Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research
Abstract
:1. Introduction
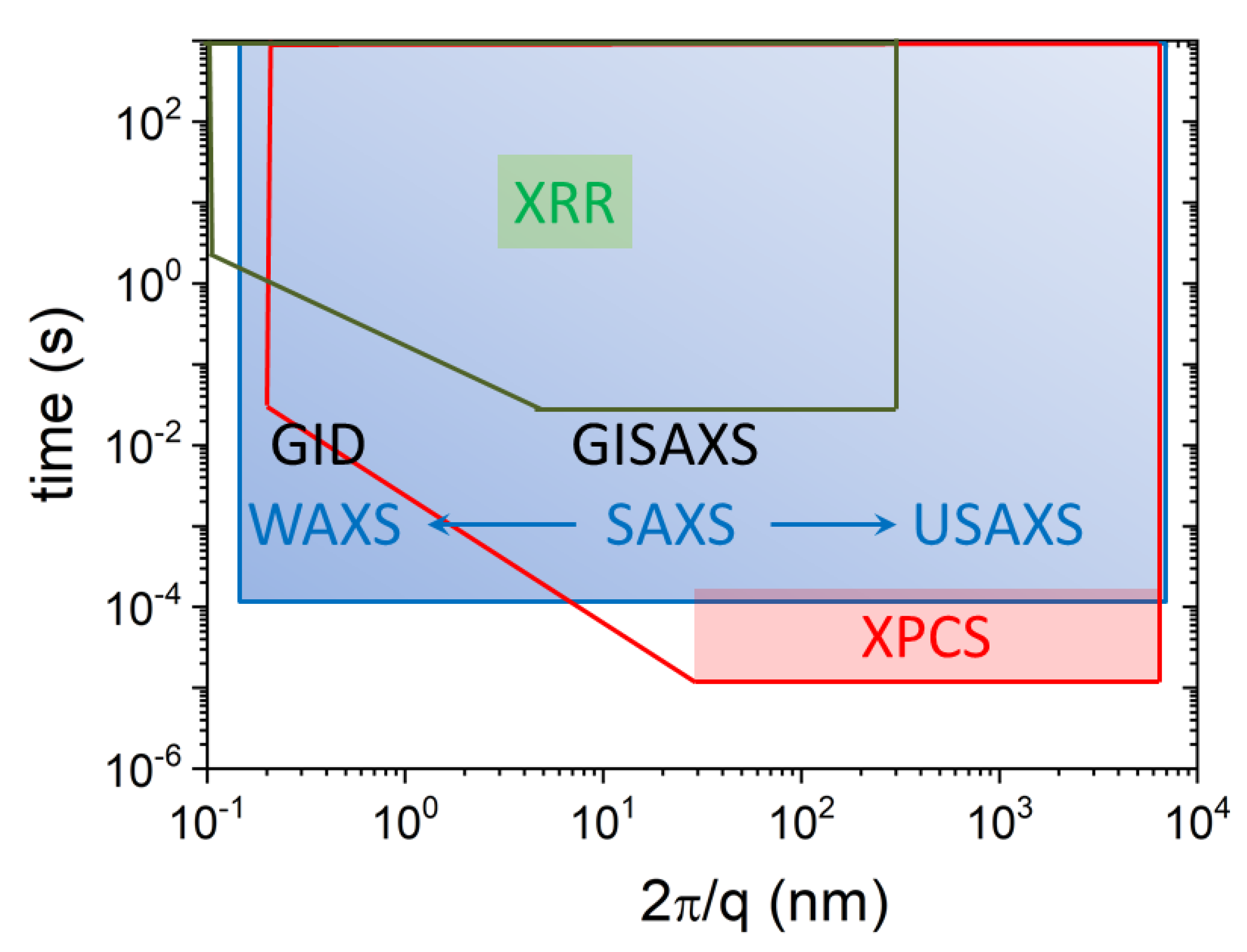
2. X-ray Scattering Methods
2.1. Small-Angle X-ray Scattering
2.2. X-ray Photon Correlation Spectroscopy
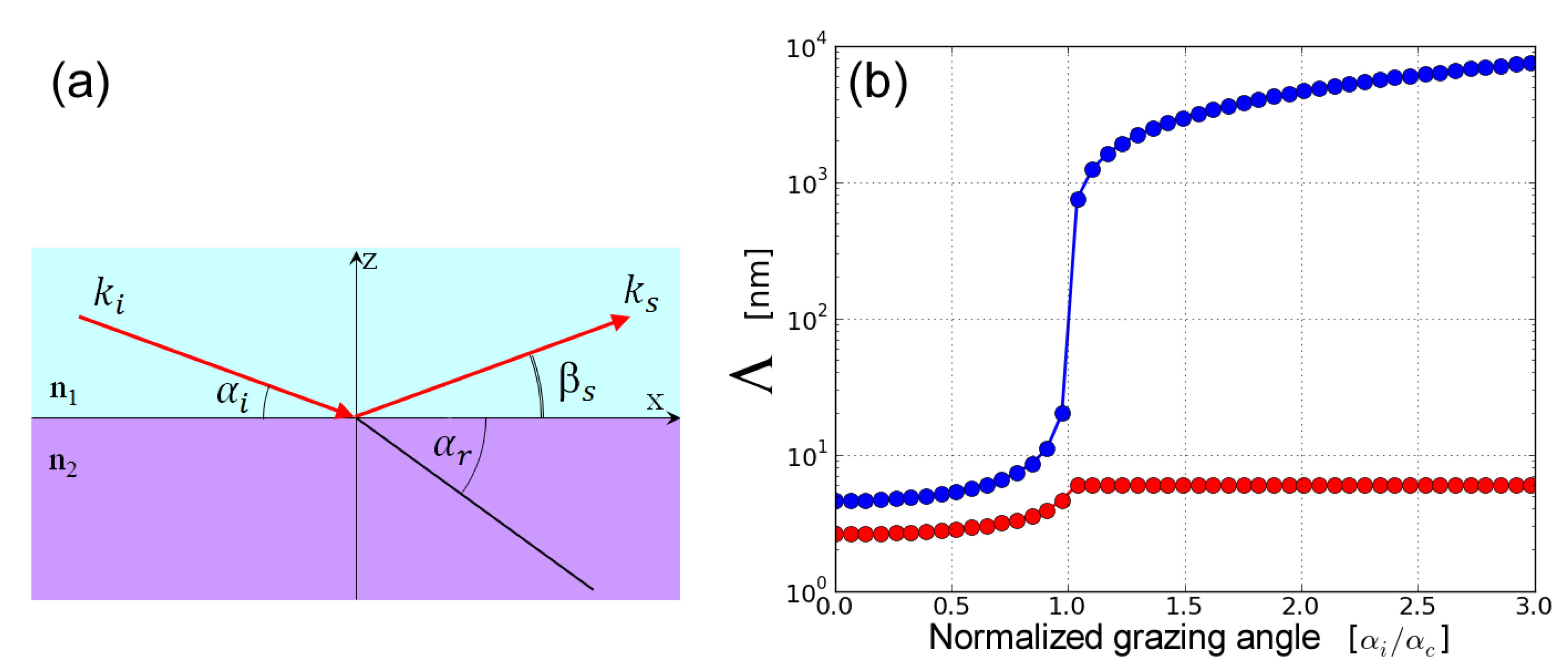
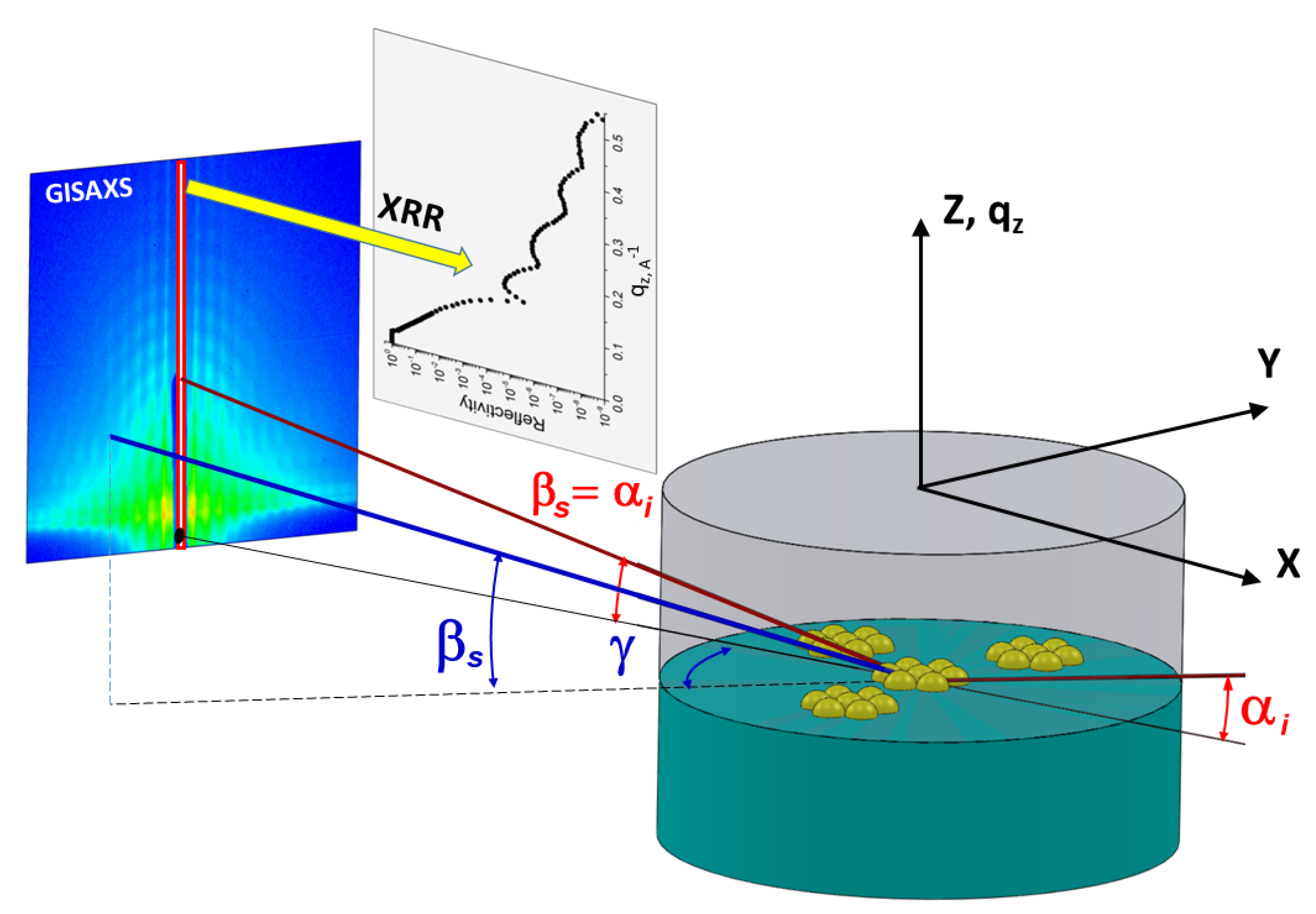
2.3. Surface and Interface X-ray Scattering
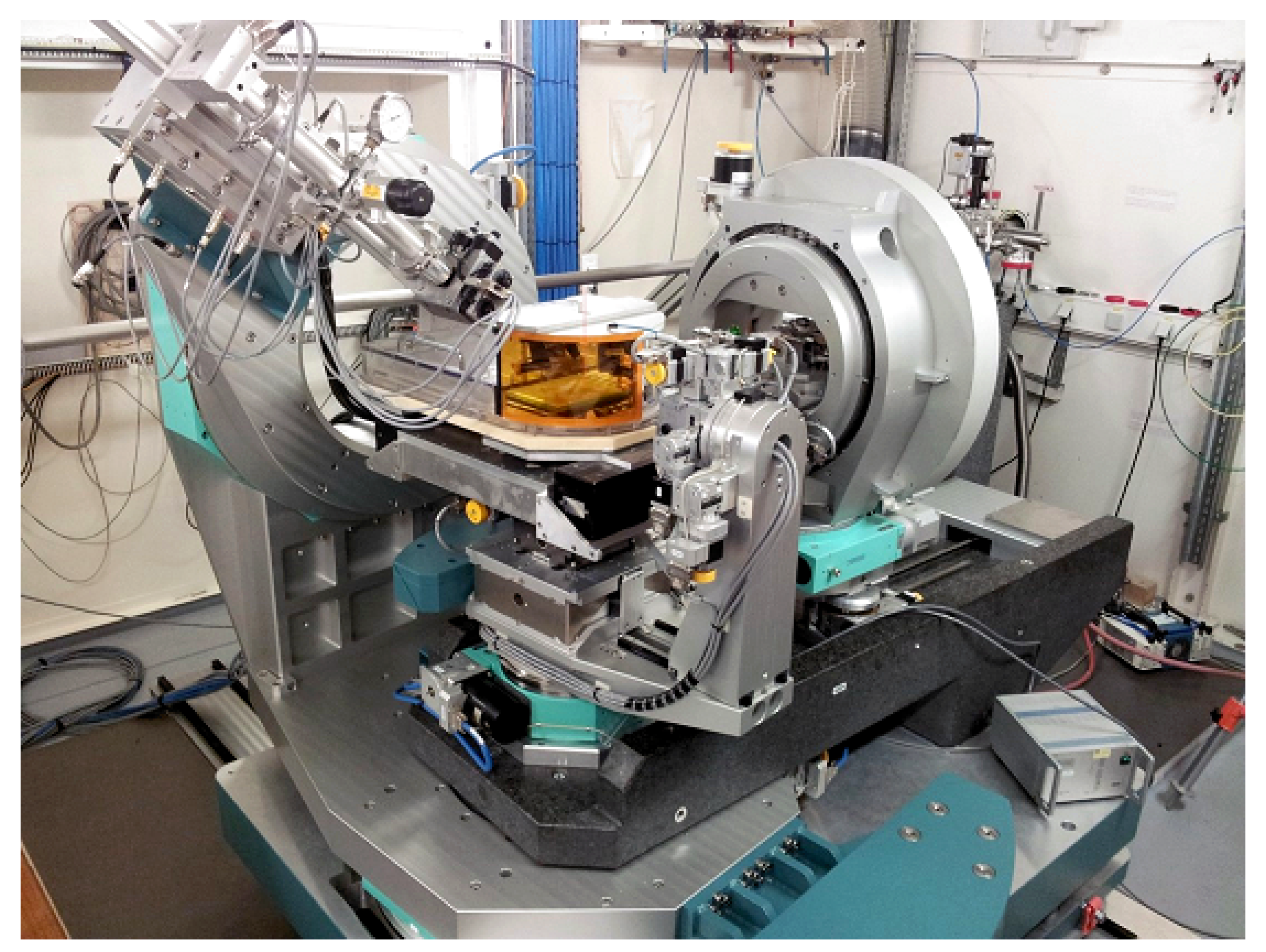
2.4. Instrumentation for Bulk and Interface Scattering
3. Investigations of Structure and Dynamics in Bulk Samples
3.1. Equilibrium Nanostructure and Interactions
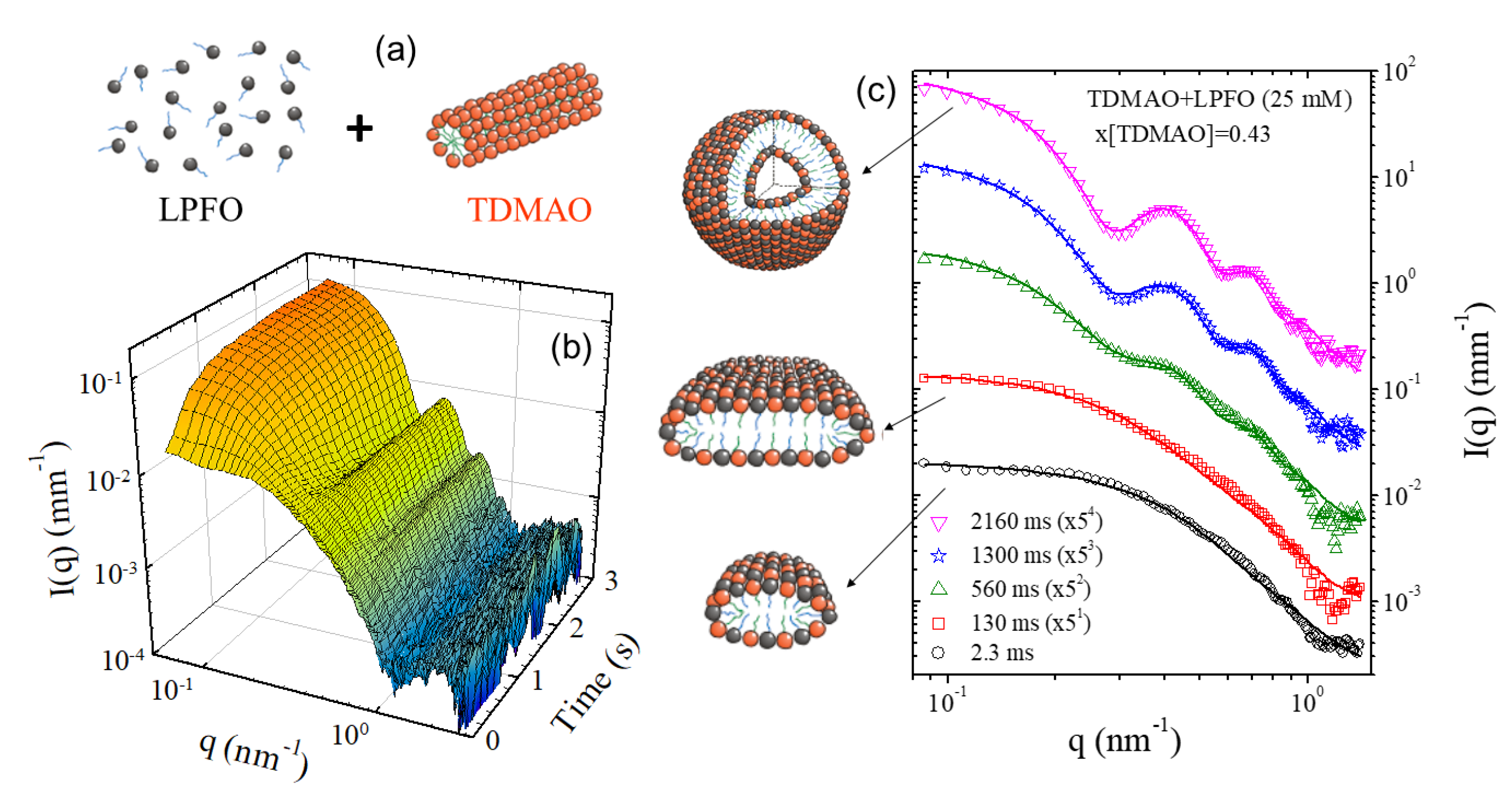
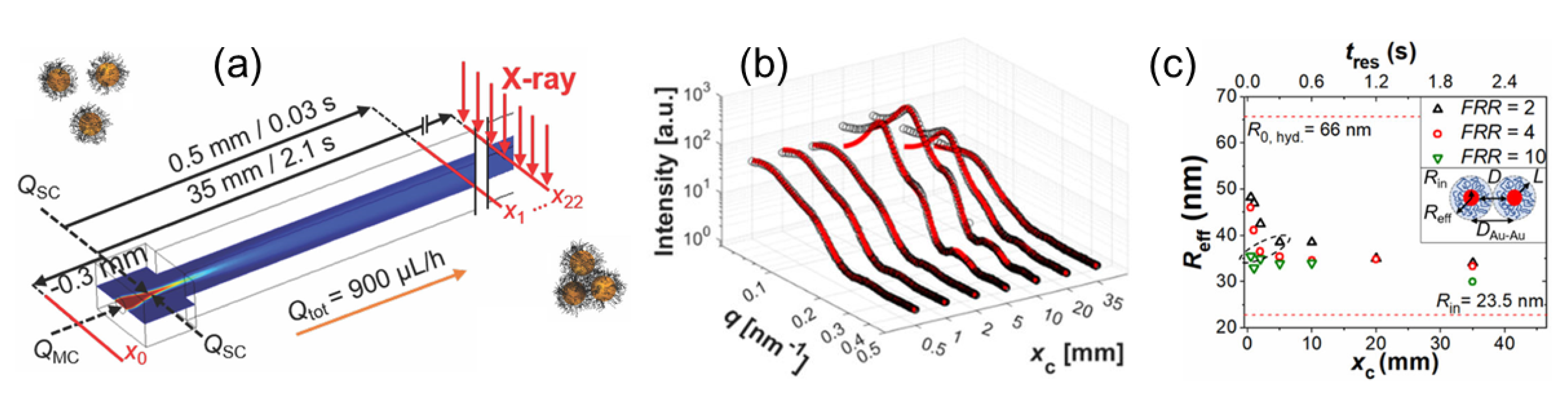
3.2. Probing the Pathways of Self-Assembly
3.3. Assembly of Biomacromolecular Complexes
3.4. In Situ Studies of Nucleation and Growth
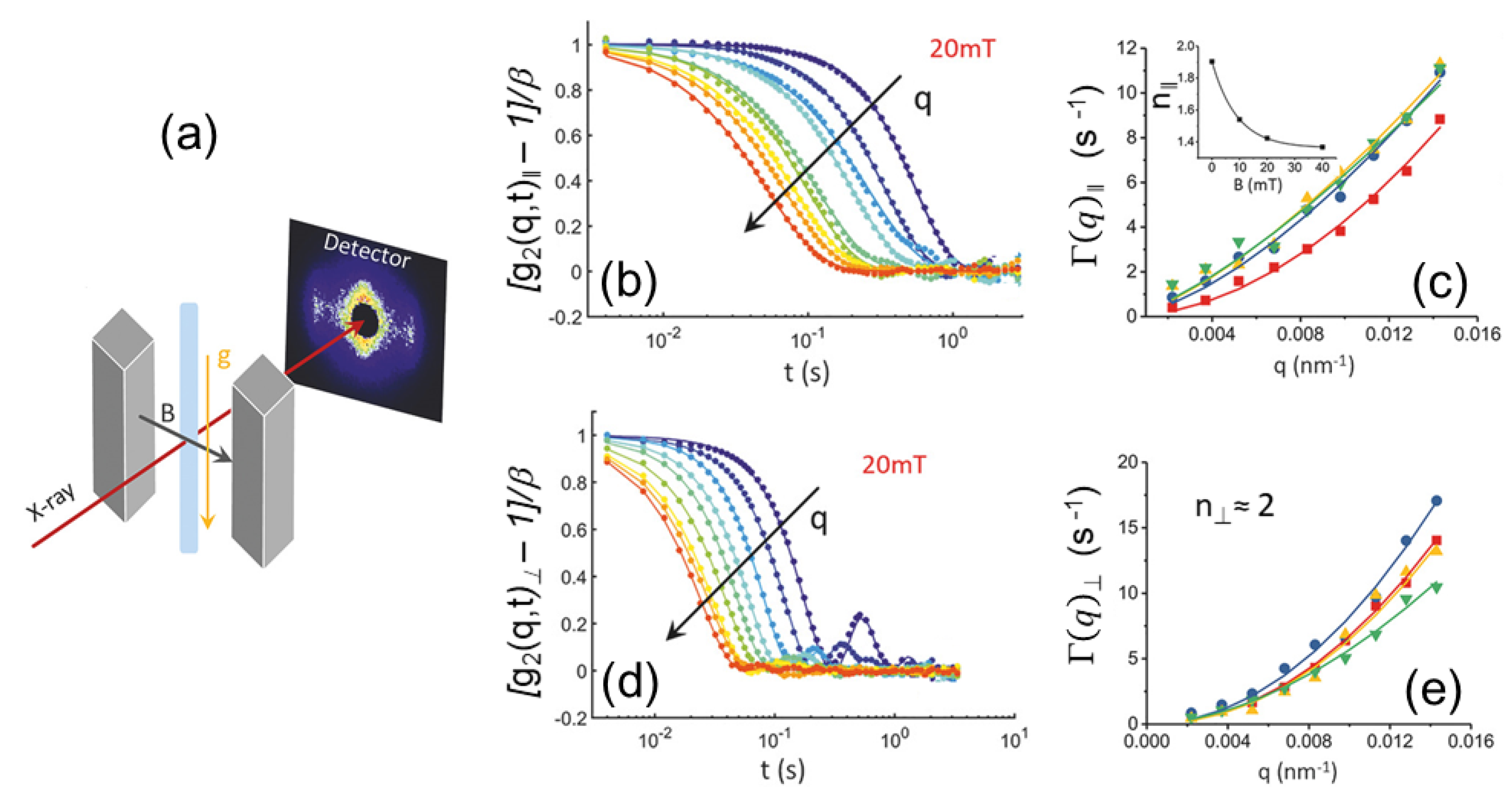
3.5. Equilibrium Dynamics
3.6. Flow-Induced Structures
3.7. High Resolution Diffraction and Imaging
4. Surface and Interface Studies
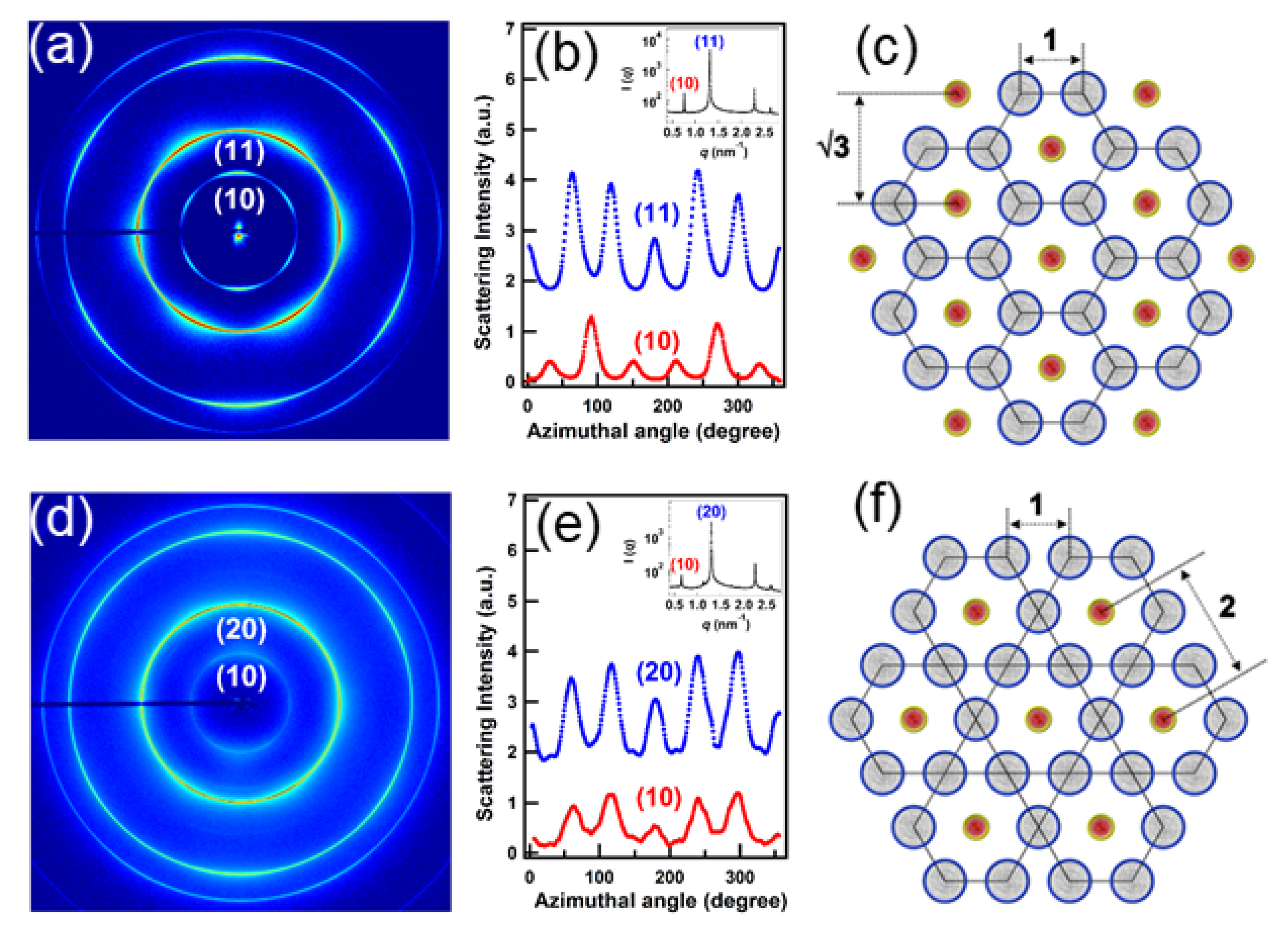
4.1. Nanoparticles at Interfaces
4.2. Model Biological Membranes
4.3. Liquid Interfaces
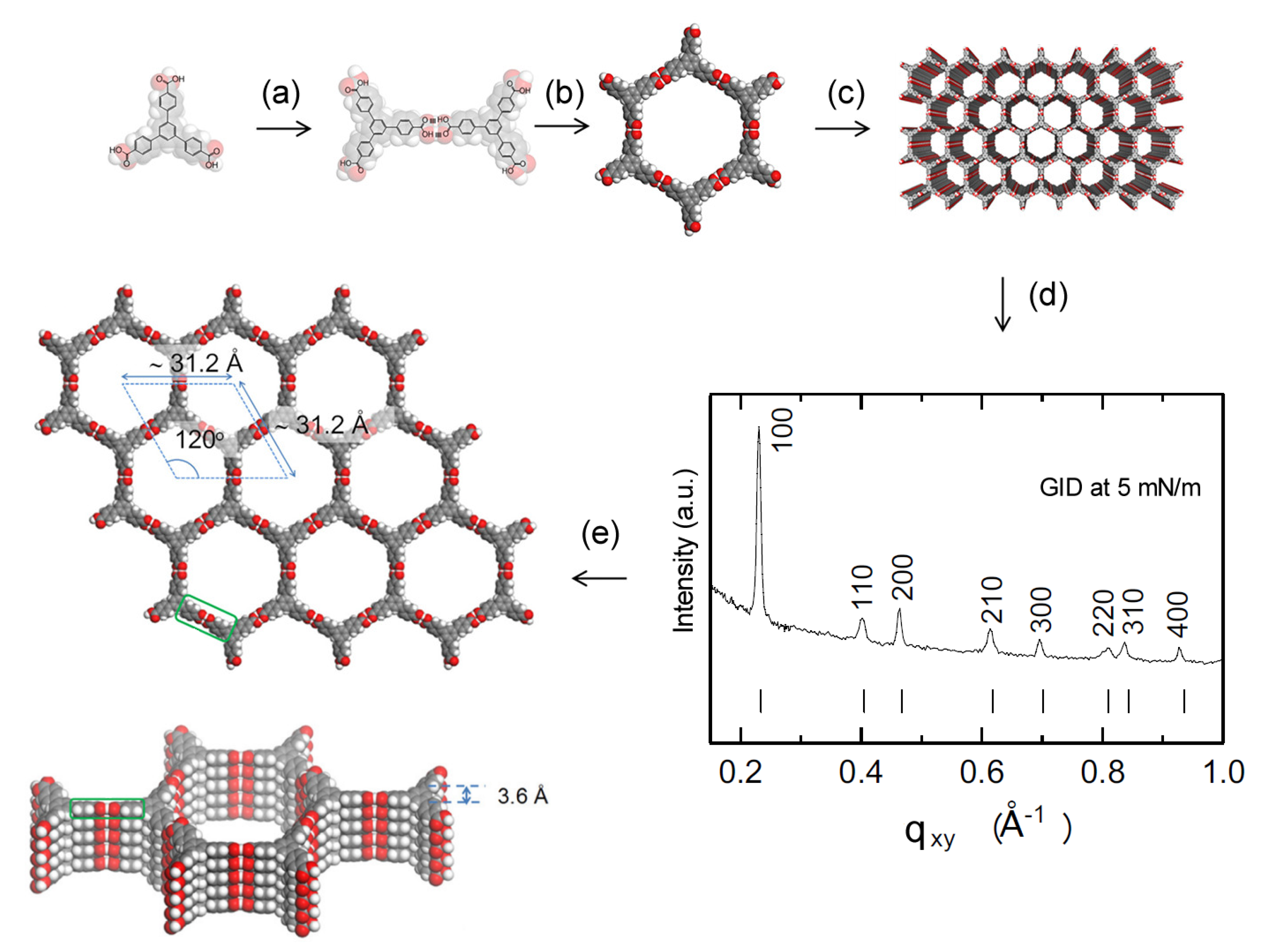
4.4. Polymer Films
4.5. Block Copolymer Nanostructures
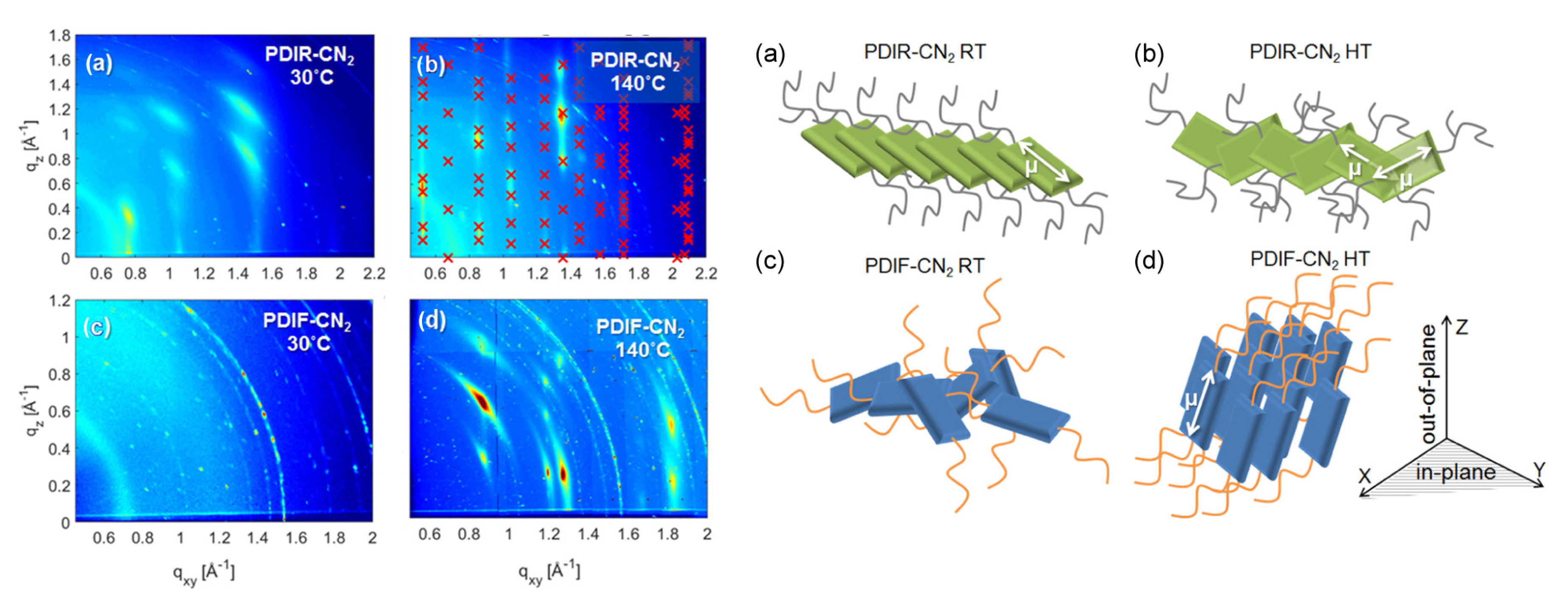
4.6. Polymer Photovoltaics
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 2DM | Two-dimensional materials |
| 3D | Three-dimensional |
| AFM | Atomic force microscope |
| CDI | Coherent diffractive imaging |
| DMF | N,N-dimethylformamide |
| FET | field effect transistors |
| GI | Grazing incidence |
| GID | Grazing incidence diffraction |
| GISAXS | Grazing incidence small-angle X-ray scattering |
| GIWAXS | Grazing incidence wide-angle X-ray scattering |
| GIXF | Grazing incidence X-ray fluorescence |
| GIXPCS | Grazing incidence X-ray photon correlation spectroscopy |
| LB | Langmuir-Blodgett |
| LED | light-emitting diodes |
| LS | Langmuir–Schaefer |
| OPV | Organic photovoltaics |
| OSC | Organic semiconductors |
| RTIL | room temperature ionic liquids |
| SAM | self-assembled monolayers |
| SANS | Small-angle neutron scattering |
| SAXD | Small-angle X-ray diffraction |
| SAXS | Small-angle X-ray scattering |
| SLD | scattering length density |
| TR-SAXS | Time-resolved small-angle X-ray scattering |
| USAXS | Ultra small-angle X-ray scattering |
| WAXS | Wide-angle X-ray scattering |
| XFEL | X-ray free-electron laser |
| XPCS | X-ray photon correlation spectroscopy |
| XRR | X-ray reflectivity |
References
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; World Scientific: Singapore, 2011. [Google Scholar]
- Hamley, I.W. Introduction to Soft Matter: Synthetic and Biological Self-Assembling Materials; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Narayanan, T.; Wacklin, H.; Konovalov, O.; Lund, R. Recent applications of synchrotron radiation and neutrons in the study of soft matter. Crystallogr. Rev. 2017, 23, 160–226. [Google Scholar] [CrossRef]
- Richman, E.K.; Hutchison, J.E. The nanomaterial characterization bottleneck. ACS Nano 2009, 3, 2441–2446. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, T. Small-Angle Scattering. In Structure from Diffraction Methods; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 259–324. [Google Scholar]
