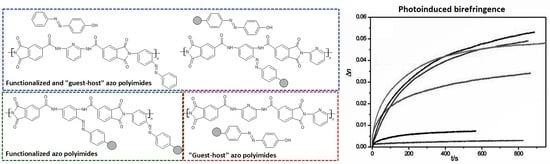
Azobenzene Functionalized “T-Type” Poly(Amide Imide)s vs. Guest-Host Systems—A Comparative Study of Structure-Property Relations
Abstract
:1. Introduction
2. Results and Discussion
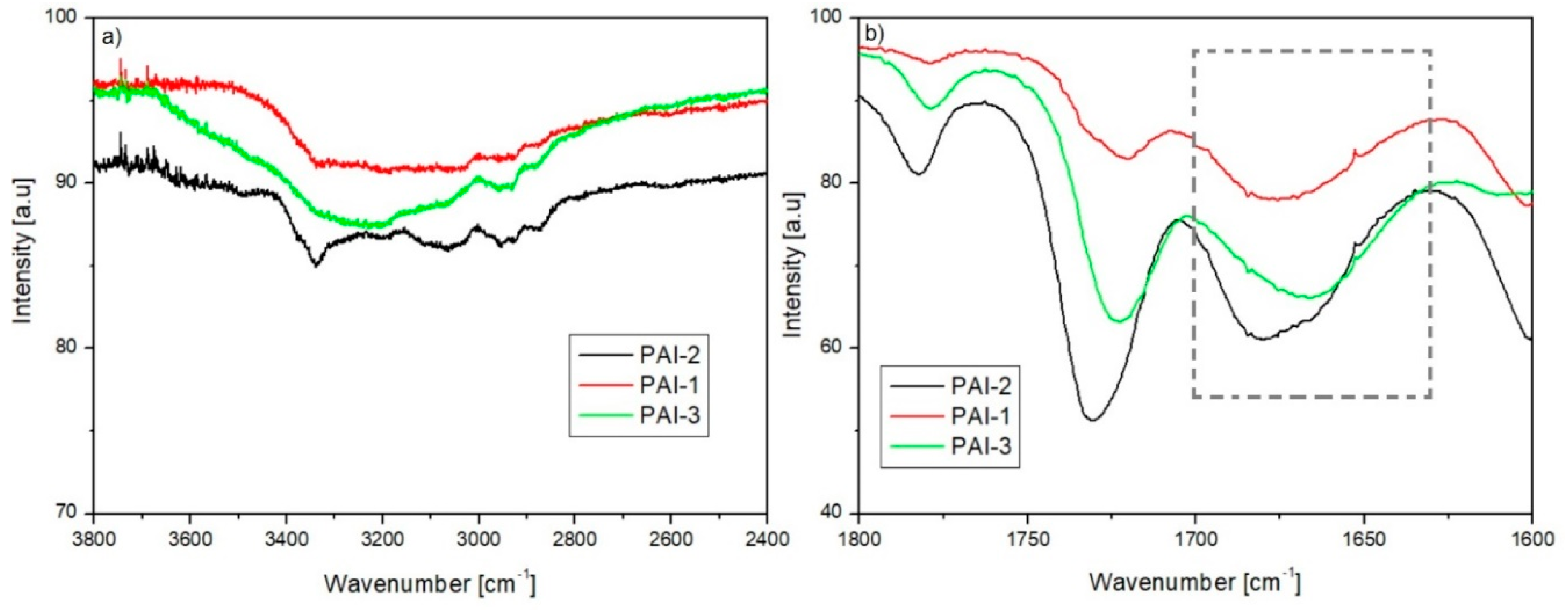
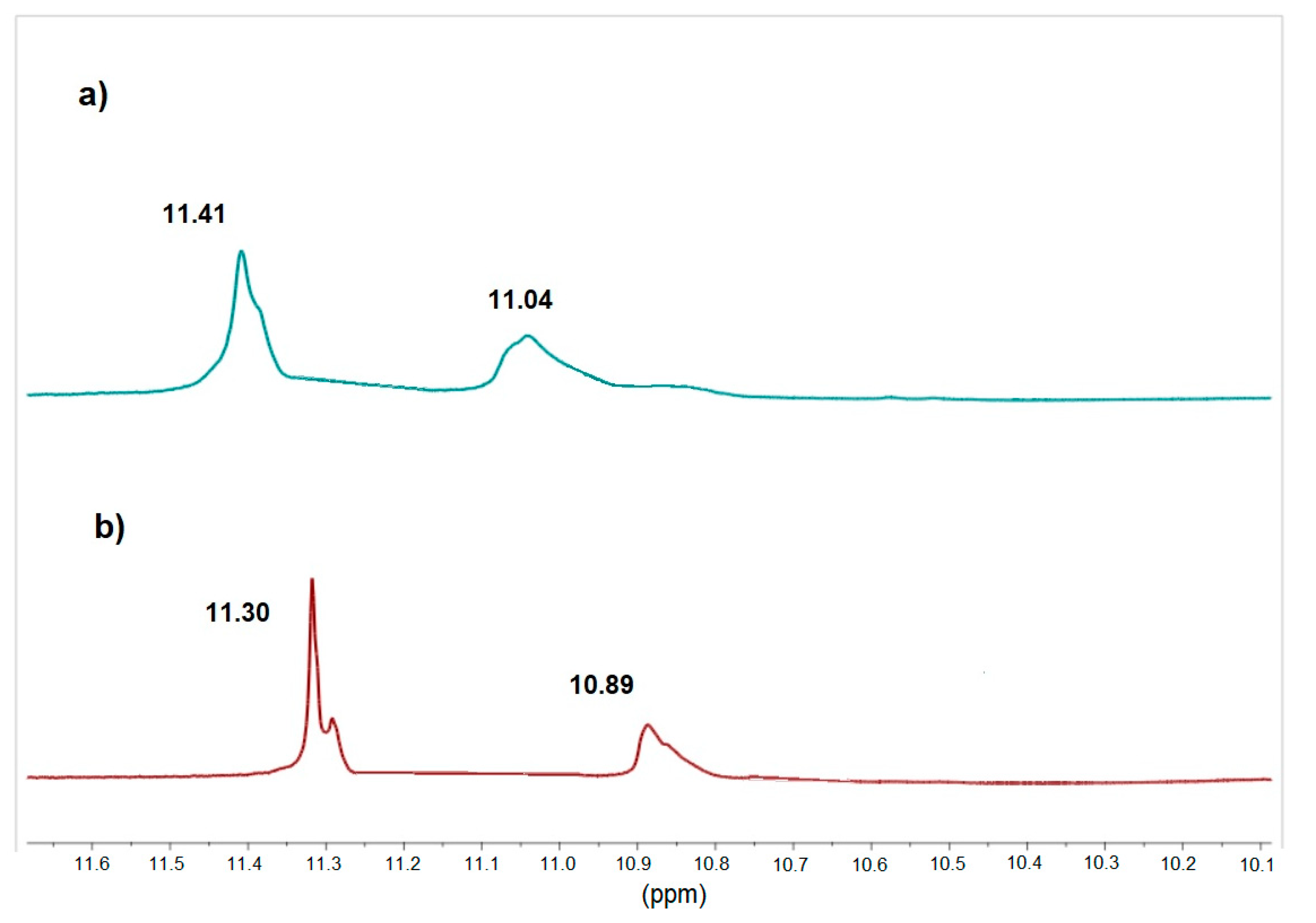
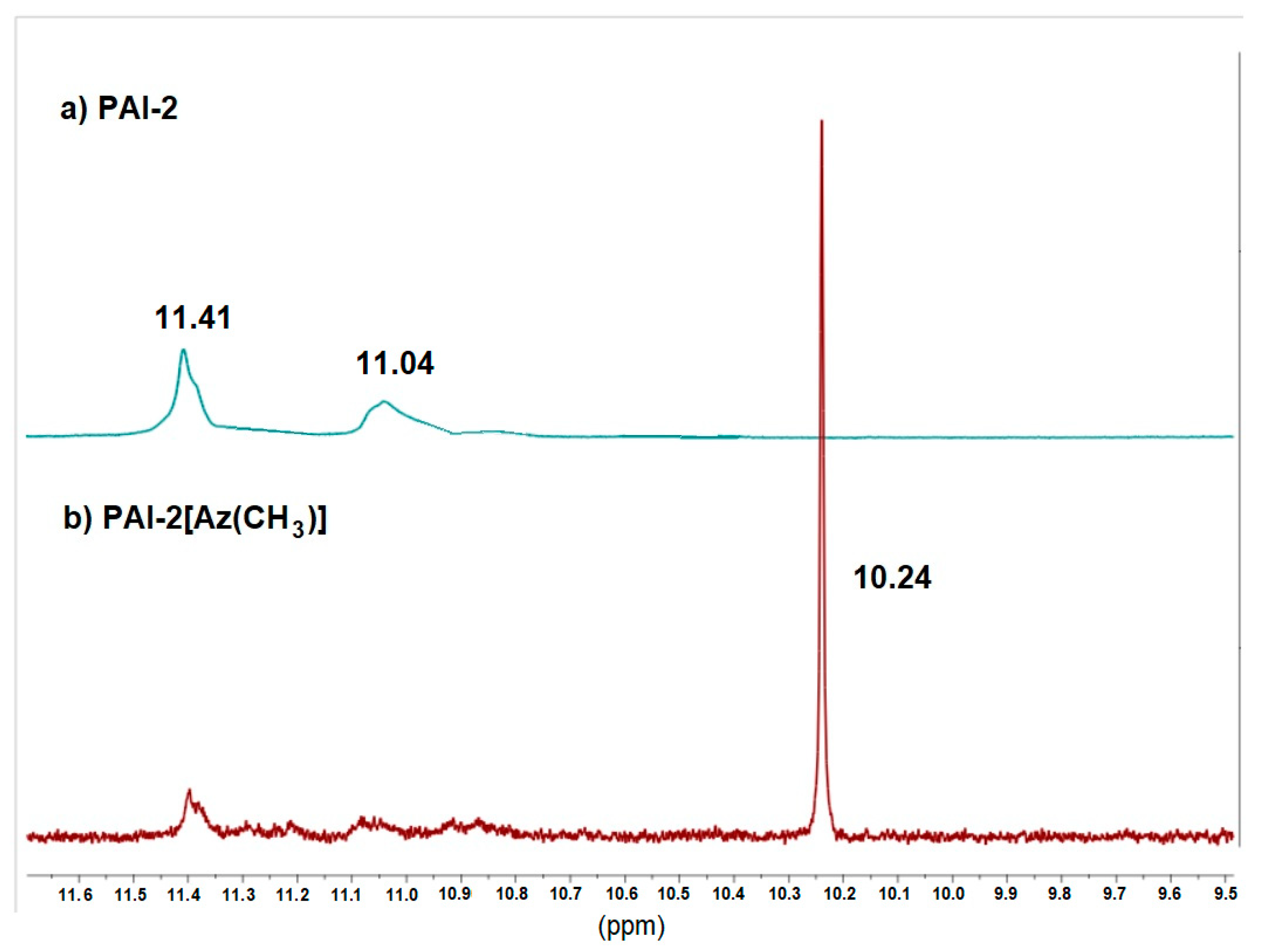
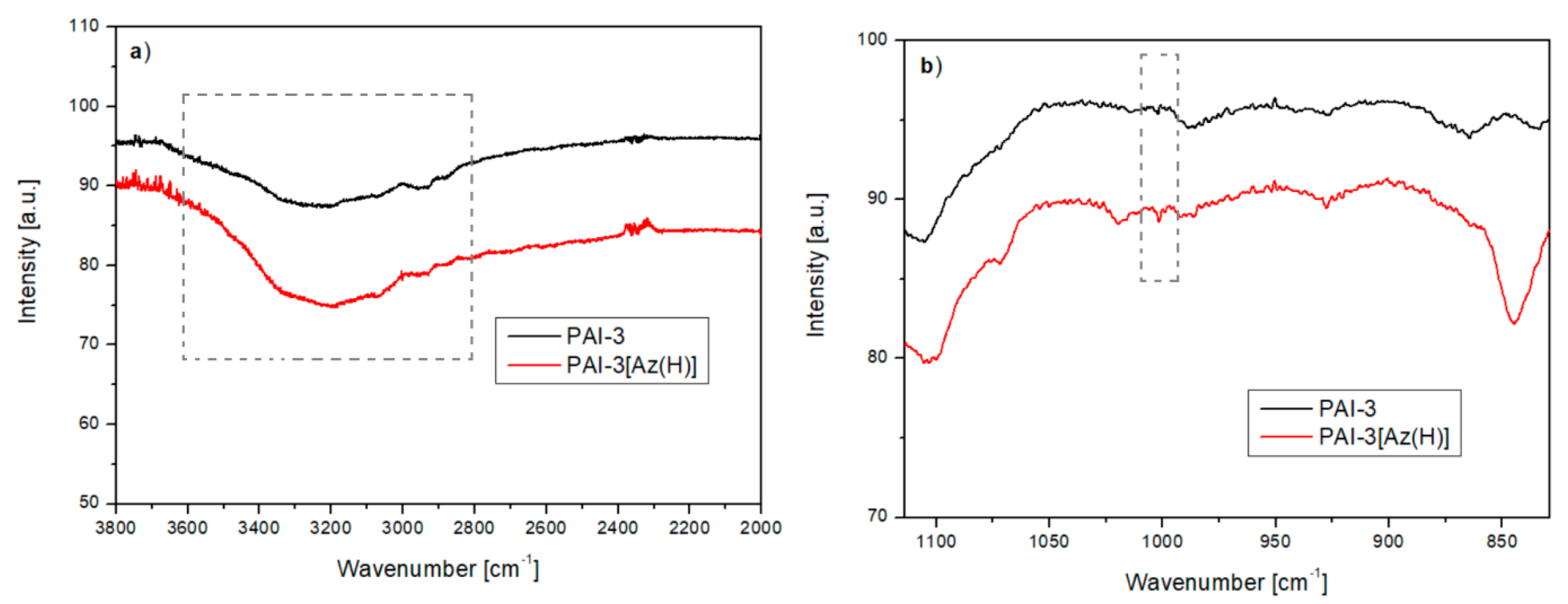
2.1. Intermolecular Hydrogen Bonds Formation
2.2. Polymer Characterization
2.3. Thermal Properties
- Polymer with pyridine rings in the backbone exhibited higher Tg than their analogue with phenyl rings (PAI-3 vs. PAI-5).
- The incorporation of the azobenzene group between the amide linkages decreased the Tg (PAI-1) in comparison to PAI-3 containing chromophores between imide rings.
- The incorporation of two covalently bonded azo chromophores to the polymer repeating unit did not influence Tg’s in comparison to the polymer with one azo group per structural unit, despite a significantly higher average for molar masses.
- The guest-host azo systems were characterized by lower Tg in comparison to polymer matrices, because of the plastification effect. A much greater reduction of Tg was observed for PAI-9[Az(CH3)] than for the other guest-host systems due to the higher content of non-covalently bonded azo dye (25 wt.% vs. 45 wt.%).
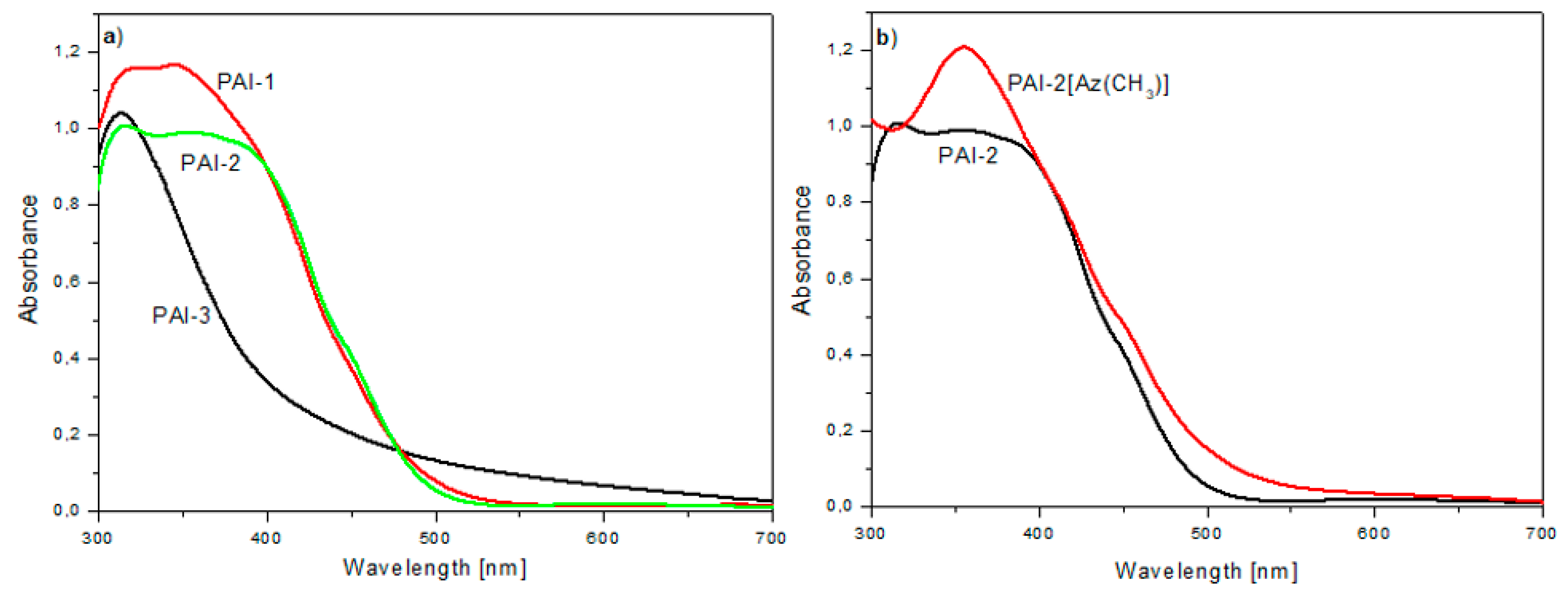
2.4. Linear Optical Properties
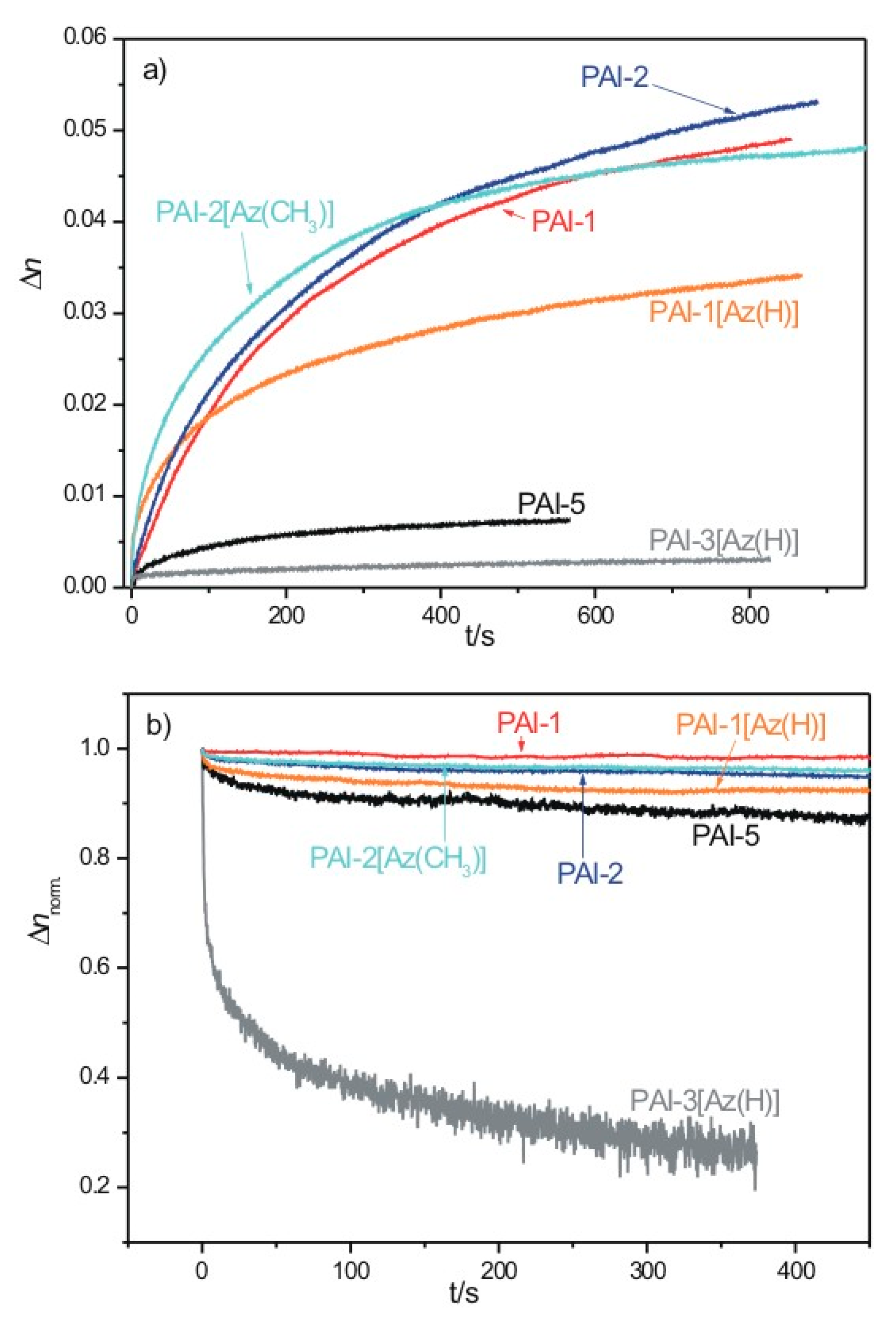
2.5. Photoinduced Birefringence
3. Materials and Methods
3.1. Materials
3.2. Measurements
3.3. Synthesis of Azo Chromophores
3.4. Synthesis of Diamines
3.5. Synthesis of Dianhydrides
3.6. Synthesis of Chromophore-Functionalized Polymers
3.7. Preparation of Non-Covalent Azo Polyimides
3.8. Polymer Film Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yaroshchuk, O.V.; Dumont, M.; Zakrevskyy, Y.A.; Bidna, T.V.; Lindau, J. Molecular structure of azopolymers and photoinduced 3D orientational order. 1. Azobenzene polyesters. J. Phys. Chem. B 2004, 108, 4647–4658. [Google Scholar] [CrossRef]
- Lee, T.S.; Kim, D.-Y.; Jiang, X.L.; Li, L.; Kumar, J. STripathy, Photoinduced surface relief gratings in high-Tg main-chain azoaromatic polymer films. J. Polym. Sci. A Polym. Chem. 1998, 36, 283–289. [Google Scholar] [CrossRef]
