Preparation of Ti3C2Tx/NiZn Ferrite Hybrids with Improved Electromagnetic Properties
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Experiments
2.2. Characteristics
3. Results and Discussion
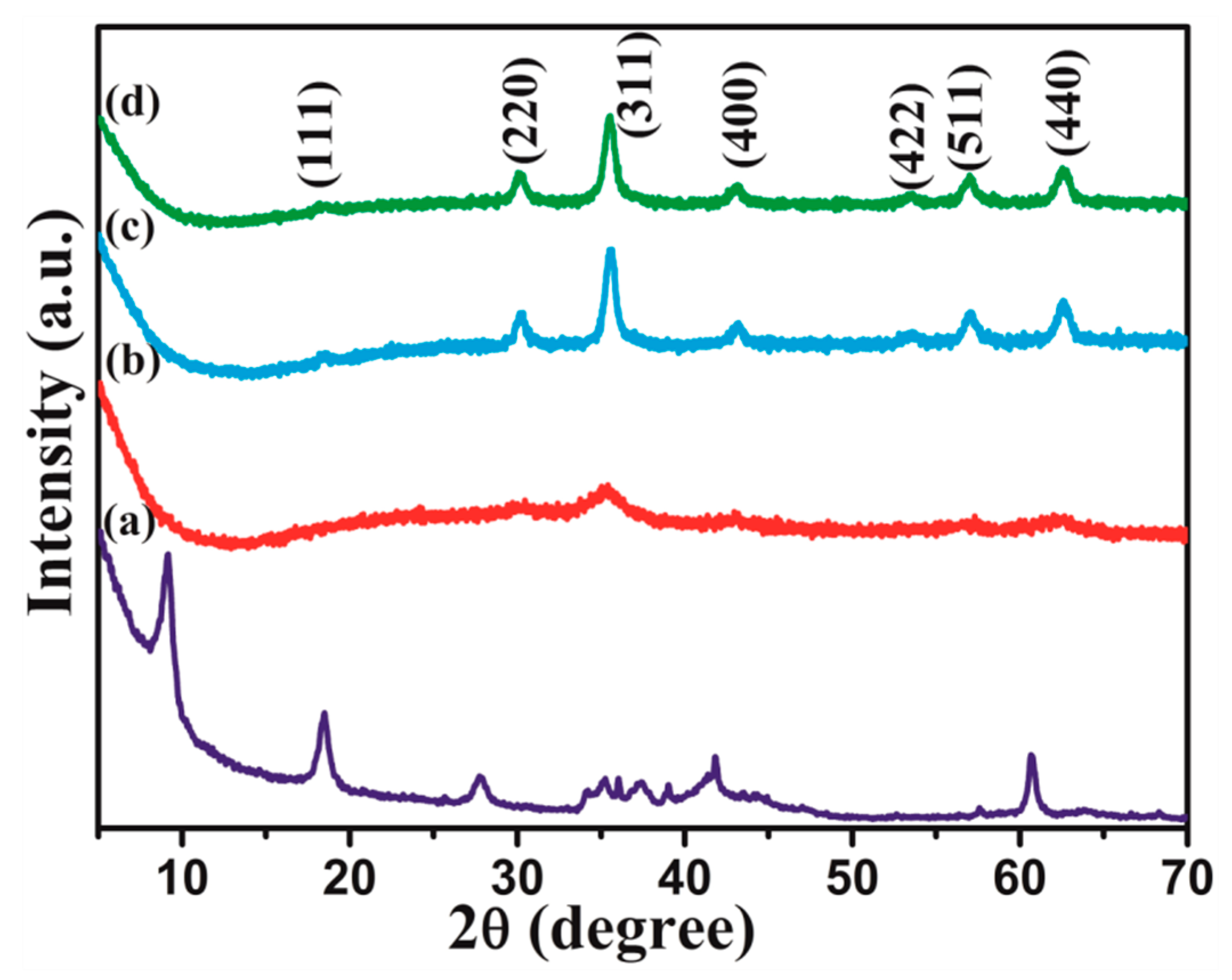
3.1. Phase and Microstructure Morphology of Ti3C2Tx/NiZn Ferrite Powders
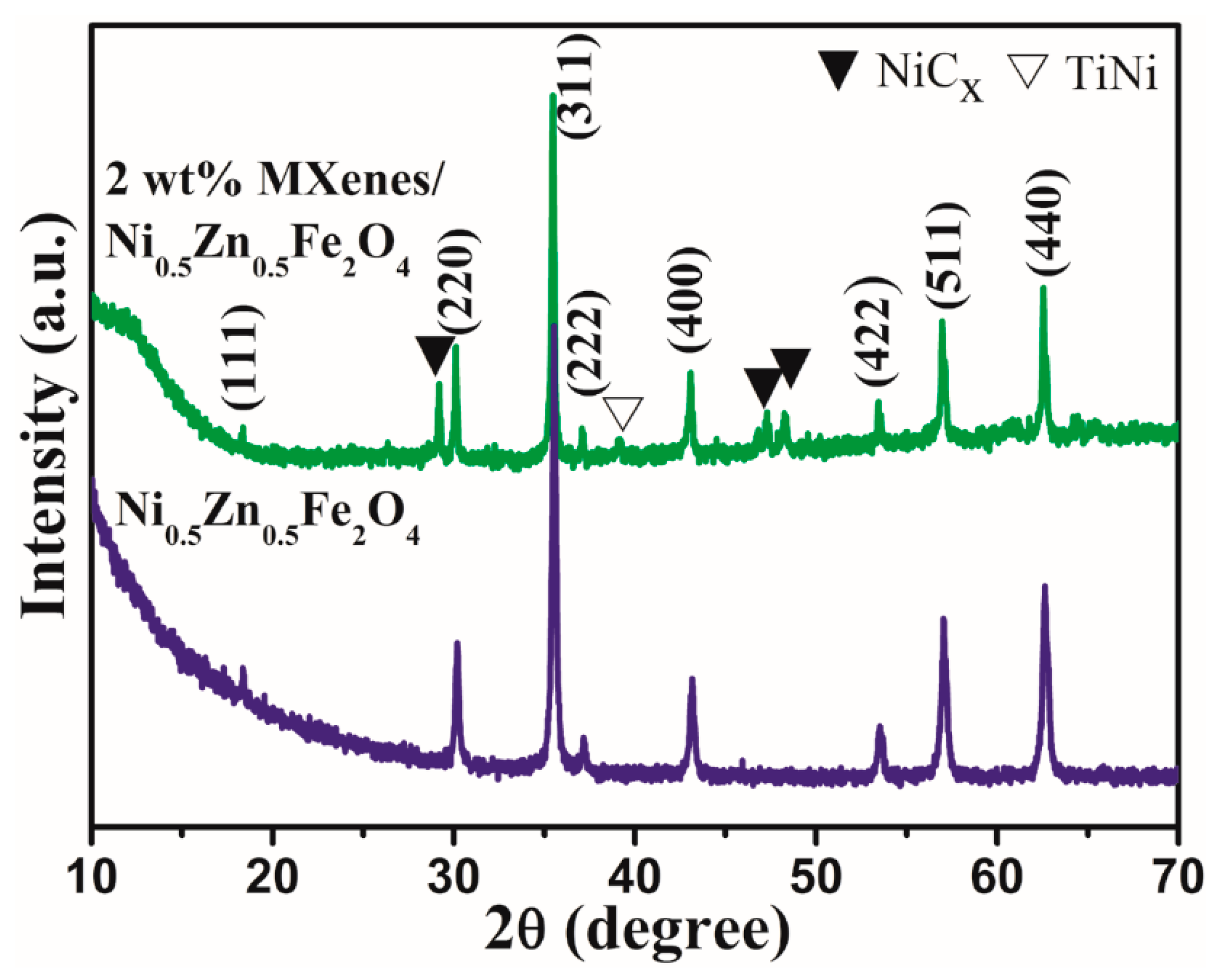
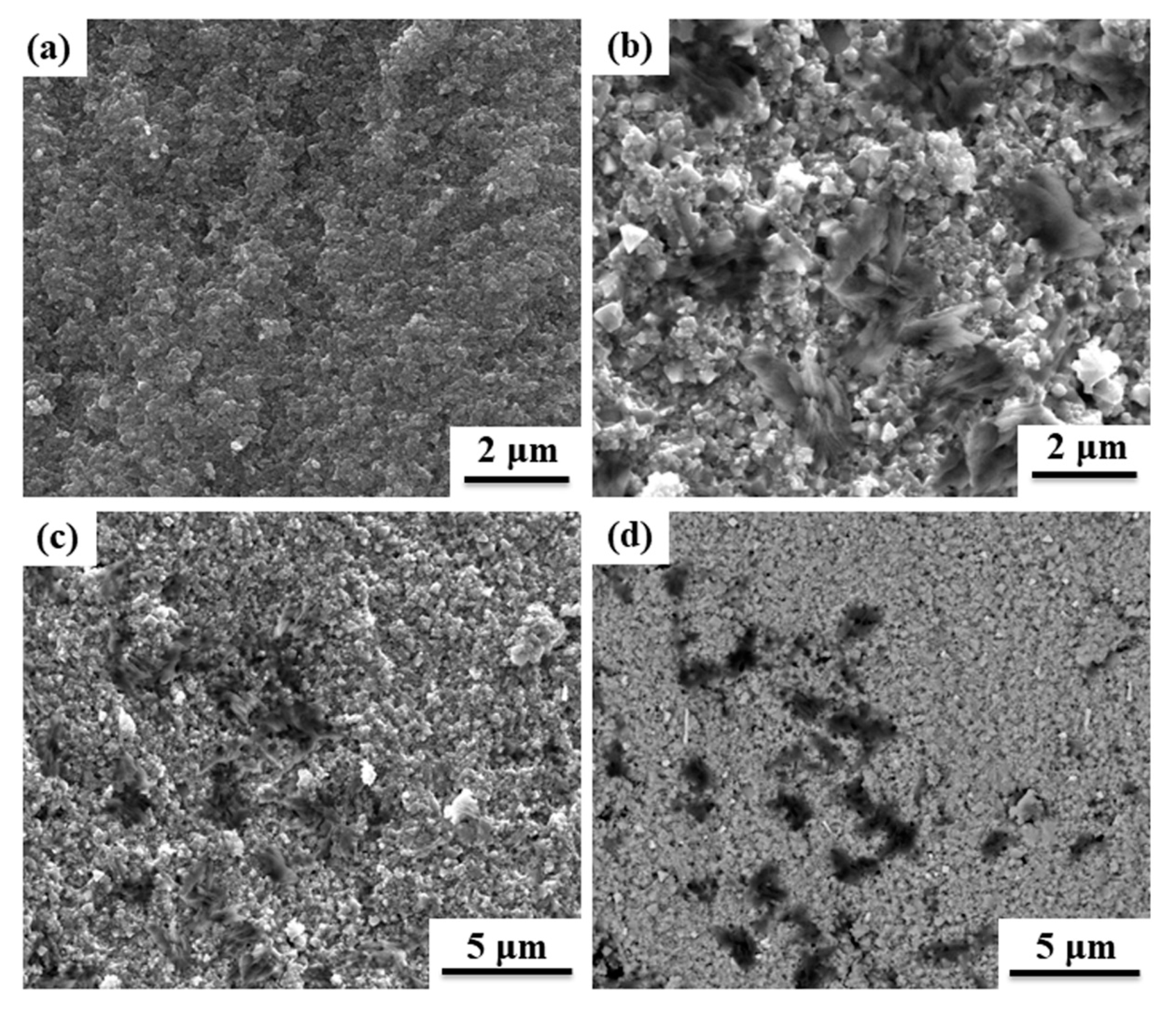
3.2. Phase and Microstructure Morphology of Ti3C2Tx/NiZn Ferrite Ceramics
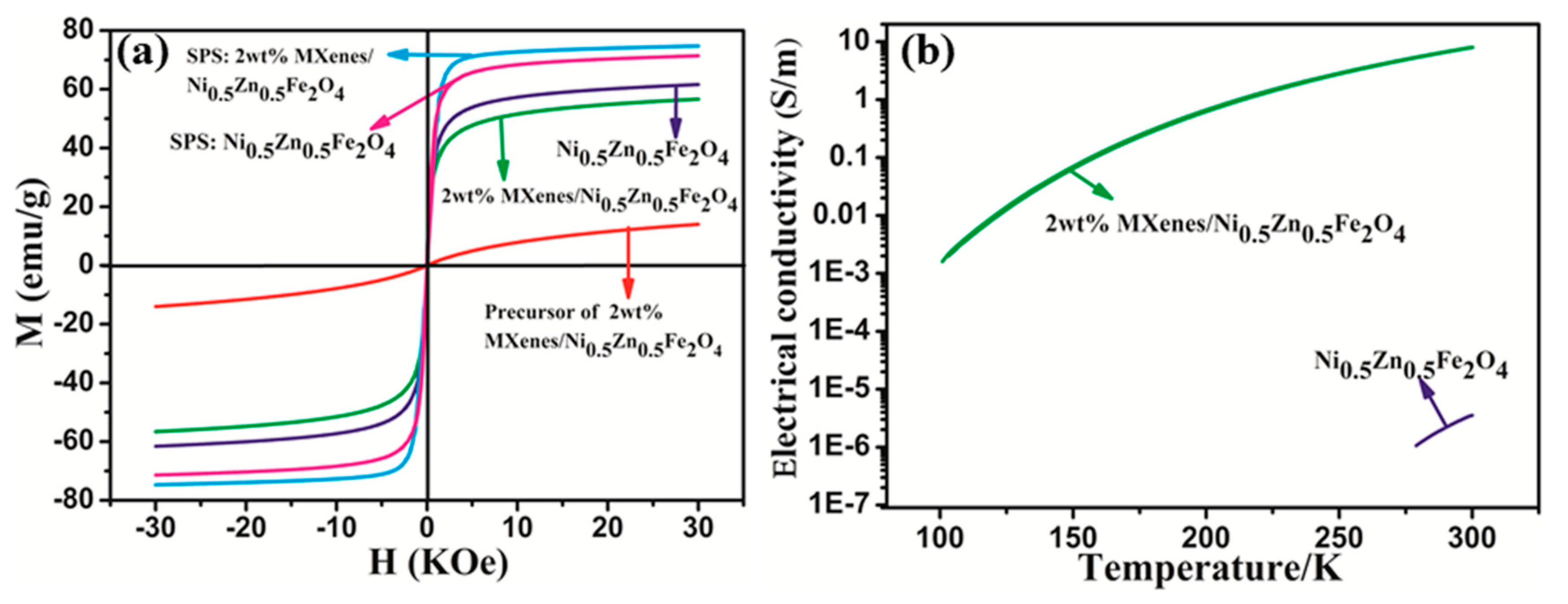
3.3. Electromagnetic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, M.; Deng, L. Doping effect of multiwall carbon nanotubes on the microwave electromagnetic properties of NiCoZn spinel ferrites. Appl. Phys. Lett. 2007, 90, 11108. [Google Scholar] [CrossRef]
- Zhou, X.-B.; Shen, L.; Li, L.; Huang, T.-M.; Hu, C.-F.; Pan, W.-M.; Jin, X.-H.; Sun, J.; Gao, L.; Huang, Q. Preparation of nanocrystalline-coated carbon nanotube/Ni0.5Zn0.5Fe2O4 composite with excellent electromagnetic property as microwave absorber. J. Phys. D Appl. Phys. 2013, 46, 145002. [Google Scholar] [CrossRef]
- Dippong, T.; Deac, I.G.; Cadar, O.; Levei, E.A.; Diamandescu, L.; Borodi, G. Effect of Zn content on structural, morphological and magnetic behavior of ZnxCo1−xFe2O4/SiO2 nanocomposites. J. Alloys Compd. 2019, 792, 432–443. [Google Scholar] [CrossRef]