- Pershan, P.S.; Schlossman, M. Liquid Surfaces and Interfaces: Synchrotron X-ray Methods; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Ilavsky, J.; Zhang, F.; Andrews, R.N.; Kuzmenko, I.; Jemian, P.R.; Levine, L.E.; Allen, A.J. Development of combined microstructure and structure characterization facility for in situ and operando studies at the Advanced Photon Source. J. Appl. Crystallogr. 2018, 51, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Petukhov, A.V.; Meijer, J.M.; Vroege, G.J. Particle shape effects in colloidal crystals and colloidal liquid crystals: Small-angle X-ray scattering studies with microradian resolution. Curr. Opin. Colloid Interface Sci. 2015, 20, 272–281. [Google Scholar] [CrossRef]
- Sinha, S.K.; Jiang, Z.; Lurio, L.B. X-ray Photon Correlation Spectroscopy Studies of Surfaces and Thin Films. Adv. Mater. 2014, 26, 7764–7785. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ishikawa, T.; Robinson, I.K.; Murnane, M.M. Beyond crystallography: Diffractive imaging using coherent X-ray light sources. Science 2015, 348, 530–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibaud, A.; Vignaud, G. Specular reflectivity from smooth and rough surfaces. In X-ray and Neutron Reflectivity; Springer: Berlin, Germany, 2009; pp. 85–131. [Google Scholar]
- Schneck, E.; Schubert, T.; Konovalov, O.V.; Quinn, B.E.; Gutsmann, T.; Brandenburg, K.; Oliveira, R.G.; Pink, D.A.; Tanaka, M. Quantitative determination of ion distributions in bacterial lipopolysaccharide membranes by grazing-incidence X-ray fluorescence. Proc. Natl. Acad. Sci. USA 2010, 107, 9147–9151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, T.; Sztucki, M.; Van Vaerenbergh, P.; Léonardon, J.; Gorini, J.; Claustre, L.; Sever, F.; Morse, J.; Boesecke, P. A multipurpose instrument for time-resolved ultra-small-angle and coherent X-ray scattering. J. Appl. Crystallogr. 2018, 51, 1511–1524. [Google Scholar] [CrossRef]
- Berlinghof, M.; Bär, C.; Haas, D.; Bertram, F.; Langner, S.; Osvet, A.; Chumakov, A.; Will, J.; Schindler, T.; Zech, T.; et al. Flexible sample cell for real-time GISAXS, GIWAXS and XRR: Design and construction. J. Synchrotron Radiat. 2018, 25, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, S. Recent developments in polymer applications of synchrotron small-angle X-ray scattering. Polym. Int. 2017, 66, 237–249. [Google Scholar] [CrossRef]
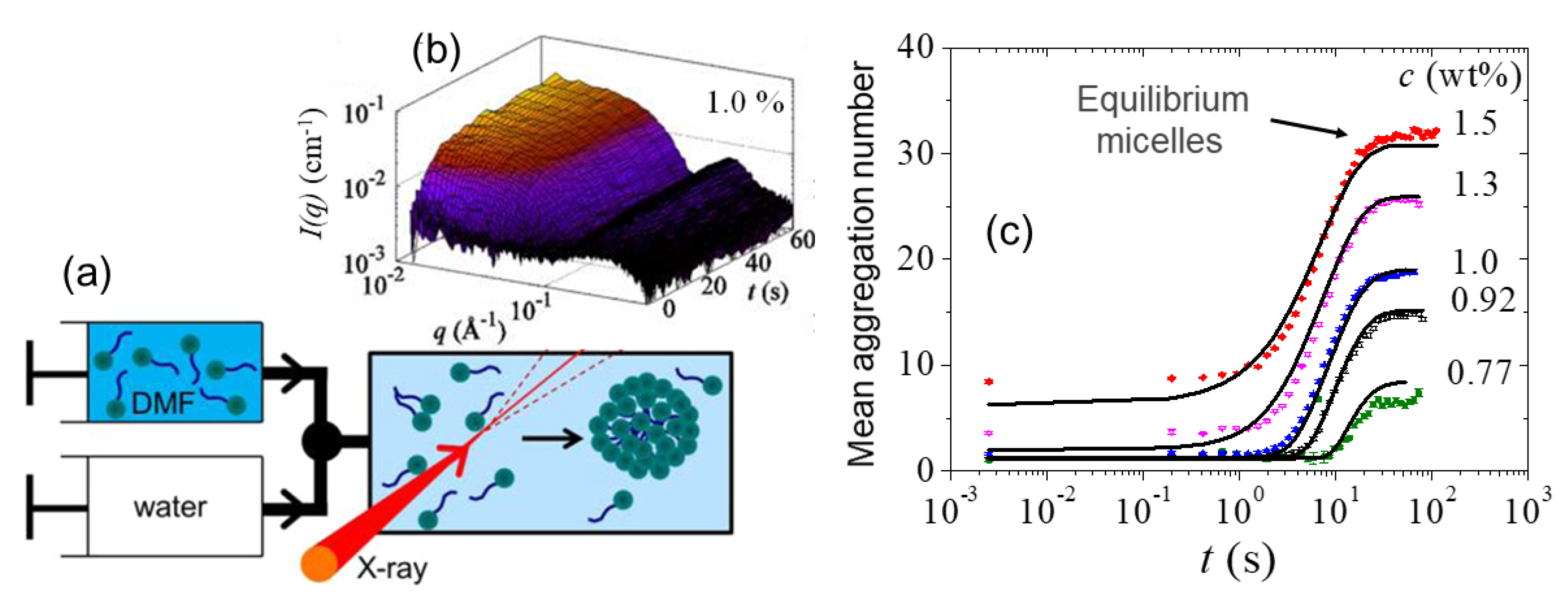
- Jensen, G.V.; Lund, R.; Gummel, J.; Monkenbusch, M.; Narayanan, T.; Pedersen, J.S. Direct observation of the formation of surfactant micelles under nonisothermal conditions by synchrotron SAXS. J. Am. Chem. Soc. 2013, 135, 7214–7222. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, T.; Gummel, J.; Gradzielski, M. Probing the self-assembly of unilamellar vesicles using time-resolved SAXS. In Advances in Planar Lipid Bilayers and Liposomes; Elsevier: Amsterdam, The Netherlands, 2014; Volume 20, pp. 171–196. [Google Scholar]
- Pabst, G.; Kučerka, N.; Nieh, M.P.; Rheinstädter, M.; Katsaras, J. Applications of neutron and X-ray scattering to the study of biologically relevant model membranes. Chem. Phys. Lipids 2010, 163, 460–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Svergun, D.I.; Koch, M.H.; Timmins, P.A.; May, R.P. Small Angle X-ray and Neutron Scattering from Solutions of Biological Macromolecules; Oxford University Press: Oxford, UK, 2013; Volume 19. [Google Scholar]
