Electrocatalytic Behavior and Determination of Amitriptyline Drug with MWCNT@Celllulose Composite Modified Glassy Carbon Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of MWCNT/Cellulose Composites
2.3. Fabrication of Electrode with GCE and MWCNT/Cellulose Composite
2.4. Preparation of Human Urinal Samples
2.5. Characterization of MWCNT/Cellulose Nanocomposite
3. Results and Discussions
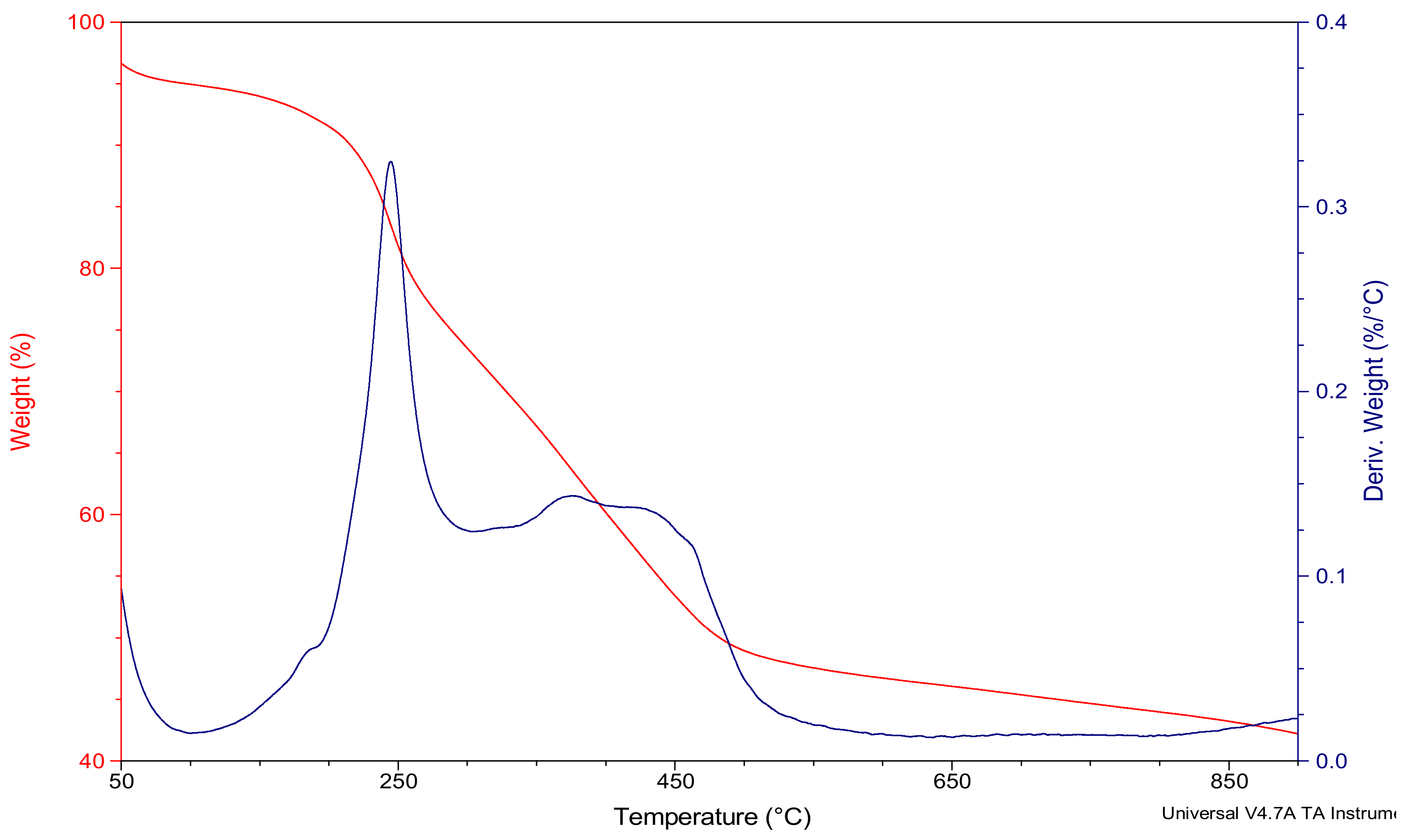
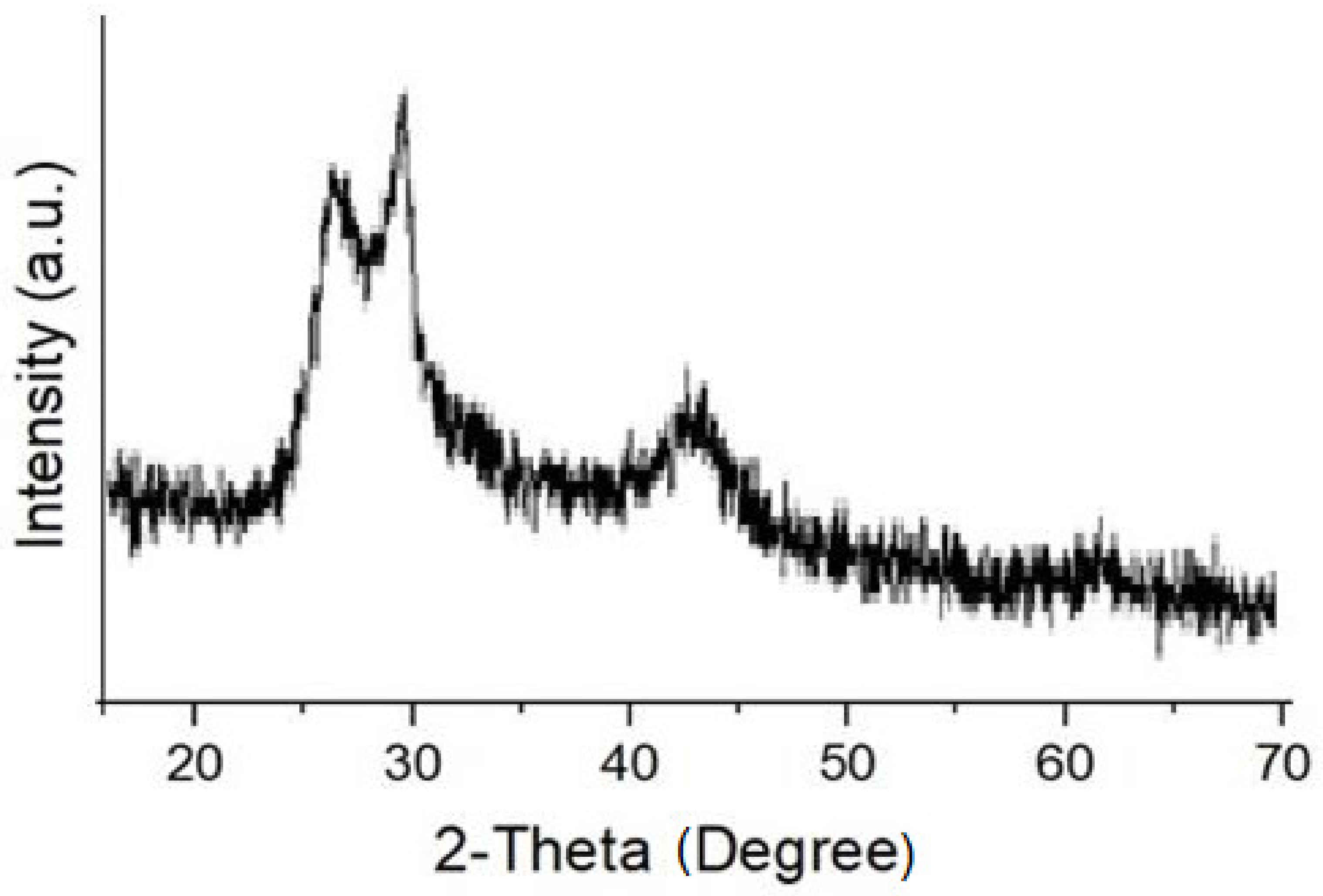
3.1. Study of Morphological and Thermal Analysis
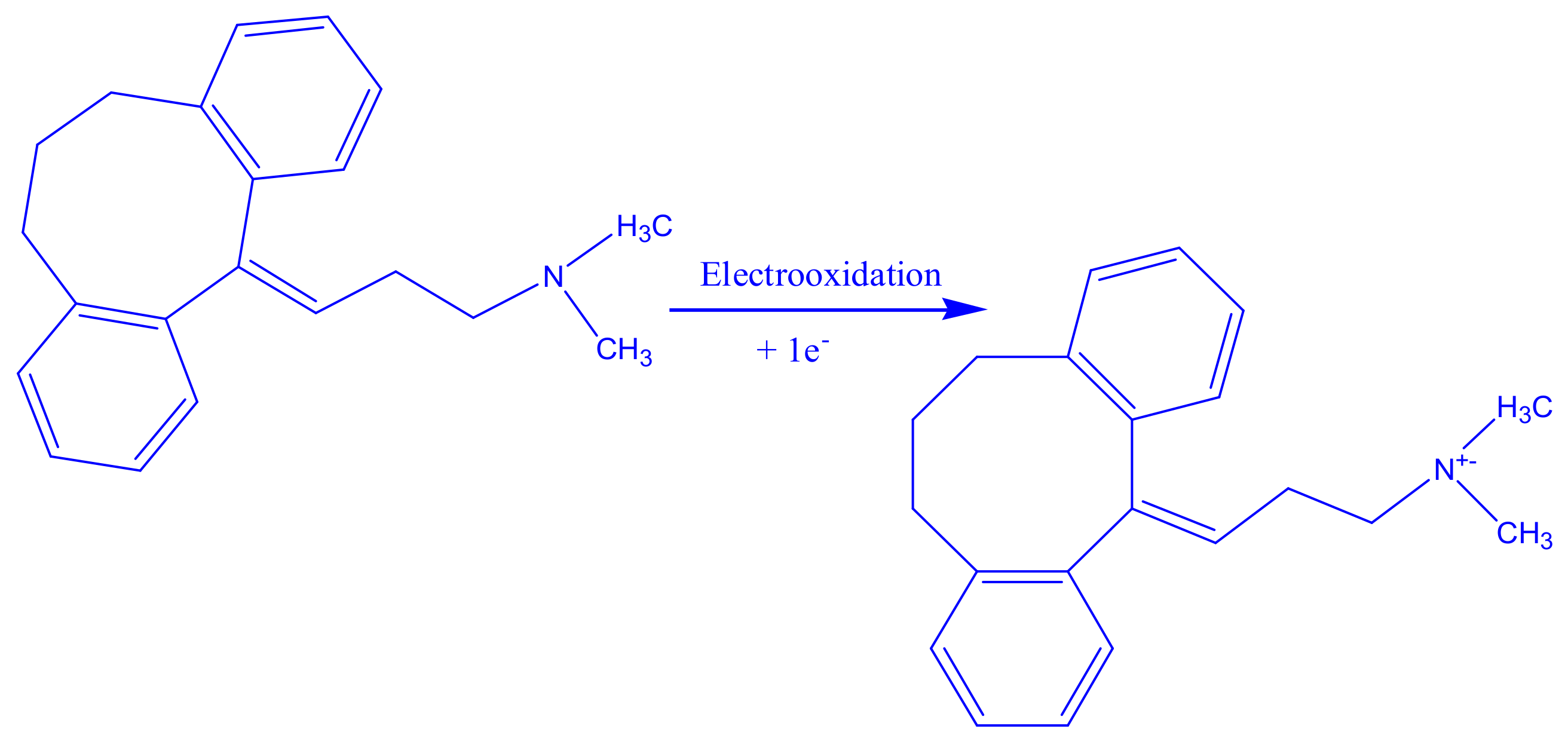
3.2. Electrocatalytic Oxidation of Amitriptyline at Multi-walled Carbon/Cellulose Nanocomposite
3.3. Electrochemical Performance of AMT on MWCNT/Cellulose/GCE
3.4. The Influence of pH of AMT on MWCNT/Cellulose/GCE
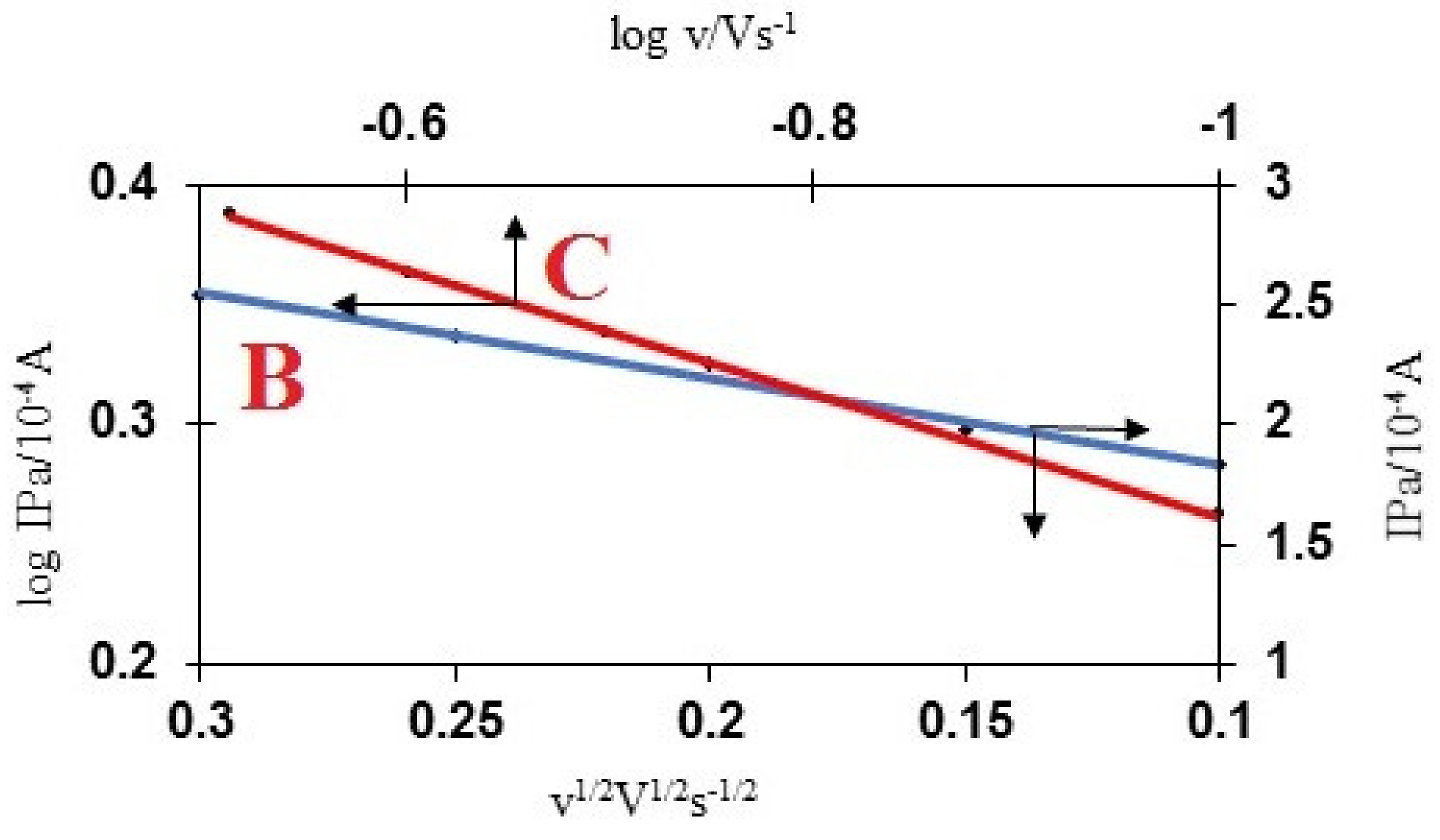
3.5. The Influence of Scan Rate on MWCNT/Cellulose/GCE for AMT
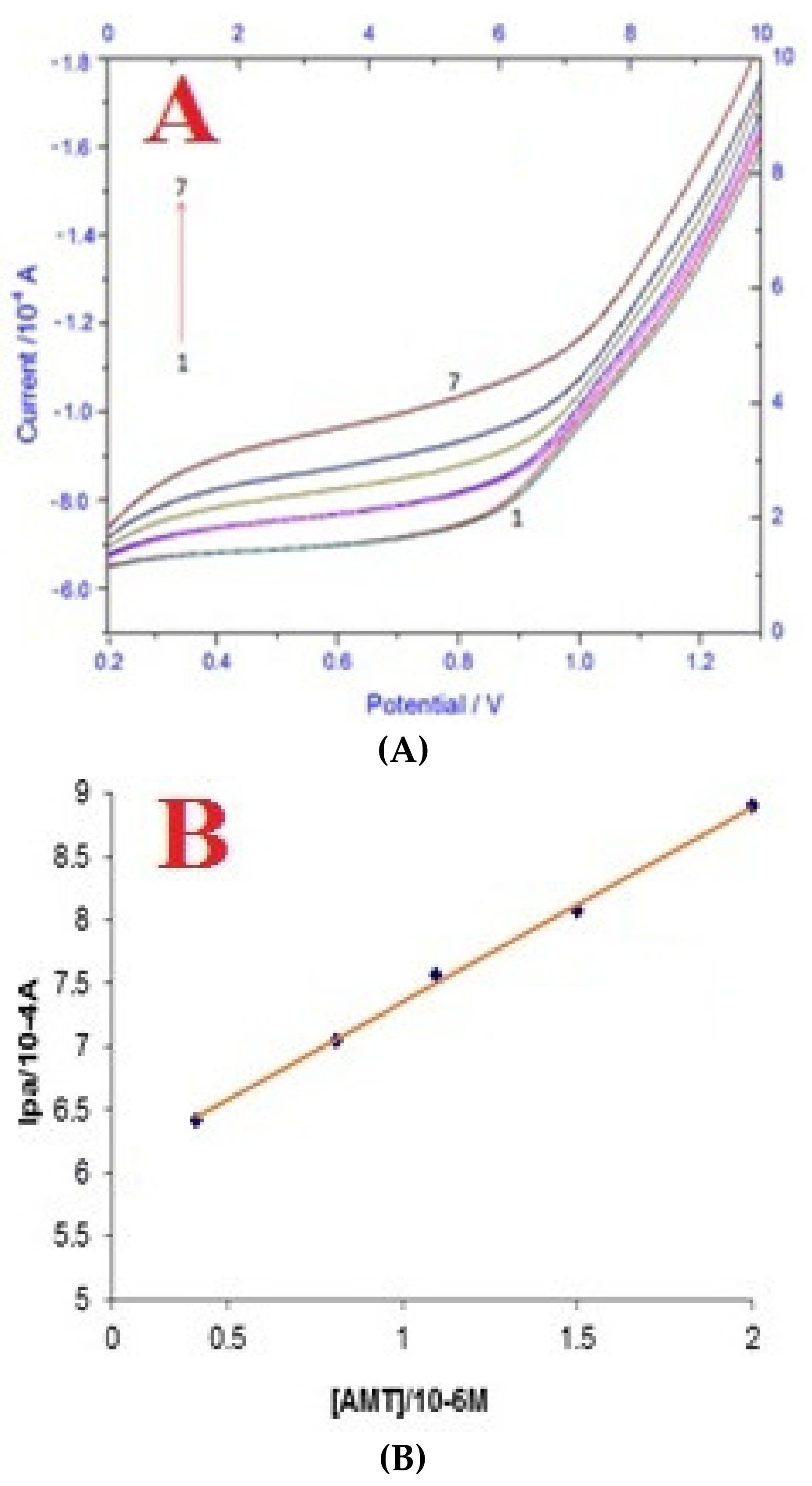
3.6. Validation Performance of MWCNT/Cellulose/GCE for AMT
3.7. Application to Real Sample Analysis
4. Conclusions
Funding
Conflicts of Interest
References
- Khalil, H.A.; Bhat, A.H.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Yoshiharu, N.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar]
- Jackson, J.K.; Letchford, K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int. J. Nanomed. 2011, 6, 321–330. [Google Scholar]
- Tongdesoontorn, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 6, 321. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Liu, X.; Wu, W.; Li, Y.; Wang, H. Highly elastic and conductive graphene/carboxymethylcellulose aerogels for flexible strain-sensing materials. J. Mater. Sci. 2017, 52, 12540–12552. [Google Scholar] [CrossRef]