- Xie, S.; Natansohn, A.; Rochon, P. Recent developments in aromatic azo polymers research. Chem. Mater. 1993, 5, 403–411. [Google Scholar] [CrossRef]
- Barrett, C.J.; Natansohn, A.L.; Rochon, P.L. Mechanism of optically inscribed high-efficiency diffraction gratings in azo polymer films. J. Phys. Chem. 1996, 100, 8836–8842. [Google Scholar] [CrossRef]
- Mendonça, C.R.; dos Santos, D.S., Jr.; Balogh, D.T.; Dhanabalan, A.; Giacometti, J.A.; Zilio, S.C.; Oliveira, O.N., Jr. Optical storage in mixed Langmuir–Blodgett (LB) films of azopolymers and cadmium stearate. Polymer 2001, 42, 6539–6544. [Google Scholar] [CrossRef]
- Buffeteau, T.; Pezolet, M. Photoinduced orientation in azopolymers studied by infrared spectroscopy: Cooperative and biaxial orientation in semicrystalline polymers. Macromolecules 1998, 31, 2631–2635. [Google Scholar] [CrossRef]
- Meng, X.; Natansohn, A.; Barrett, C.; Rochon, P. Azo polymers for reversible optical storage. 10. Cooperative motion of polar side groups in amorphous polymers. Macromolecules 1996, 29, 946–952. [Google Scholar] [CrossRef]
- Sava, I.; Burescu, A.; Stoica, I.; Musteata, V.; Cristea, M.; Mihaila, I.; Pohoata, V.; Topala, I. Properties of some azo-copolyimide thin films used in the formation of photoinduced surface relief gratings. RSC Adv. 2015, 5, 10125–10133. [Google Scholar] [CrossRef]
- Priimagi, A.; Vapaavuori, J.; Rodriguez, F.J.; Faul, C.F.J.; Heino, M.T.; Ikkala, O.; Kauranen, M.; Kaivola, M. Hydrogen-bonded polymer-azobenzene complexes: Enhanced photoinduced birefringence with high temporal stability through interplay of intermolecular interactions. Chem. Mater. 2008, 20, 6358–6363. [Google Scholar] [CrossRef]
- Vapaavuori, J.; Valtavirta, V.; Alasaarela, T.; Mamiya, J.-I.; Priimagi, A.; Shishido, A.; Kaivola, M. Efficient surface structuring and photoalignment of supramolecular polymer–azobenzene complexes through rational chromophore design. J. Mater. Chem. 2011, 21, 15437–15441. [Google Scholar] [CrossRef]
- Koskela, J.E.; Vapaavuori, J.; Hautala, J.; Priimagi, A.; Faul, C.F.J.; Kaivola, M.; Ras, R.H.A. Surface-relief gratings and stable birefringence inscribed using light of broad spectral range in supramolecular polymer-bisazobenzene complexes. J. Phys. Chem. C 2012, 116, 2363–2370. [Google Scholar] [CrossRef]
- Hu, D.; Chen, K.; Zou, G.; Zhang, Q. Preparation and characterization of hydrogen-bonded P4VP-bisazobenzene complexes. J. Polym. Res. 2012, 19, 9983. [Google Scholar] [CrossRef]