- Ingham, B. X-ray scattering characterisation of nanoparticles. Crystallogr. Rev. 2015, 21, 229–303. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small angle X-ray scattering for nanoparticle research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef]
- Bauer, P.; Amenitsch, H.; Baumgartner, B.; Köberl, G.; Rentenberger, C.; Winkler, P. In situ aerosol nanoparticle characterization by small angle X-ray scattering at ultra-low volume fraction. Nat. Commun. 2019, 10, 1122. [Google Scholar] [CrossRef]
- Meir, Y.; Jerby, E.; Barkay, Z.; Ashkenazi, D.; Mitchell, J.; Narayanan, T.; Eliaz, N.; LeGarrec, J.L.; Sztucki, M.; Meshcheryakov, O. Observations of ball-lightning-like plasmoids ejected from silicon by localized microwaves. Materials 2013, 6, 4011–4030. [Google Scholar] [CrossRef]
- Ulama, J.; Zackrisson Oskolkova, M.; Bergenholtz, J. Polymer-graft-mediated interactions between colloidal spheres. Langmuir 2016, 32, 2882–2890. [Google Scholar] [CrossRef]
- Jonsson, G.K.; Ulama, J.; Persson, R.A.; Oskolkova, M.Z.; Sztucki, M.; Narayanan, T.; Bergenholtz, J. Stabilizing colloidal particles against salting-out by shortening surface grafts. Langmuir 2019, 35, 11836–11842. [Google Scholar] [CrossRef] [Green Version]
- Angelini, R.; Zaccarelli, E.; de Melo Marques, F.A.; Sztucki, M.; Fluerasu, A.; Ruocco, G.; Ruzicka, B. Glass–glass transition during aging of a colloidal clay. Nat. Commun. 2014, 5, 4049. [Google Scholar] [CrossRef] [Green Version]
- Paineau, E.; Krapf, M.E.M.; Amara, M.S.; Matskova, N.V.; Dozov, I.; Rouziere, S.; Thill, A.; Launois, P.; Davidson, P. A liquid-crystalline hexagonal columnar phase in highly-dilute suspensions of imogolite nanotubes. Nat. Commun. 2016, 7, 10271. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Bahadur, D.; Dufresne, E.M.; Grybos, P.; Kmon, P.; Leheny, R.L.; Maj, P.; Narayanan, S.; Szczygiel, R.; Ramakrishnan, S.; et al. Dynamic scaling of colloidal gel formation at intermediate concentrations. Phys. Rev. Lett. 2017, 119, 178006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Allen, A.J.; Levine, L.E.; Tsai, D.H.; Ilavsky, J. Structure and dynamics of bimodal colloidal dispersions in a low-molecular-weight polymer solution. Langmuir 2017, 33, 2817–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, J.; Grobelny, S.; Schulze, J.; Bieder, S.; Steffen, A.; Erlkamp, M.; Paulus, M.; Tolan, M.; Winter, R. Reentrant liquid–liquid phase separation in protein solutions at elevated hydrostatic pressures. Phys. Rev. Lett. 2014, 112, 028101. [Google Scholar] [CrossRef] [PubMed]
- Matsarskaia, O.; Roosen-Runge, F.; Lotze, G.; Möller, J.; Mariani, A.; Zhang, F.; Schreiber, F. Tuning phase transitions of aqueous protein solutions by multivalent cations. Phys. Chem. Chem. Phys. 2018, 20, 27214–27225. [Google Scholar] [CrossRef] [Green Version]
- Henzler, K.; Haupt, B.; Rosenfeldt, S.; Harnau, L.; Narayanan, T.; Ballauff, M. Interaction strength between proteins and polyelectrolyte brushes: A small angle X-ray scattering study. Phys. Chem. Chem. Phys. 2011, 13, 17599–17605. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Henzler, K.; Lu, Y.; Wang, J.; Han, H.; Tian, Y.; Wang, Y.; Zhou, Z.; Lotze, G.; et al. Protein immobilization onto cationic spherical polyelectrolyte brushes studied by small angle X-ray scattering. Biomacromolecules 2017, 18, 1574–1581. [Google Scholar] [CrossRef]