- Hajian, A.; Fu, Q.; Berglund, L.A. Recyclable and superelastic aerogels based on carbon nanotubes and carboxymethyl cellulose. Compos. Sci. Technol. 2018, 159, 1–10. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Al-tawbini, B.; Bukhari, A.A.; Abuilaiwi, F.A.; Fettouhi, M.B. Effect of Carboxylic Functional Group Functionalized on Carbon Nanotubes Surface on the Removal of Lead from Water. Bioinorg. Chem. Appl. 2010, 9, 603978. [Google Scholar] [CrossRef]
- Wyngaarden, J. SmithL-Cecil’s Textbook of Medicine, 18th ed.; W B Saunders: Philadelphia, PA, USA, 1988; p. 1229. [Google Scholar]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800. [Google Scholar] [CrossRef]
- Nugent, J.M.; Santhanam, K.S.V.; Rubio, A.; Ajayan, P.M. Fast Electron Transfer Kinetics on Multiwalled Carbon Nanotube. Microbun. Electrodes. Nano Lett. 2001, 1, 87–91. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, L.; Li, G.; Du, L.; Zhao, X.; Yan, J.; Cheng, Y.; Dang, A.; Li, T. Preparation and electrochemical property of CMC/MWCNT composite using ionic liquid as solvent. Chin. Sci. Bull. 2008, 57, 11620–11625. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.L.; Meng, L.J.; Lu, Q.H. Preparation of Cellulose/SWNTs Complex Using Ionic Liquids as Solvent. J. Mater. Eng. 2008, 8, 26–30. [Google Scholar]
- Nadagouda, M.N.; Varma, R.S. Microwave-Assisted Synthesis of Crosslinked Poly, vinyl alcohol, Nanocomposites Comprising Single-Walled Carbon Nanotubes and Multi-Walled Carbon Nanotubes. Macromol. Rapid Commun. 2007, 28, 842–847. [Google Scholar] [CrossRef]
- Fukushima, T.; Aida, T. Ionic Liquids for Soft Functional Materials with Carbon Nanotubes. Chem. Eur. J. 2007, 13, 5048–5058. [Google Scholar] [CrossRef]