- Stefanescu, M.; Stoia, M.; Caizer, C.; Dippong, T.; Barvinschi, P. Preparation of CoxFe3-XO4 nanoparticles by thermal decomposition of some organo-metallic precursors. J. Them. Anal. Calorim. 2009, 97, 245–250. [Google Scholar] [CrossRef]
- Shemelin, V.; Liepe, M.; Padamsee, H. Characterization of ferrites at low temperature and high frequency. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2006, 557, 268–271. [Google Scholar] [CrossRef]
- Shemelin, V.; Barnes, P.; Gillett, B.; Liepe, M.; Medjidzade, V.; Padamsee, H.; Roy, R.; Sears, J. Status of HOM load for for Cornell ERL injector. Proc. EPAC 2006, 2006, 478–480. [Google Scholar]
- Zhou, X.; Shen, L.; Li, L.; Zhou, S.; Huang, T.; Hu, C.; Pan, W.; Jing, X.; Sun, J.; Gao, L.; et al. Microwave sintering carbon nanotube/Ni0.5Zn0.5Fe2O4 composites and their electromagnetic performance. J. Eur. Ceram. Soc. 2013, 33, 2119–2126. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Aken, K.L.V.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. Two-Dimensional Titanium Carbide for Efficiently Reductive Removal of Highly Toxic Chromium(VI) from Water. ACS Appl. Mater. Interfaces 2015, 7, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique Lead Adsorption Behavior of Activated Hydroxyl Group in Two-Dimensional Titanium Carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Come, J.; Naguib, M.; Rozier, P.; Barsoum, M.W.; Gogotsi, Y.; Taberna, P.L.; Morcrette, M.; Simon, P. A Non-Aqueous Asymmetric Cell with a Ti2C-Based Two-Dimensional Negative Electrode. J. Electrochem. Soc. 2012, 159, A1368–A1373. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Ti3C2 MXenes with Modified Surface for High-Performance Electromagnetic Absorption and Shielding in the X-Band. ACS Appl. Mater. Interfaces 2016, 8, 21011–21019. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, X.; Wang, J.; Deng, Q.; Li, M.; Du, S.; Han, Y.-H.; Lee, J.; Huang, Q. Facile preparation of in situ coated Ti3C2Tx/Ni0.5Zn0.5Fe2O4composites and their electromagnetic performance. RSC Adv. 2017, 7, 24698–24708. [Google Scholar] [CrossRef] [Green Version]
- Millot, N.; Le Gallet, S.; Aymes, D.; Bernard, F.; Grin, Y. Spark plasma sintering of cobalt ferrite nanopowders prepared by coprecipitation and hydrothermal synthesis. J. Eur. Ceram. Soc. 2007, 27, 921–926. [Google Scholar] [CrossRef]
- Borodianska, O.V.H.; Sakka, Y.; Demirskyi, D. Flash spark plasma sintering of ultrafine yttria-stabilized zirconia ceramics. Scr. Mater. 2016, 121, 32–36. [Google Scholar]
- Dong, P.; Wang, Z.; Wang, W.X.; Chena, S.P.; Zhou, J. Understanding the spark plasma sintering from the view of materials joining. Scr. Mater. 2016, 123, 118–121. [Google Scholar] [CrossRef]