- Steinschulte, A.A.; Gelissen, A.P.; Jung, A.; Brugnoni, M.; Caumanns, T.; Lotze, G.; Mayer, J.; Pergushov, D.V.; Plamper, F.A. Facile screening of various micellar morphologies by blending miktoarm stars and diblock copolymers. ACS Macro Lett. 2017, 6, 711–715. [Google Scholar] [CrossRef]
- Russina, O.; Lo Celso, F.; Plechkova, N.V.; Triolo, A. Emerging evidences of mesoscopic-scale complexity in neat ionic liquids and their mixtures. J. Phys. Chem. Lett. 2017, 8, 1197–1204. [Google Scholar] [CrossRef]
- Patil, N.; Narayanan, T.; Michels, L.; Skjønsfjell, E.T.B.; Guizar-Sicairos, M.; Van den Brande, N.; Claessens, R.; Van Mele, B.; Breiby, D.W. Probing Organic Thin Films by Coherent X-ray Imaging and X-ray Scattering. ACS Appl. Polym. Mater. 2019, 1, 1787–1797. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Tournilhac, F.; Leibler, L. Phase Separation and Self-Assembly in Vitrimers: Hierarchical Morphology of Molten and Semicrystalline Polyethylene/Dioxaborolane Maleimide Systems. Macromolecules 2018, 52, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Mizuta, R.; Devos, J.; Webster, J.; Ling, W.L.; Narayanan, T.; Round, A.; Munnur, D.; Mossou, E.; Farahat, A.; Boykin, D.; et al. Dynamic self-assembly of DNA minor groove-binding ligand DB921 into nanotubes triggered by an alkali halide. Nanoscale 2018, 10, 5550–5558. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, R.; Kawauchi, H.; Kameta, N.; Lee, J.H.; Fujii, S.; Shimizu, T.; Sakurai, K. Observing the Kinetic Pathway of Nanotube Formation from Bolaamphiphiles by Time-Resolved Small-Angle X-ray Scattering. J. Phys. Chem. B 2019, 123, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Ouhajji, S.; Landman, J.; Prévost, S.; Jiang, L.; Philipse, A.P.; Petukhov, A.V. In situ observation of self-assembly of sugars and surfactants from nanometres to microns. Soft Matter 2017, 13, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Dhasaiyan, P.; Prévost, S.; Baccile, N.; Prasad, B.L. pH-and Time-Resolved in Situ SAXS Study of Self-Assembled Twisted Ribbons Formed by Elaidic Acid Sophorolipids. Langmuir 2018, 34, 2121–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cola, E.; Grillo, I.; Ristori, S. Small angle X-ray and neutron scattering: Powerful tools for studying the structure of drug-loaded liposomes. Pharmaceutics 2016, 8, 10. [Google Scholar] [CrossRef]
- Narayanan, T.; Weerakkody, D.; Karabadzhak, A.G.; Anderson, M.; Andreev, O.A.; Reshetnyak, Y.K. pHLIP peptide interaction with a membrane monitored by SAXS. J. Phys. Chem. B 2016, 120, 11484–11491. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J.E.; Bjørnestad, V.A.; Lund, R. Resolving the structural interactions between antimicrobial peptides and lipid membranes using small-angle scattering methods: The case of indolicidin. Soft Matter 2018, 14, 8750–8763. [Google Scholar] [CrossRef]