- Marcilla, R.; Curri, M.L.; Cozzoli, P.D. Nano-Objects on a Round Trip from Water to Organics in a Polymeric Ionic Liquid Vehicle. Small 2006, 2, 507–512. [Google Scholar] [CrossRef]
- Yun, S.; Kim, J. Multi-walled carbon nanotubes–cellulose paper for a chemical vapor sensor. Sen. Actuators B Chem. 2010, 150, 308–313. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Edit. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Sun, H.; Miao, J.; Yu, Y.; Zhang, L. Dissolution of cellulose with a novel solvent and formation of regenerated cellulose fiber. Appl. Phys. A Mater. Sci. Proc. 2015, 119, 539–546. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, D.; Yu, Y.; Miao, J.; Liu, Y.; Zhang, L. Composite fibers prepared from multi-walled carbon nanotubes/cellulose dispersed/dissolved in ammonium/dimethyl sulfoxide mixed solvent. RSC Adv. 2017, 7, 2186–2192. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices—Review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Hamedi, M.M.; Hajian, A.; Fall, A.B.; Hakansson, K.; Salajkova, M.; Lundell, F.; Wagberg, L.; Berglund, L.A. Highly conducting.; strong nanocomposites based on nanocellulose-assisted aqueous dispersions of single-wall carbon nanotubes. ACS Nano 2014, 8, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M.; Wågberg, L. Understanding the dispersive action of nanocellulose for carbon nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Gao, K.; Shao, Z. Cellulose nanofiber/single-walled carbon nanotube hybrid non-woven macrofiber mats as novel wearable supercapacitors with excellent stability, tailorability and reliability. Nanoscale 2014, 6, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Chun, S.J.; Lee, S.S.; Kim, B.Y.; Kim, J.H.; Chung, H.; Lee, S.Y.; Kim, W. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers.; carbon nanotubes and triblock-copolymer ion gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Shao, Z.; Wang, X.; Zhang, Y.; Wang, W.; Wang, F. Cellulose nanofibers/multi-walled carbon nanotube nanohybrid aerogel for all-solid-state flexible supercapacitors. RSC Adv. 2013, 3, 15058–15064. [Google Scholar] [CrossRef]
- Geetha, B.; Gaspar, L.J.M.; Mandal, A.B. Surface Characteristics of Comblike Copolymers from Hexadecylacrylamide and Acrylic Acid at the Air/Water Interface. Langmuir 2003, 19, 9051–9057. [Google Scholar]
- Ramya, R.S.G.; Thennarasu, S.; Mandal, A.B. Self-assembling characteristics of 5-α-acetamido-α-benzyl, methyl-5′-methyl imidazolidine-2.; 4-dione-A hydantoin drug. Chem. Phys. 2003, 291, 195–205. [Google Scholar]
- Ramya, R.S.G.; Thennarasu, S.; Mandal, A.B. Self-Assembling Characteristics of a New Nonionic Gemini Surfactant. J. Phys. Chem. B 2004, 108, 8806–8816. [Google Scholar]
- Attwood, D.; Florence, A.T. Surface Activity and Colloidal Properties of Drugs and Naturally Occurring Substances; Surfactant Systems; Chapman and Hall: London, UK, 1985; pp. 124–228. [Google Scholar]
- Vahedi, H.; Merat, S.; Momtahen, S.; Kazzazi, A.S.; Ghaffari, N. Clinical trial: The effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Alim. Pharmacol. Ther. 2008, 27, 678–684. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Yao, X.; Gao, S.; Xie, J.; Xu, H.; Lin, N. Electrochemical chiral sensor based on cellulose nanocrystals and multiwall carbon nanotubes for discrimination of tryptophan enantiomers. Cellulose 2018, 25, 3861–3871. [Google Scholar] [CrossRef]
- Rezaei, B.; Damiri, S. Voltammetric behavior of multi-walled carbon nanotubes modified electrode-hexacyanoferrate, II, electrocatalyst system as a sensor for determination of captopril. Sens. Actuators B 2008, 134, 324–331. [Google Scholar] [CrossRef]