- González-Vila, Á.; Debliquy, M.; Lahem, D.; Zhang, C.; Mégret, P.; Caucheteur, C. Molecularly imprinted electropolymerization on a metal-coated optical fiber for gas sensing applications. Sens. Actuators B Chem. 2017, 244, 1145–1151. [Google Scholar] [CrossRef]
- Aldaba, A.L.; González-Vila, Á.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018, 254, 1087–1093. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X. Amphiphilic azo polymers: Molecular engineering, self-assembly and photoresponsive properties. Prog. Polym. Sci. 2013, 38, 271–301. [Google Scholar] [CrossRef]
- Oliveira, O.N., Jr.; dos Santos, D.S., Jr.; Balogh, D.T.; Zucolotto, V.; Mendonc, C.R. Optical storage and surface-relief gratings in azobenzene-containing nanostructured films. Adv. Coll. Interface Sci. 2005, 116, 179–192. [Google Scholar] [CrossRef]
- Yesodha, S.K.; Pillai, C.K.S.; Tsutsumi, N. Stable polymeric materials for nonlinear optics: A review based on azobenzene systems. Prog. Polym. Sci. 2004, 29, 48–74. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Schab-Balcerzak, E. Supramolecular azopolymers based on hydrogen bonds. Polimery 2015, 60, 423–434. [Google Scholar] [CrossRef]
- Raut, P.; Swanson, N.; Kulkarni, A.; Pugh, C.; Jana, S.C. Exploiting arene-perfluoroarene interactions for dispersion of carbon black in rubber compounds. Polymer 2018, 148, 247–258. [Google Scholar] [CrossRef]
- Yager, K.G.; Barrett, C.J. Novel photo-switching using azobenzene functional materials. J. Photochem. Photobiol. A Chem. 2006, 182, 250–261. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Siwy, M.; Jarzabek, B.; Kozanecka-Szmigiel, A.; Switkowski, K.; Pura, B. Post and prepolymerization strategies to develop novel photochromic poly(ester imide)s. J. Appl. Polym. Sci. 2011, 120, 631–643. [Google Scholar] [CrossRef]
- Kozanecka-Szmigiel, A.; Switkowski, K.; Schab-Balcerzak, E.; Szmigiel, D. Photoinduced birefringence of azobenzene polymer at blue excitation wavelengths. Appl. Phys. B 2015, 119, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Schab-Balcerzak, E.; Sobolewska, A.; Miniewicz, A.; Jurusik, J. Chromophore concentration effect on holographic grating formation efficiency in novel azobenzene-functionalized polymers. Polym. Eng. Sci. 2008, 48, 1755–1767. [Google Scholar] [CrossRef]