- Sreij, R.; Dargel, C.; Hannappel, Y.; Jestin, J.; Prévost, S.; Dattani, R.; Wrede, O.; Hellweg, T. Temperature dependent self-organization of DMPC membranes promoted by intermediate amounts of the saponin aescin. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, E.F.; Devos, J.M.; Porcar, L.; Forsyth, V.T.; Narayanan, T. In vivo analysis of the Escherichia coli ultrastructure by small-angle scattering. IUCrJ 2017, 4, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Sreij, R.; Prévost, S.; Dargel, C.; Dattani, R.; Hertle, Y.; Wrede, O.; Hellweg, T. Interaction of the Saponin Aescin with Ibuprofen in DMPC Model Membranes. Mol. Pharm. 2018, 15, 4446–4461. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Garrastazu, G.; del Favero, E.; Rondelli, V.; Cantù, L.; Padula, C.; Santi, P.; Nicoli, S. Microemulsions based on TPGS and isostearic acid for imiquimod formulation and skin delivery. Eur. J. Pharm. Sci. 2018, 125, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Clemente, I.; Menicucci, F.; Colzi, I.; Sbraci, L.; Benelli, C.; Giordano, C.; Gonnelli, C.; Ristori, S.; Petruccelli, R. Unconventional and sustainable nanovectors for phytohormone delivery: Insights on Olea europaea. ACS Sustain. Chem. Eng. 2018, 6, 15022–15031. [Google Scholar] [CrossRef]
- Sanada, Y.; Akiba, I.; Sakurai, K.; Shiraishi, K.; Yokoyama, M.; Mylonas, E.; Ohta, N.; Yagi, N.; Shinohara, Y.; Amemiya, Y. Hydrophobic molecules infiltrating into the poly (ethylene glycol) domain of the core/shell interface of a polymeric micelle: Evidence obtained with anomalous small-angle X-ray scattering. J. Am. Chem. Soc. 2013, 135, 2574–2582. [Google Scholar] [CrossRef]
- Al-Ayoubi, S.R.; Schinkel, P.K.; Berghaus, M.; Herzog, M.; Winter, R. Combined effects of osmotic and hydrostatic pressure on multilamellar lipid membranes in the presence of PEG and trehalose. Soft Matter 2018, 14, 8792–8802. [Google Scholar] [CrossRef]
- Nack, A.; Seifert, J.; Passow, C.; Wagner, J. Hindered nematic alignment of hematite spindles in poly (N-isopropylacrylamide) hydrogels: A small-angle X-ray scattering and rheology study. J. Appl. Crystallogr. 2018, 51, 87–96. [Google Scholar] [CrossRef]
- Eliseev, A.A.; Eliseev, A.A.; Trusov, L.A.; Chumakov, A.P.; Boesecke, P.; Anokhin, E.O.; Vasiliev, A.V.; Sleptsova, A.E.; Gorbachev, E.A.; Korolev, V.V.; et al. Rotational dynamics of colloidal hexaferrite nanoplates. Appl. Phys. Lett. 2018, 113, 113106. [Google Scholar] [CrossRef]
- Zákutná, D.; Falke, Y.; Dresen, D.; Prévost, S.; Bender, P.; Honecker, D.; Disch, S. Morphological and crystallographic orientation of hematite spindles in an applied magnetic field. Nanoscale 2019, 11, 7149–7156. [Google Scholar] [CrossRef]
- Saranathan, V.; Forster, J.D.; Noh, H.; Liew, S.F.; Mochrie, S.G.; Cao, H.; Dufresne, E.R.; Prum, R.O. Structure and optical function of amorphous photonic nanostructures from avian feather barbs: A comparative small angle X-ray scattering (SAXS) analysis of 230 bird species. J. R. Soc. Interface 2012, 9, 2563–2580. [Google Scholar] [CrossRef] [Green Version]
- Burg, S.L.; Parnell, A.J. Self-assembling structural colour in nature. J. Phys. Condens. Matter 2018, 30, 413001. [Google Scholar] [CrossRef]
