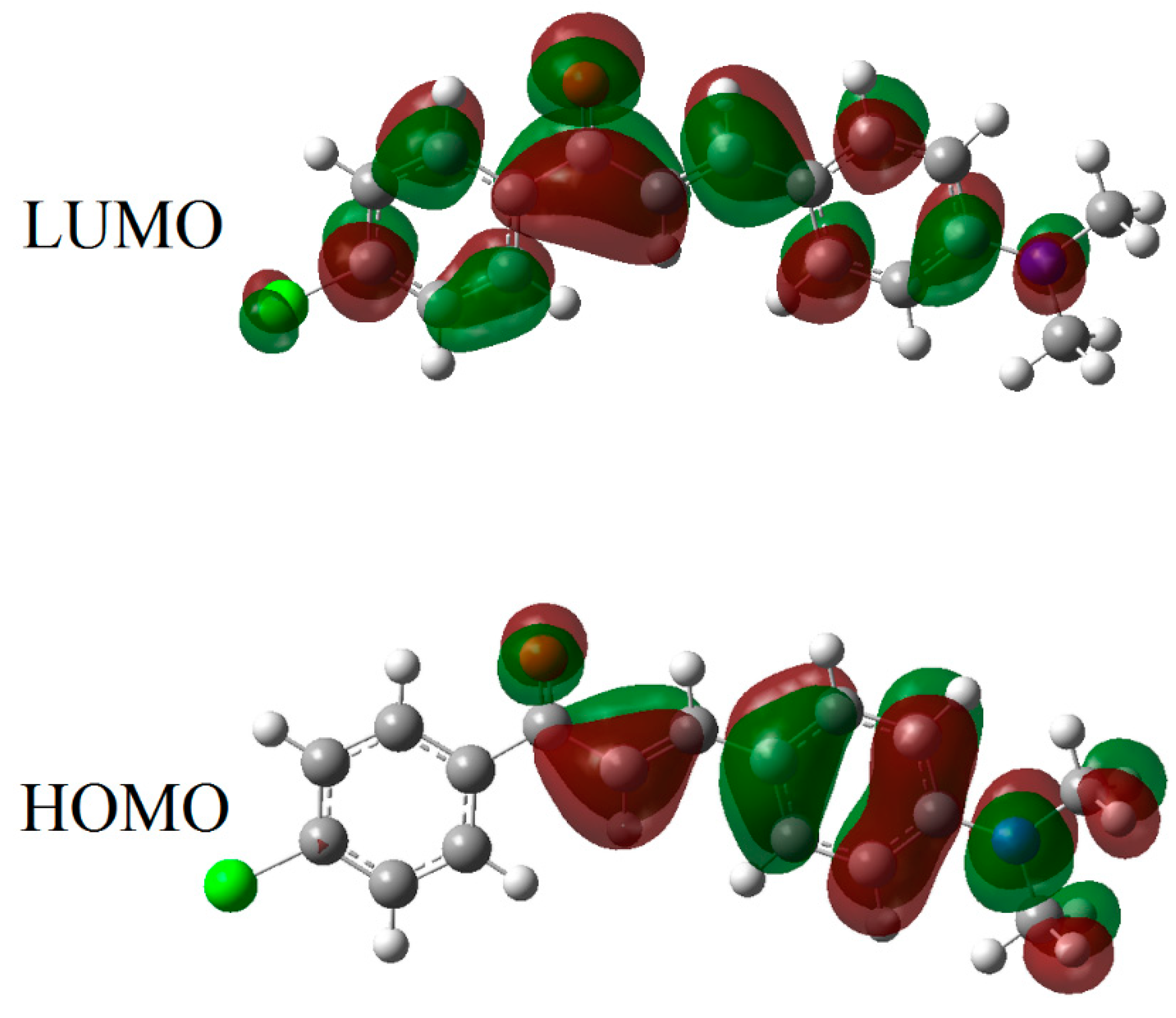
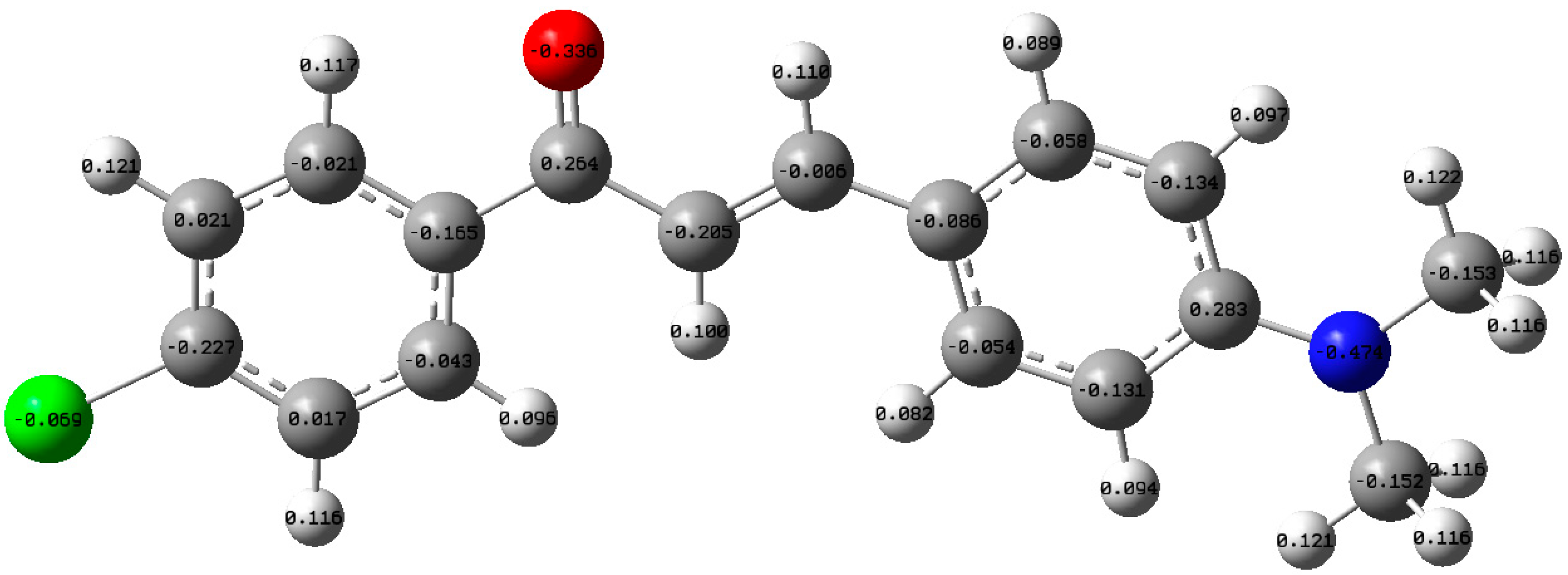
3.5. Theoretical Calculations
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adachi, C.; Sandanayaka, A.S. The Leap from Organic Light-Emitting Diodes to Organic Semiconductor Laser Diodes. CCS Chem. 2020, 2, 1203–1216. [Google Scholar] [CrossRef]
- Badgurjar, D.; Shan, B.; Nayak, A.; Wu, L.; Chitta, R.; Meyer, T.J. Electron-Withdrawing Boron Dipyrromethene Dyes As Visible Light Absorber/Sensitizers on Semiconductor Oxide Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 7768–7776. [Google Scholar] [CrossRef]
- Tamm, C.; Schnier, D. A Tunable Three-level Neodymium-doped Fiber Laser and Its Application to Depletion of the 4f14 5d 2D32 Level in Optically Excited, Trapped Ytterbium Ions. Opt. Commun. 1992, 87, 240–244. [Google Scholar] [CrossRef]
- Singh, N.; Patel, H.K.; Dixit, S.K.; Vora, H.S. Fluorescence Fluctuation of Rhodamine 6G Dye for High Repetition Rate Laser Excitation. J. Lumin. 2013, 134, 607–613. [Google Scholar] [CrossRef]
- Ibnaouf, K.H.; Masilamani, V.; Aldwayyan, A.S.; Alsalhi, M.S. Dual ASE Spectra from “Superexciplex” TICT States of Dye Molecules. Laser Phys. Lawrence 2005, 15, 1536. [Google Scholar]
- Ibnaouf, K.H.; Prasad, S.; Aldwayyan, A.S.; AlSalhi, M.S.; Masilamani, V. Amplified Spontaneous Emission Spectra from the Superexciplex of Coumarin 138. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.; El-Daly, S.A.; Fayed, T.A.; El-Sayed, Y.S. Photophysical Properties, Laser Activity and Photoreactivity of a Heteroaryl Chalcone: A Model of Solvatochromic Fluorophore. Opt. Laser Technol. 2008, 40, 528–537. [Google Scholar] [CrossRef]
- Rurack, K.; Bricks, J.L.; Reck, G.; Radeglia, R.; Resch-Genger, U. Chalcone-analogue Dyes Emitting in the Near-infrared (NIR): Influence of Donor-acceptor Substitution and Cation Complexation on Their Spectroscopic Properties and X-ray Structure. J. Phys. Chem. A 2000, 104, 3087–3109. [Google Scholar] [CrossRef]
- Tejkiran, P.; Teja, M.S.B.; Kumar, P.S.S.; Sankar, P.; Philip, R.; Naveen, S.; Lokanath, N.K.; Rao, G.N. DA-π-D Synthetic Approach for Thienyl Chalcones–NLO–a Structure Activity Study. J. Photochem. Photobiol. A Chem. 2016, 324, 33–39. [Google Scholar] [CrossRef]