- He, T.C.; Wang, C.S.; Pan, X.; Zhang, C.Z.; Lu, G.Y. The nonlinear optical property and photoinduced anisotropy of a novel azobenzene-containing fluorinated polyimide. Appl. Phys. B 2009, 94, 653–659. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Janeczek, H.; Małecki, J.; Trzebicka, B.; Szmigiel, D.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E. Noncovalent azopoly(ester imide)s: Experimental study on structure-property relations and theoretical approach for prediction of glass transition temperature and hydrogen bond formation. Polymer 2017, 113, 53–66. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Sobolewska, A.; Stumpe, J.; Hamryszak, L.; Bujak, P. Surface relief gratings in azobenzene supramolecular systems based on polyimides. Opt. Mater. 2012, 35, 155–167. [Google Scholar] [CrossRef]
- Saccone, M.; Dichiarante, V.; Forni, A.; Goulet-Hanssens, A.; Cavallo, G.; Vapaavuori, J.; Terraneo, G.; Barrett, C.J.; Resnati, G.; Metrangolo, P.; et al. Supramolecular hierarchy among halogen and hydrogen bond donors in light-induced surface patterning. J. Mater. Chem. C 2015, 3, 759–768. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Vapaavuori, J.; Wang, X.; Sabat, R.G.; Pellerin, C.; Bazuin, C.G. Influence of supramolecular interaction type on photoresponsive azopolymer complexes: A surface relief grating formation study. Macromolecules 2016, 49, 4923–4934. [Google Scholar] [CrossRef]
- Chen, J.P.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Highly stable optically induced birefringence and holographic surface gratings on a new azo-carbazole-based polyimide. Macromolecules 1999, 32, 8572–8579. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Wojtowicz, M.; Sobolewska, A.; Noga, J.; Jarczyk-Jedryka, A.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E. Thermal, optical and photoinduced properties of a series of homo and co-polyimides with two kinds of covalently bonded azo-dyes and their supramolecular counterparts. Opt. Mater. 2015, 48, 139–149. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Flakus, H.; Jarczyk-Jedryka, A.; Konieczkowska, J.; Siwy, M.; Bijak, K.; Sobolewska, A.; Stumpe, J. Photochromic supramolecular azopolyimides based on hydrogen bonds. Opt. Mater. 2015, 47, 501–511. [Google Scholar] [CrossRef]