- Manimaran, P.; Saravanan, S.P.; Sanjay, M.R.; Siengchin, S.; Jawaid, M.; Khan, A. Characterization of new cellulosic fiber: Dracaena reflexa as a reinforcement for polymer composite structures. J. Mater. Res. Technol. 2019, 8, 1952–1963. [Google Scholar] [CrossRef]
- Popescu, M.C.; Dogaru, B.I.; Popescu, C.M. The influence of cellulose nanocrystals content on the water sorption properties of bio-based composite films. Mater. Des. 2017, 132, 170–177. [Google Scholar] [CrossRef]
- Meng, X.; Bocharova, V.; Tekinalp, H.; Cheng, S.; Kisliuk, A.; Sokolov, A.P.; Kunc, V.; Peter, W.H.; Ozcan, S. Toughening of nanocelluose/PLA composites via bio-epoxy interaction: Mechanistic study. Mater. Des. 2018, 139, 188–197. [Google Scholar] [CrossRef]
- Mulakkal, M.C.; Trask, R.S.; Ting, V.P.; Seddon, A.M. Responsive cellulose-hydrogel composite ink for 4D printing. Mater. Des. 2018, 160, 108–118. [Google Scholar] [CrossRef]
- Madhu, P.; Sanjay, M.R.; Pradeep, S.; Bhat, K.S.; Yogesha, B.; Siengchin, S. Characterization of cellulosic fibre from Phoenixpusilla leaves as potential reinforcement for polymeric composites. J. Mater. Res. Technol. 2019, 8, 2597–2604. [Google Scholar] [CrossRef]
- Morsi, M.A.; Oraby, A.H.; Elshahawy, A.G.; El-Hady, R.M.A.; Oraby, A.G.; Elshahawy, R.M.; Abd, E.H. Preparation.; structural analysis.; morphological investigation and electrical properties of gold nanoparticles filled polyvinyl alcohol/carboxymethyl cellulose blend. J. Mater. Res. Technol. 2019, 8, 5996–6010. [Google Scholar] [CrossRef]