- Pannipara, M.; Asiri, A.M.; Alamry, K.A.; Arshad, M.N.; El-Daly, S.A. Synthesis, Spectral Behaviour and Photophysics of Donor–acceptor Kind of Chalcones: Excited State Intramolecular Charge Transfer and Fluorescence Quenching Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1893–1902. [Google Scholar] [CrossRef]
- Miguel, F.B.; Dantas, J.A.; Amorim, S.; Andrade, G.F.S.; Costa, L.A.S.; Couri, M.R.C. Synthesis, Spectroscopic and Computational Characterization of the Tautomerism of Pyrazoline Derivatives from Chalcones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 318–326. [Google Scholar] [CrossRef] [PubMed]
- El-Daly, S.A.; Asiri, A.M.; Khan, S.A.; Alamry, K.A.; Hussein, M.A. Effect of Medium Acidity and Photostability of 3-(4-Dimethylamino-phenyl)-1-(2, 5-dimethyl-thiophen-3-yl)-propenone (DDTP): A New Green Emitting Laser Dye. Chin. J. Chem. 2011, 29, 2557–2561. [Google Scholar] [CrossRef]
- Ibnaouf, K.H.; Prasad, S.; Al Salhi, M.S.; Hamdan, A.; Zaman, M.B.; El Mir, L. Influence of the Solvent Environments on the Spectral Features of CdSe Quantum Dots with and without ZnS Shell. J. Lumin. 2014, 149, 369–373. [Google Scholar] [CrossRef]
- Somasundaram, G.; Ramalingam, A. Gain Studies of Coumarin 1 Dye-doped Polymer Laser. J. Lumin. 2000, 90, 1–5. [Google Scholar] [CrossRef]
- Masilamani, V.; Aldwayyan, A. Structural and Solvent Dependence of Superexciplex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, A.A.; Al-Dwayyan, A.S.; Masilamani, V.; Al-Saud, T.S.M.; Al-Salhi, M.S. Superexciplex of Coumarin Molecules Using Tunable Ti-sapphire Laser. Jpn. J. Appl. Phys. 2003, 42, 6610. [Google Scholar] [CrossRef]
- El-Daly, S.A.; Asiri, A.M.; Obeid, A.Y.; Khan, S.A.; Alamry, K.A.; Hussien, M.A.; Al-Sehemi, A.G. Photophysical Parameters and Laser Activity of 3 (4-dimethylamino-phenyl)-1-(2, 5-dimethyl-thiophen-3-yl)-propenone (DDTP): A New Potential Laser Dye. Opt. Laser Technol. 2013, 45, 605–612. [Google Scholar] [CrossRef]
- Das, P.K.; Pramanik, R.; Banerjee, D.; Bagchi, S. Studies of Solvation of Ketocyanine Dyes in Homogeneous and Heterogeneous Media by UV/Vis Spectroscopic Method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 2763–2773. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, G.; Dong, C. Spectral and Photophysical Properties of Intramolecular Charge Transfer Fluorescence Probe: 4′-Dimethylamino-2, 5-dihydroxychalcone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 987–990. [Google Scholar] [CrossRef]