- Węgłowski, R.; Piecek, W.; Kozanecka-Szmigiel, A.; Konieczkowska, J.; Schab-Balcerzak, E. Poly(esterimide) bearing azobenzene units as photoaligning layer for liquid crystals. Opt. Mater. 2015, 49, 224–229. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Schab-Balcerzak, E.; Siwy, M.; Switkowski, K.; Kozanecka-Szmigiel, A. Large and highly stable photoinduced birefringence in poly(amideimide)s with two azochromophores per structural unit. Opt. Mater. 2015, 39, 199–206. [Google Scholar] [CrossRef]
- Kozanecka-Szmigiel, A.; Konieczkowska, J.; Switkowski, K.; Antonowicz, J.; Trzebicka, B.; Szmigiel, D.; Schab-Balcerzak, E. Influence of supramolecular interactions on photoresponsive behavior of azobenzene poly(amide imide)s. J. Photochem. Photobiol. A Chem. 2016, 318, 114–123. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Kozanecka-Szmigiel, A.; Piecek, W.; Węgłowski, R.; Schab-Balcerzak, E. Azopolyimides—Influence of chemical structure on azochromophore photo-orientation efficiency. Polimery 2018, 63, 481–487. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Kozanecka-Szmigiel, A.; Janeczek, H.; Małecki, J.; Wójtowicz, M.; Schab-Balcerzak, E. No effect of the hydrogen bonds on the physicochemical properties of the guest-host poly(amide imide) azosystems and efficiency of chromophore orientation. Dyes. Pigm. 2018, 156, 250–259. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Janeczek, H.; Małecki, J.; Schab-Balcerzak, E. The comprehensive approach towards study of (azo)polymers fragility parameter: Effect of architecture, intra– and intermolecular interactions and backbone conformation. Eur. Polym. J. 2018, 109, 489–498. [Google Scholar] [CrossRef]
- Aiba, M.; Tokuyama, T.; Matsumoto, H.; Tomioka, H.; Higashihara, T.; Ueda, M. Effect of N-methyl amide linkage on hydrogen bonding behavior and water transport properties of partially N-methylated random aromatic copolyamides. J. Polym. Sci. 2014, 52, 3453–3462. [Google Scholar] [CrossRef]
- Wu, S.; Duan, S.; Lei, Z.; Su, W.; Zhang, Z.; Wang, K.; Zhang, Q. Supramolecular bisazopolymers exhibiting enhanced photoinduced birefringence and enhanced stability of birefringence for four-dimensional optical recording. J. Mater. Chem. 2010, 20, 5202–5209. [Google Scholar] [CrossRef]