- Parveen, S.; Pichandi, S.; Goswami, P.; Rana, S. Novel glass fibre reinforced hierarchical composites with improved interfacial; mechanical and dynamic mechanical properties developed using cellulose microcrystals. Mater. Des. 2020, 188, 108448. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Dumanlı, A.G.; Windle, A.H. Carbon fibres from cellulosic precursors: A review. J. Mater. Sci. 2012, 47, 4236–4250. [Google Scholar] [CrossRef]
- Rahman, M.J.; Mieno, T. Conductive cotton textile from safely functionalized carbon nanotubes. J. Nanomat. 2015, 2015, 978484. [Google Scholar] [CrossRef]
- Maria, K.H.; Mieno, T. Production and properties of carbon nanotube/cellulose composite paper. J. Nanomat. 2017, 2017, 6745029. [Google Scholar] [CrossRef]
- Ceylan, O.; Landuyt, L.V.; Rahier, H.; De, C.K. The effect of water immersion on the thermal degradation of cotton fibers. Cellulose 2013, 20, 1603–1612. [Google Scholar] [CrossRef]
- Zavadskii, A.E. X-ray diffraction method of determining the degree of crystallinity of cellulose materials of different anisotropy. Fibre Chem. 2004, 36, 425–430. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, A.; Alam, M.M.; Asiri, A.M.; Uddin, J.; Rahman, M.M. SDBS-functionalized MWCNT/poly(o-toluidine) nanowires modified glassy carbon electrode as a selective sensing platform for Ce3+ in real samples. J. Mol. Liq. 2019, 279, 392–399. [Google Scholar] [CrossRef]
- Khan, A.A.P.; Khan, A.; Rahman, M.M.; Asiri, A.M. Conventional surfactant-doped poly (o-anisidine)/GO nanocomposites for benzaldehyde chemical sensor development. J. Sol Gel Sci. Technol. 2016, 77, 361–370. [Google Scholar] [CrossRef]
- Pattar, V.P.; Nandibewoor, S.T. Electroanalytical method for the determination of 5-fluorouracil using a reduced graphene oxide/chitosan modified sensor. RSC Adv. 2015, 5, 34292–34301. [Google Scholar] [CrossRef]