- Valenzuela, R.; Beji, Z.; Herbst, F.; Ammar, S. Ferromagnetic resonance behavior of spark plasma sintered Ni–Zn ferrite nanoparticles produced by a chemical route. J. Appl. Phys. 2011, 109, 7. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Lukatskaya, M.R.; Dyatkin, B.; Zhang, C.; Presser, V.; Gogotsi, Y.; Barsoum, M.W. One-step synthesis of nanocrystalline transition metal oxides on thin sheets of disordered graphic carbon by oxidation of MXenes. Chem. Commun. 2014, 50, 7420–7423. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Hu, Q.; Cao, X. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, L.B.; Sun, D.D.; Zhang, Y.D.; Liu, B.Z.; Hu, Q.K.; Zhou, A.G. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Mater. Sci. Eng. B 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Cullity, B.D. Element of X-ray Differaction; Adison-Wesley: London, UK, 1956; Volume 261. [Google Scholar]
- Sun, J.; Li, J.; Sun, G.; Qu, W. Synthesis of dense NiZn ferrites by spark plasma sintering. Ceram. Int. 2002, 28, 855–858. [Google Scholar] [CrossRef]
- Goswami, P.P.; Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Sonochemical Synthesis and Characterization of Manganese Ferrite Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 17848–17855. [Google Scholar] [CrossRef]
- Gabal, M.; El-Shishtawy, R.M.; Al Angari, Y. Structural and magnetic properties of nano-crystalline Ni–Zn ferrites synthesized using egg-white precursor. J. Magn. Magn. Mater. 2012, 324, 2258–2264. [Google Scholar] [CrossRef]