- Priimagi, A.; Kaivola, M.; Rodriguez, F.J.; Kauranen, M. Enhanced photoinduced birefringence in polymer-dye complexes: Hydrogen bonding makes a difference. Appl. Phys. Lett. 2007, 90, 121103. [Google Scholar] [CrossRef] [Green Version]
- Barrio, J.; Blasco, E.; Oriol, L.; Alcal, R.; Sanchez-Somolinos, C. Diblock copolymer–azobenzene complexes through hydrogen bonding: Self-assembly and stable photoinduced optical anisotropy. J. Polym. Sci. A 2013, 51, 1716–1725. [Google Scholar] [CrossRef]
- Yan, S.; Chen, W.; Yang, X.; Chen, C.; Huang, M.; Xu, Z.; Yeung, K.W.K.; Yi, C. Soluble polyimides based on a novel pyridine-containing diamine m,p-PAPP and various aromatic dianhydrides. Polym. Bull. 2011, 66, 1191–1206. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Y.-F.; Ma, T.; Zhang, S.; Gong, C. Synthesis and characterization of novel polyimides derived from 2,6-bis[4-(3,4-dicarboxyphenoxy)benzoyl]pyridine dianhydride and aromatic diamines. Polymer 2006, 47, 3774–3783. [Google Scholar] [CrossRef]
- Tamami, B.; Yeganeh, H. Preparation and properties of novel polyimides derived from 4-Aryl-2,6 Bis(4-amino phenyl)pyridine. J. Polym. Sci. A Polym. Chem. 2001, 39, 3826–3831. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, S.; Ma, T.; Shao, Y.; Zhao, X. Synthesis and characterization of novel polyimides derived from pyridine-bridged aromatic dianhydride and various diamines. Eur. Polym. J. 2006, 42, 1229–1239. [Google Scholar] [CrossRef]
- Wang, K.-L.; Liou, W.-T.; Liaw, D.-J.; Huang, S.-T. High glass transition and thermal stability of new pyridine-containing polyimides: Effect of protonation on fluorescence. Polymer 2008, 49, 1538–1546. [Google Scholar] [CrossRef]
- Bujak, K.; Orlikowska, H.; Małecki, J.G.; Schab-Balcerzak, E.; Bartkiewicz, S.; Bogucki, J.; Sobolewska, A.; Konieczkowska, J. Fast dark cis-trans isomerization of azopyridine derivatives in comparison to their azobenzene analogues: Experimental and computational study. Dyes. Pigm. 2019, 160, 654–662. [Google Scholar] [CrossRef]
- Sava, I.; Sacarescu, L.; Stoica, I.; Apostol, I.; Damian, V.; Hurduc, N. Photochromic properties of polyimide and polysiloxane azopolymers. Polym. Int. 2009, 58, 163–170. [Google Scholar] [CrossRef]