- Grigoryants, V.M.; Anisimov, O.A.; Molin, Y.N. Study of the radical-cations of triethylamine and benzene derivatives by the optical detection of the EPR spectra of radical-ion Pairs. J. Struct. Chem. 1982, 23, 327–333. [Google Scholar] [CrossRef]
- Jurva, U.; Wikström, H.V.; Weidolf, L.; Bruins, A.P. Comparison between electrochemistry/mass spectrometry and cytochrome P450 catalyzed oxidation reactions. Rapid Commun. Mass Spectrom. 2003, 17, 800–810. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltammetry: Simulation Analysis of Reaction Mechanisms. Synth. React. Inorg. Metal Organ. Chem. 1994, 24, 1237–1238. [Google Scholar]
- Tran, H.V.; Piro, B.; Reisberg, S.; Duc, H.T.; Pham, M.C. Antibodies Directed to RNA/DNA Hybrids: An Electrochemical Immunosensor for MicroRNAs Detection using Graphene-Composite Electrodes. Anal. Chem. 2013, 85, 8469–8474. [Google Scholar] [CrossRef]
- Duarte, E.H.; Dos, S.W.P.; Hudari, F.F.; Bott, N.J.L.; Sartori, E.R.; Dall, A.L.H.; Pereira, A.C.; Teixeira, T.C.R. A highly improved method forsensitive determination of amitriptyline in pharmaceuticalformulations using an unmodified carbon nanotubeelectrode in the presence of sulfuric acid. Talanta 2014, 127, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beitollahi, H.; Nejad, F.G.; Tajik, S.; Jahani, S.; Biparva, P. Voltammetric determination of amitriptyline based on raphite screen printed electrode modified with a opper Oxide nanoparticles. Int. J. Nano Dimens. 2017, 8, 197–205. [Google Scholar]
- Eslami, E.; Farjami, F. Electrochemical determinationof amitriptyline using a nanocomposite carbon pasteelectrode in human body fluids. J. Phys. Chem. Electrochem. 2014, 4, 111–117. [Google Scholar]