- DiCesare, N.; Lakowicz, J.R. New Sensitive and Selective Fluorescent Probes for Fluoride Using Boronic Acids. Anal. Biochem. 2002, 301, 111–116. [Google Scholar] [CrossRef]
- Ibnaouf, K. Amplified Spontaneous Emission Spectra of Poly (9, 9-dioctylfluorenyl-2, 7-diyl) under Pulsed Laser Excitation. Synth. Met. 2015, 209, 534–543. [Google Scholar] [CrossRef]
- Shettigar, V.; Patil, P.S.; Naveen, S.; Dharmaprakash, S.M.; Sridhar, M.A.; Prasad, J.S. Crystal Growth and Characterization of New Nonlinear Optical Chalcone Derivative: 1-(4-Methoxyphenyl)-3-(3, 4-dimethoxyphenyl)-2-propen-1-one. J. Cryst. Growth 2006, 295, 44–49. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone Synthesis, Properties and Medicinal Applications: A Review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- N Choudhary, A.; Kumar, A.; Juyal, V. Design, Synthesis and Evaluation of Chalcone Derivatives as Anti-inflammatory, Antioxidant and Antiulcer Agents. Lett. Drug Des. Discov. 2012, 9, 479–488. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Results obtained with the correlation energy density functionals. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785. [Google Scholar]
- Frisch, A. Gaussian 09W Reference; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Ibnaouf, K.H. Excimer State of a Conjugated Polymer (MEH-PPV) in Thin Films. Opt. Laser Technol. 2013, 48, 401–404. [Google Scholar] [CrossRef]
- Mataga, N.; Nishimoto, K. Electronic Structure and Spectra of Nitrogen Heterocycles. Z. Phys. Chem. 1957, 13, 140. [Google Scholar] [CrossRef]
- Janardhana, K.; Ravindrachary, V.; Kumar, P.R. Third Order Nonlinear Optical Studies of 1-(4-chloro phenyl)-3-(4-dimethylamino phenyl) prop-2-en-1-one. J. Cryst. Growth 2013, 368, 11–20. [Google Scholar] [CrossRef]
- Ibnaouf, K.H.; Taha, K.K.; Idriss, H.; Aldaghri, O. Amplified Spontaneous Emission (ASE) Properties of a Laser Dye (LD-473) in Solid State. J. Eur. Opt. Soc. Rapid Publ. 2017, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Cerdán, L.; Costela, A.; Durán-Sampedro, G.; García-Moreno, I. Random Lasing from Sulforhodamine Dye-doped Polymer Films with High Surface Roughness. Appl. Phys. B 2012, 108, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Arias, D.H.; Stone, K.W.; Vlaming, S.M.; Walker, B.J.; Bawendi, M.G.; Silbey, R.J.; Bulovic, V.; Nelson, K.A. Thermally-limited Exciton Delocalization in Superradiant Molecular Aggregates. J. Phys. Chem. B 2013, 117, 4553–4559. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.D.B.; Benjamin, S.C.; Stace, T.M.; Milburn, G.J.; Lovett, B.W.; Gauger, E.M. Superabsorption of Light via Quantum Engineering. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miasojedovas, A.; Kazlauskas, K.; Armonaite, G.; Sivamurugan, V.; Valiyaveettil, S.; Grazulevicius, J.V.; Jursenas, S. Concentration Effects on Emission of Bay-substituted Perylene Diimide Derivatives in a Polymer Matrix. Dyes Pigment. 2012, 92, 1285–1291. [Google Scholar] [CrossRef]
- Khajehzadeh, M.; Sadeghi, N. Molecular Structure, the Effect of Solvent on UV–vis and NMR, FT–IR and FT–Raman Spectra, NBO, Frontier Molecular Orbital Analysis of Mitomycin Anticancer Drug. J. Mol. Liq. 2018, 256, 238–246. [Google Scholar] [CrossRef]







| Solvent | Dipole Factor | Absorption Peak (nm) | Fluorescence Peak (nm) | Short | Long | Φf (%) |
|---|---|---|---|---|---|---|
| ASE Peak (nm) | ||||||
| Benzene | 0.0016 | 420 | 487 | - | - | 16 |
| Toluene | 0.0132 | 413 | 480 | - | - | 12 |
| Chloroform | 0.1483 | 424 | 521 | - | - | 91 |
| Acetic acid | 0.2022 | 429 | 520 | - | - | 25 |
| Tetrahydrofuran | 0.2096 | 413 | 516 | 538 | - | 88 |
| Acetone | 0.2843 | 418 | 532 | 547 | - | 70 |
| Ethanol | 0.2887 | 425 | 535 | - | - | 40 |
| Methanol | 0.3086 | 427 | 533 | - | - | 20 |
| Dimethylformamide | 0.2744 | 427 | 541 | 546 | 566 | 95 |
| Acetonitrile | 0.3054 | 417 | 538 | 545 | 565 | 44 |
| ET (eV) | EHOMO (eV) | ELUMO (eV) | Eg (eV) | µD |
|---|---|---|---|---|
| −33,954.992 | −5.432 | −2.103 | 3.332 | 8.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldaghri, O. Spectral Characteristics and Molecular Structure of (E)-1-(4-Chlorophenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-en-1-One (DAP). Materials 2021, 14, 2766. https://doi.org/10.3390/ma14112766
Aldaghri O. Spectral Characteristics and Molecular Structure of (E)-1-(4-Chlorophenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-en-1-One (DAP). Materials. 2021; 14(11):2766. https://doi.org/10.3390/ma14112766
Chicago/Turabian StyleAldaghri, O. 2021. "Spectral Characteristics and Molecular Structure of (E)-1-(4-Chlorophenyl)-3-(4-(Dimethylamino)Phenyl)Prop-2-en-1-One (DAP)" Materials 14, no. 11: 2766. https://doi.org/10.3390/ma14112766