- Sava, I.; Burescu, A.; Lisa, G. Study of thermal behavior of polyimides containing pendent-substituted azobenzene units. Polym. Bull. 2014, 71, 1359–1373. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Janeczek, H.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E. Poly(amic acid)s and their poly(amide imide) counterparts containing azobenzene moieties: Characterization, imidization kinetics and photochromic properties. Mater. Chem. Phys. 2016, 180, 203–212. [Google Scholar] [CrossRef]
- Kozanecka-Szmigiel, A.; Rutkowska, K.A.; Nieborek, M.; Kwasny, M.; Karpierz, M.A.; Schab-Balcerzak, E.; Konieczkowska, J.; Szmigiel, D. Photopatterned azo poly(amide imide) layers as aligning substrates of holographic liquid crystal diffraction gratings for beam steering applications. J. Mater. Chem. C 2020, 8, 968–976. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Sapich, B.; Stumpe, J. Photoinduced optical anisotropy in new poly(amide imide)s with azobenzene units. Polymer 2005, 46, 49–59. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Sobolewska, A.; Miniewicz, A.; Jarusik, J.; Jarzabek, B. Photoinduced holographic gratings in azobenzene-functionalized poly(amideimide)s. Polym. J. 2007, 39, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Schab-Balcerzak, E.; Konieczkowska, J.; Siwy, M.; Sobolewska, A.; Wojtowicz, M.; Wiacek, M. Comparative studies of polyimides with covalently bonded azo-dyes with their supramolecular analoges: Thermo-optical and photoinduced properties. Opt. Mater. 2014, 36, 892–902. [Google Scholar] [CrossRef]
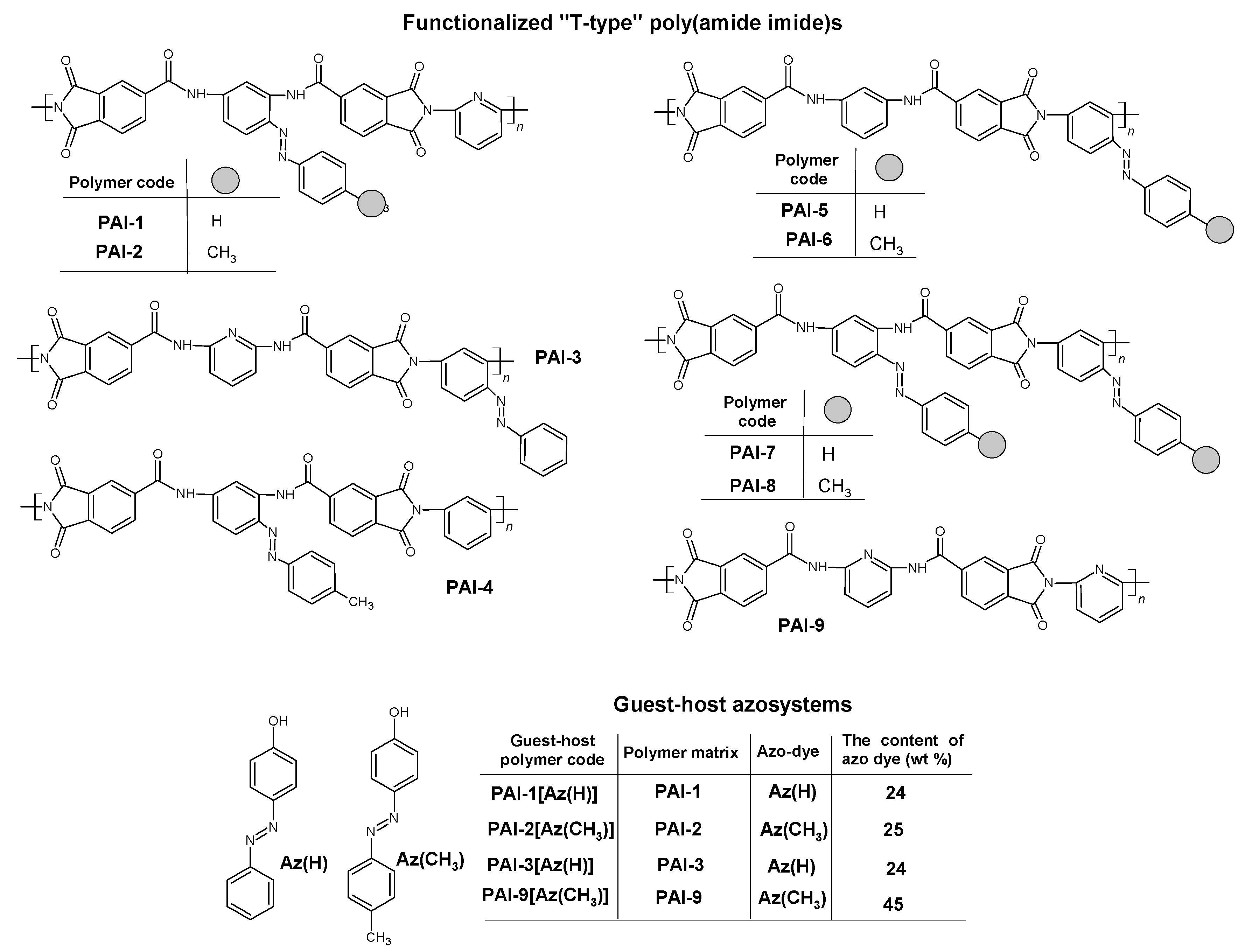
| Polymer Code | Mn × 103 (g/mol) | Mw × 103 (g/mol) | Mw/Mn |
|---|---|---|---|
| PAI-1 | 3.2 | 6.2 | 1.9 |
| PAI-2 | 3.2 | 5.2 | 1.7 |
| PAI-3 | 1.1 | 1.7 | 1.5 |
| PAI-5 [37] | 2.1 | 3.85 | 1.8 |
| PAI-6 [34,35] | 1.1 | 1.9 | 1.7 |
| PAI-7 [35] | 4.3 | 6.8 | 1.6 |
| PAI-8 [34,35] | 10.3 | 14.9 | 1.5 |
| Polymer Code | DSC | TGA (N2) | |||
|---|---|---|---|---|---|
| Tg (°C) | aT5 (°C) | bT10 (°C) | cTmax (°C) | d Residual Weight (%) | |
| PAI-1 | 244 | 419 | 455 | 432; 612 | 53 |
| PAI-2 | 281 | 426 | 489 | 427; 596 | 54 |
| PAI-3 | 300 | 357 | 416 | 390; 533 | 49 |
| PAI-5 | 282 | 331 | 408 | 356; 463; 552 | 53 |
| PAI-6 | 247 [35] | 290 [34,35] | 407 | 434; 598 | 50 |
| PAI-7 [35] | 254 | 371 | 412 | 429; 606 | 49 |
| PAI-8 [33,34,35] | nd | 258 | 389 | - | 33 |
| PAI-9 [36] | 265 | 356 | 412 | 454; 565 | 69 |
| PAI-1[Az(H)] | 198 | 202 | 221 | 214; 437; 593 | 38 |