- Marco, J.P.; Borges, K.B.; Tarley, C.R.T.; Ribeiro, E.S.; Pereira, A.C. Development and application of an electrochemical biosensor based on carbon paste and silica modified with niobium oxide.; alumina and DNA, SiO2/Al2O3/b2O5/DNA, for amitriptyline. J. Electroanal. Chem. 2013, 704, 159–168. [Google Scholar] [CrossRef]





| Linearity range (μM) Slope of the calibration plot Intercept Correlation coefficient (r) RSD of slope a (%) RSD of intercept a (%) LOD (μM) LOQ (μM) | 0.5–20.0 5.458 0.8207 0.9985 0.5812 1.5417 0.0845 0.282 |
| Modifier | LOD | Ref. |
|---|---|---|
| Carbon nanotube CuO nanoparticles Montmorillonite nanoclay and ionic liquid CNT/SiO2/Al2O3/Nb2O5/DNA Multi-walledcarbon@celllulose nanocomposite | 1.16 μM 0.4 μM 24 nM 0.12 μM 0.0845 μM | [54] [55] [56] [57] This work |
| Sample | Spiked (μM) | Found a (μM) | Recovery (%) | Bias (%) | S.D. ± R.S.D (%) |
|---|---|---|---|---|---|
| 1 | 0.5 | 0.502 | 100.4 | 0.40 | 0.021 ± 0.022 |
| 2 | 1.0 | 1.001 | 100.1 | 0.10 | 0.015 ± 0.011 |
| 3 | 1.5 | 1.481 | 98.70 | −1.30 | 0.030 ± 0.016 |
| 4 | 2.0 | 1.972 | 98.60 | −1.40 | 0.065 ± 0.032 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.P. Electrocatalytic Behavior and Determination of Amitriptyline Drug with MWCNT@Celllulose Composite Modified Glassy Carbon Electrode. Materials 2020, 13, 1708. https://doi.org/10.3390/ma13071708
Khan AAP. Electrocatalytic Behavior and Determination of Amitriptyline Drug with MWCNT@Celllulose Composite Modified Glassy Carbon Electrode. Materials. 2020; 13(7):1708. https://doi.org/10.3390/ma13071708
Chicago/Turabian StyleKhan, Aftab Aslam Parwaz. 2020. "Electrocatalytic Behavior and Determination of Amitriptyline Drug with MWCNT@Celllulose Composite Modified Glassy Carbon Electrode" Materials 13, no. 7: 1708. https://doi.org/10.3390/ma13071708