- Vatankhah-Varnosfaderani, M.; Keith, A.N.; Cong, Y.; Liang, H.; Rosenthal, M.; Sztucki, M.; Clair, C.; Magonov, S.; Ivanov, D.A.; Dobrynin, A.V.; et al. Chameleon-like elastomers with molecularly encoded strain-adaptive stiffening and coloration. Science 2018, 359, 1509–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brotherton, E.E.; Hatton, F.L.; Cockram, A.A.; Derry, M.J.; Czajka, A.; Cornel, E.J.; Topham, P.D.; Mykhaylyk, O.O.; Armes, S.P. In situ small-angle X-ray scattering studies during reversible addition–fragmentation chain transfer aqueous emulsion polymerization. J. Am. Chem. Soc. 2019, 141, 13664–13675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mable, C.J.; Derry, M.J.; Thompson, K.L.; Fielding, L.A.; Mykhaylyk, O.O.; Armes, S.P. Time-resolved saxs studies of the kinetics of thermally triggered release of encapsulated silica nanoparticles from block copolymer vesicles. Macromolecules 2017, 50, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Kriechbaum, M.; Liu, L.E. In situ capabilities of Small Angle X-ray Scattering. Nanotechnol. Rev. 2019, 8, 352–369. [Google Scholar] [CrossRef]
- Gummel, J.; Sztucki, M.; Narayanan, T.; Gradzielski, M. Concentration dependent pathways in spontaneous self-assembly of unilamellar vesicles. Soft Matter 2011, 7, 5731–5738. [Google Scholar] [CrossRef] [Green Version]
- Bressel, K.; Muthig, M.; Prévost, S.; Gummel, J.; Narayanan, T.; Gradzielski, M. Shaping vesicles–controlling size and stability by admixture of amphiphilic copolymer. ACS Nano 2012, 6, 5858–5865. [Google Scholar] [CrossRef]
- Jensen, G.V.; Lund, R.; Gummel, J.; Narayanan, T.; Pedersen, J.S. Monitoring the transition from spherical to polymer-like surfactant micelles using small-angle X-ray scattering. Angew. Chem. Int. Ed. 2014, 53, 11524–11528. [Google Scholar] [CrossRef]
- Jensen, G.V.; Lund, R.; Narayanan, T.; Pedersen, J.S. Transformation from Globular to Cylindrical Mixed Micelles through Molecular Exchange that Induces Micelle Fusion. J. Phys. Chem. Lett. 2016, 7, 2039–2043. [Google Scholar] [CrossRef]
- Takahashi, R.; Matsumoto, S.; Fujii, S.; Narayanan, T.; Sakurai, K. Monitoring the Discontinuous Dodecamer–Icosamer Transition of a Calix [4] arene-Derived Surfactant by Time-Resolved Small-Angle X-ray Scattering. Angew. Chem. Int. Ed. 2017, 56, 6734–6738. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Narayanan, T.; Drechsler, M.; Stepanek, P.; Couvreur, P.; Lesieur, S. DNA/fusogenic lipid nanocarrier assembly: Millisecond structural dynamics. J. Phys. Chem. Lett. 2013, 4, 1959–1964. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Stepanek, P.; Lesieur, S. Multicompartment lipid cubic nanoparticles with high protein upload: Millisecond dynamics of formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef]
- Varga, Z.; Wacha, A.; Bóta, A. Osmotic shrinkage of sterically stabilized liposomes as revealed by time-resolved small-angle X-ray scattering. J. Appl. Crystallogr. 2014, 47, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Yaghmur, A.; Rappolt, M. Structural characterization of lipidic systems under nonequilibrium conditions. Eur. Biophys. J. 2012, 41, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.Y.D.; Brooks, N.J.; Ces, O.; Seddon, J.M.; Templer, R.H. Structural studies of the lamellar to bicontinuous gyroid cubic () phase transitions under limited hydration conditions. Soft Matter 2015, 11, 1991–1997. [Google Scholar] [CrossRef] [Green Version]
- Barriga, H.; Tyler, A.; McCarthy, N.; Parsons, E.; Ces, O.; Law, R.; Seddon, J.; Brooks, N. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter 2015, 11, 600–607. [Google Scholar] [CrossRef] [Green Version]
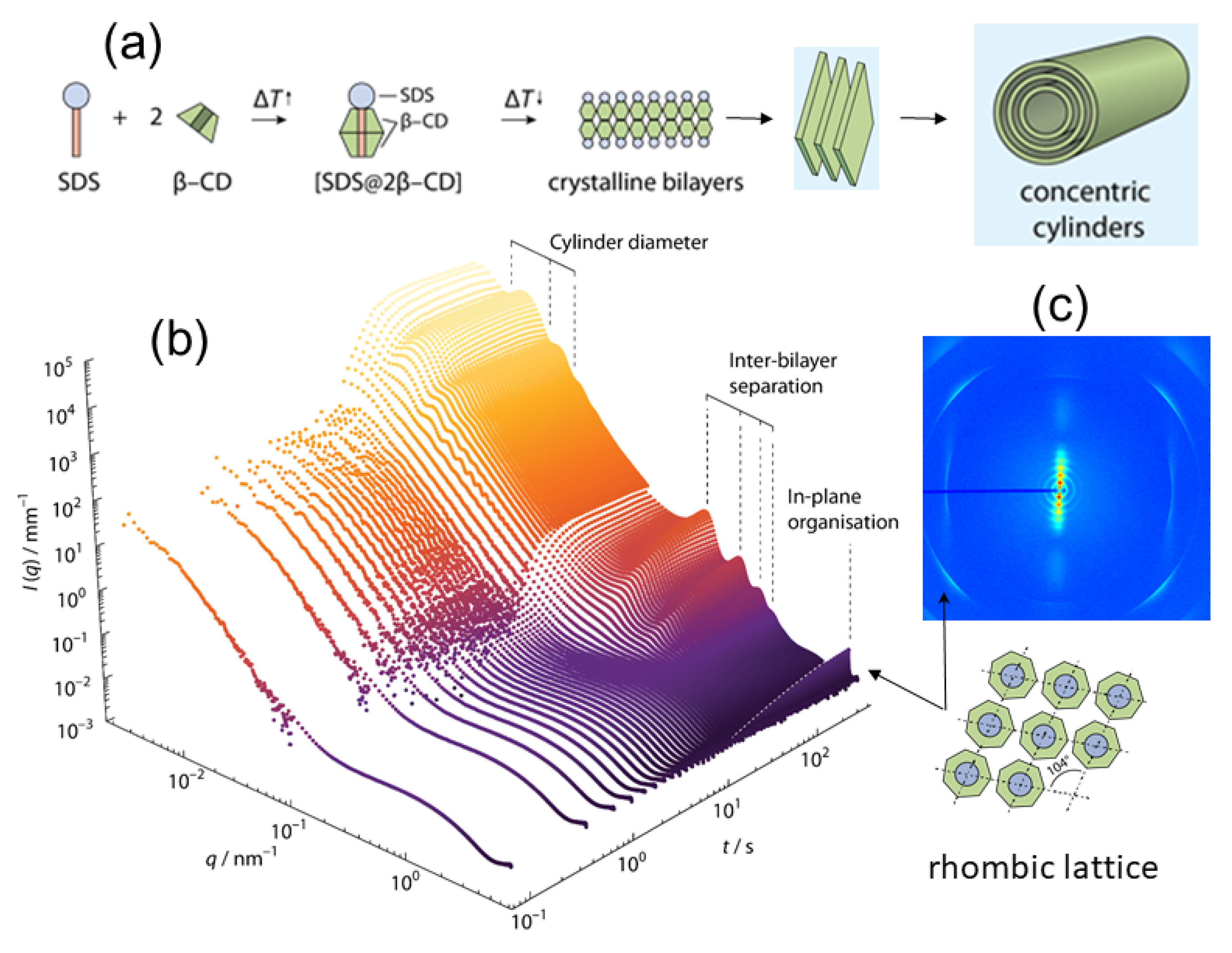