| PAI-2[Az(CH3)] | 210 | 211 | 235 | 226; 405; 581 | 42 |
| PAI-3[Az(H)] | 133 | 210 | 235 | 225; 389; 597 | 45 |
| PAI-9[Az(CH3)] [36] | 105 | 174 | 229 | 172; 362 | 40 |
| Az(H) [47] | −73 | 188 | 202 | 244 | - |
| Az(CH3) [47] | 4 | 114 | 124 | 127; 237; 289 | - |
| Polymer Code | λmax (nm) in Film | λmax (nm) in NMP |
|---|---|---|
| PAI-1 | 317; 345 | 311; 361 |
| PAI-2 | 314; 354 | 308; 381 |
| PAI-3 | 310 | 314 |
| PAI-5 [37] | below 300 | 263; 311; 444 * |
| PAI-6 [34,35] | 309 | 264 |
| PAI-7 [33] | 342 | 292 *; 338; 395 *; 440 * |
| PAI-8 [33] | 355 | 263; 350; 447 * |
| PAI-9 [36] | 323 | 301 |
| PAI-1[Az(H)] | 349 | |
| PAI-2[Az(CH3)] | 355 | |
| PAI-3[Az(H)] | 343 | |
| PAI-9[Az(CH3)] [36] | 353 |
| Polymer Code | α405 (cm−1) | d (µm) | Δnmax | Δnrelax (%) |
|---|---|---|---|---|
| PAI-1 | 5.8 × 104 | 0.40 | 0.049 | 2 |
| PAI-2 | 8.1 × 104 | 0.25 | 0.052 | 5 |
| PAI-3 | 2.7 × 104 | 0.37 | non-detectable | - |
| PAI-5 | 3.0 × 104 | 0.50 | 0.007 | 12 |
| PAI-6 [34,35] | 3.0 × 104 | 0.38 | non-detectable | - |
| PAI-7 [35] | 1.0 × 105 | 0.19 | 0.055 (after 600 s) | 1.5 (after 500 s) |
| PAI-8 [34,35] | 1.0 × 105 | 0.13 | 0.060 (after 1000 s) | 5 (after 500 s) |
| PAI-1[Az(H)] | 7.2 × 104 | 0.25 | 0.034 | 8 |
| PAI-2[Az(CH3)] | 8.5 × 104 | 0.25 | 0.047 | 4 |
| PAI-3[Az(H)] | 3.5 × 104 | 0.61 | 0.003 | 73 |
| PAI-9[Az(CH3)] [36] | 3.1 × 104 | 0.35 | 0.012 (after 600 s) | 25 (after 500 s) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bujak, K.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E.; Konieczkowska, J. Azobenzene Functionalized “T-Type” Poly(Amide Imide)s vs. Guest-Host Systems—A Comparative Study of Structure-Property Relations. Materials 2020, 13, 1912. https://doi.org/10.3390/ma13081912
Bujak K, Kozanecka-Szmigiel A, Schab-Balcerzak E, Konieczkowska J. Azobenzene Functionalized “T-Type” Poly(Amide Imide)s vs. Guest-Host Systems—A Comparative Study of Structure-Property Relations. Materials. 2020; 13(8):1912. https://doi.org/10.3390/ma13081912
Chicago/Turabian StyleBujak, Karolina, Anna Kozanecka-Szmigiel, Ewa Schab-Balcerzak, and Jolanta Konieczkowska. 2020. "Azobenzene Functionalized “T-Type” Poly(Amide Imide)s vs. Guest-Host Systems—A Comparative Study of Structure-Property Relations" Materials 13, no. 8: 1912. https://doi.org/10.3390/ma13081912