- Jadhav, J.; Biswas, S.; Yadav, A.; Jha, S.; Bhattacharyya, D. Structural and magnetic properties of nanocrystalline Ni Zn ferrites: In the context of cationic distribution. J. Alloys Compd. 2017, 696, 28–41. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Yadav, M.; Arora, M.; Pant, R. Finite size effect on Ni doped nanocrystalline NixZn1−xFe2O4 (0.1 ≤ x ≤ 0.5). Thin Solid Films 2010, 519, 1056–1058. [Google Scholar] [CrossRef]

| Sample | Molar Ratio Ni(NO3)2: Zn(NO3)2: Fe(NO3)3 | Molar Ratio NO3−: OH− | Weight Present of Ti3C2Tx (wt.%) |
|---|---|---|---|
| Ni0.5Zn0.5Fe2O4 | 0.5:0.5:2 | 1:1 | 0 |
| 2 wt.% Ti3C2Tx/ Ni0.5Zn0.5Fe2O4 | 0.5:0.5:2 | 1:1 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Li, Y.; Huang, Q. Preparation of Ti3C2Tx/NiZn Ferrite Hybrids with Improved Electromagnetic Properties. Materials 2020, 13, 820. https://doi.org/10.3390/ma13040820
Zhou X, Li Y, Huang Q. Preparation of Ti3C2Tx/NiZn Ferrite Hybrids with Improved Electromagnetic Properties. Materials. 2020; 13(4):820. https://doi.org/10.3390/ma13040820
Chicago/Turabian StyleZhou, Xiaobing, Youbing Li, and Qing Huang. 2020. "Preparation of Ti3C2Tx/NiZn Ferrite Hybrids with Improved Electromagnetic Properties" Materials 13, no. 4: 820. https://doi.org/10.3390/ma13040820