- Jiang, L.; Peng, Y.; Yan, Y.; Deng, M.; Wang, Y.; Huang, J. Annular Ring microtubes formed by SDS@2β-CD complexes in aqueous solution. Soft Matter 2010, 6, 1731–1736. [Google Scholar] [CrossRef]
- Landman, J.; Ouhajji, S.; Prévost, S.; Narayanan, T.; Groenewold, J.; Philipse, A.P.; Kegel, W.K.; Petukhov, A.V. Inward growth by nucleation: Multiscale self-assembly of ordered membranes. Sci. Adv. 2018, 4, eaat1817. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, R.; Narayanan, T.; Sato, T. Growth Kinetics of Polyelectrolyte Complexes Formed from Oppositely-Charged Homopolymers Studied by Time-Resolved Ultra-Small-Angle X-ray Scattering. J. Phys. Chem. Lett. 2017, 8, 737–741. [Google Scholar] [CrossRef]
- Dähling, C.; Lotze, G.; Mori, H.; Pergushov, D.V.; Plamper, F.A. Thermoresponsive segments retard the formation of equilibrium micellar interpolyelectrolyte complexes by detouring to various intermediate structures. J. Phys. Chem. B 2017, 121, 6739–6748. [Google Scholar] [CrossRef]
- Takahashi, R.; Narayanan, T.; Yusa, S.i.; Sato, T. Kinetics of Morphological Transition between Cylindrical and Spherical Micelles in a Mixture of Anionic–Neutral and Cationic–Neutral Block Copolymers Studied by Time-Resolved SAXS and USAXS. Macromolecules 2018, 51, 3654–3662. [Google Scholar] [CrossRef]
- Amann, M.; Diget, J.S.; Lyngsø, J.; Pedersen, J.S.; Narayanan, T.; Lund, R. Kinetic Pathways for Polyelectrolyte Coacervate Micelle Formation Revealed by Time-Resolved Synchrotron SAXS. Macromolecules 2019, 52, 8227–8237. [Google Scholar] [CrossRef]
- Piazzesi, G.; Caremani, M.; Linari, M.; Reconditi, M.; Lombardi, V. Thick filament mechano-sensing in skeletal and cardiac muscles: A common mechanism able to adapt the energetic cost of the contraction to the task. Front. Physiol. 2018, 9, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
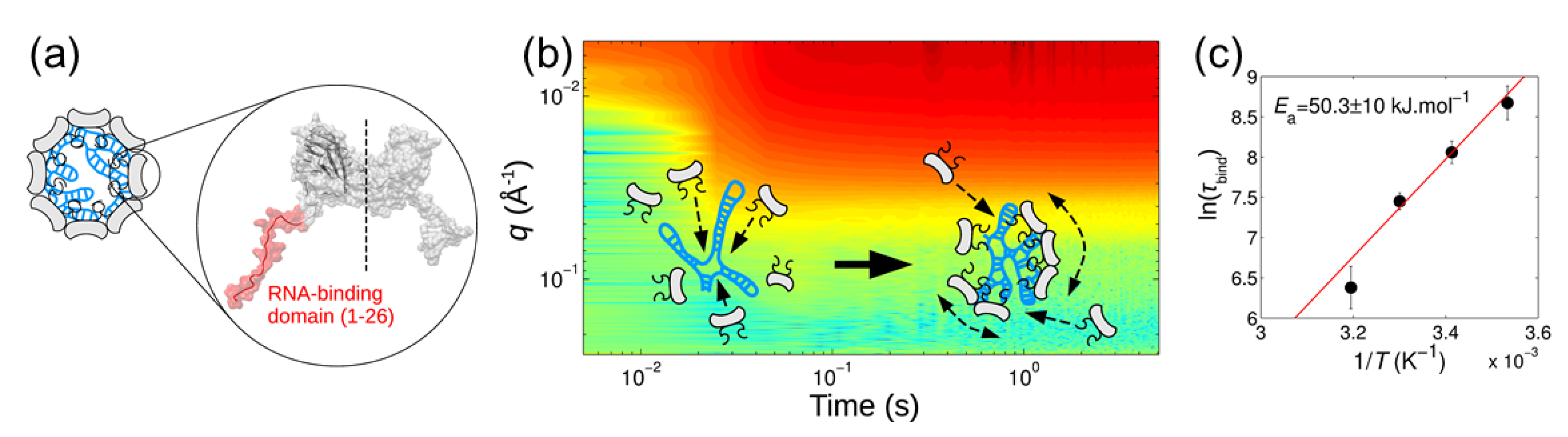
- Kler, S.; Asor, R.; Li, C.; Ginsburg, A.; Harries, D.; Oppenheim, A.; Zlotnick, A.; Raviv, U. RNA encapsidation by SV40-derived nanoparticles follows a rapid two-state mechanism. J. Am. Chem. Soc. 2012, 134, 8823–8830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresset, G.; Le Coeur, C.; Bryche, J.F.; Tatou, M.; Zeghal, M.; Charpilienne, A.; Poncet, D.; Constantin, D.; Bressanelli, S. Norovirus capsid proteins self-assemble through biphasic kinetics via long-lived stave-like intermediates. J. Am. Chem. Soc. 2013, 135, 15373–15381. [Google Scholar] [CrossRef]
- Shemesh, A.; Ginsburg, A.; Levi-Kalisman, Y.; Ringel, I.; Raviv, U. Structure, assembly, and disassembly of tubulin single rings. Biochemistry 2018, 57, 6153–6165. [Google Scholar] [CrossRef]
- Law-Hine, D.; Sahoo, A.K.; Bailleux, V.; Zeghal, M.; Prévost, S.; Maiti, P.K.; Bressanelli, S.; Constantin, D.; Tresset, G. Reconstruction of the disassembly pathway of an icosahedral viral capsid and shape determination of two successive intermediates. J. Phys. Chem. Lett. 2015, 6, 3471–3476. [Google Scholar] [CrossRef]
- Chevreuil, M.; Law-Hine, D.; Chen, J.; Bressanelli, S.; Combet, S.; Constantin, D.; Degrouard, J.; Möller, J.; Zeghal, M.; Tresset, G. Nonequilibrium self-assembly dynamics of icosahedral viral capsids packaging genome or polyelectrolyte. Nat. Commun. 2018, 9, 3071. [Google Scholar] [CrossRef] [Green Version]
- Asor, R.; Ben-nun Shaul, O.; Oppenheim, A.; Raviv, U. Crystallization, reentrant melting, and resolubilization of virus nanoparticles. ACS Nano 2017, 11, 9814–9824. [Google Scholar] [CrossRef]
- Asor, R.; Khaykelson, D.; Ben-nun Shaul, O.; Oppenheim, A.; Raviv, U. Effect of Calcium Ions and Disulfide Bonds on Swelling of Virus Particles. ACS Omega 2019, 4, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Bruetzel, L.K.; Walker, P.U.; Gerling, T.; Dietz, H.; Lipfert, J. Time-resolved small-angle X-ray scattering reveals millisecond transitions of a DNA origami switch. Nano Lett. 2018, 18, 2672–2676. [Google Scholar] [CrossRef] [Green Version]
- Da Vela, S.; Braun, M.K.; Dörr, A.; Greco, A.; Möller, J.; Fu, Z.; Zhang, F.; Schreiber, F. Kinetics of liquid–liquid phase separation in protein solutions exhibiting LCST phase behavior studied by time-resolved USAXS and VSANS. Soft Matter 2016, 12, 9334–9341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Vela, S.; Exner, C.; Schäufele, R.S.; Möller, J.; Fu, Z.; Zhang, F.; Schreiber, F. Arrested and temporarily arrested states in a protein–polymer mixture studied by USAXS and VSANS. Soft Matter 2017, 13, 8756–8765. [Google Scholar] [CrossRef] [PubMed]
- Matsarskaia, O.; Da Vela, S.; Mariani, A.; Fu, Z.; Zhang, F.; Schreiber, F. Phase Separation Kinetics in Protein-Salt Mixtures with Compositionally Tuned Interactions. J. Phys. Chem. B 2019, 123, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Abécassis, B.; Bouet, C.; Garnero, C.; Constantin, D.; Lequeux, N.; Ithurria, S.; Dubertret, B.; Pauw, B.R.; Pontoni, D. Real-time in situ probing of high-temperature quantum dots solution synthesis. Nano Lett. 2015, 15, 2620–2626. [Google Scholar] [CrossRef]
- Saha, S.; Springer, S.; Schweinefuß, M.E.; Pontoni, D.; Wiebcke, M.; Huber, K. Insight into fast nucleation and growth of zeolitic imidazolate framework-71 by in situ time-resolved light and X-ray scattering experiments. Cryst. Growth Des. 2016, 16, 2002–2010. [Google Scholar] [CrossRef]
- Schiener, A.; Schmidt, E.; Bergmann, C.; Seifert, S.; Zahn, D.; Krach, A.; Weihrich, R.; Magerl, A. The formation of CdS quantum dots and Au nanoparticles. Z. Krist.-Cryst. Mater. 2017, 232, 39–46. [Google Scholar] [CrossRef]
- Lund, R.; Brun, G.; Chevallier, E.; Narayanan, T.; Tribet, C. Kinetics of photocontrollable micelles: Light-induced self-assembly and disassembly of azobenzene-based surfactants revealed by TR-SAXS. Langmuir 2016, 32, 2539–2548. [Google Scholar] [CrossRef]
- Van Der Stam, W.; Rabouw, F.T.; Geuchies, J.J.; Berends, A.C.; Hinterding, S.O.; Geitenbeek, R.G.; Van Der Lit, J.; Prévost, S.; Petukhov, A.V.; de Mello Donega, C. In Situ Probing of Stack-Templated Growth of Ultrathin Cu2-XS Nanosheets. Chem. Mater. 2016, 28, 6381–6389. [Google Scholar] [CrossRef] [Green Version]
- Montanarella, F.; Geuchies, J.J.; Dasgupta, T.; Prins, P.T.; Van Overbeek, C.; Dattani, R.; Baesjou, P.; Dijkstra, M.; Petukhov, A.V.; Van Blaaderen, A.; et al. Crystallization of nanocrystals in spherical confinement probed by in Situ X-ray scattering. Nano Lett. 2018, 18, 3675–3681. [Google Scholar] [CrossRef]
- Lokteva, I.; Koof, M.; Walther, M.; Grübel, G.; Lehmkühler, F. Monitoring Nanocrystal Self-Assembly in Real Time Using In Situ Small-Angle X-ray Scattering. Small 2019, 15, 1900438. [Google Scholar] [CrossRef]
- Lokteva, I.; Koof, M.; Walther, M.; Grübel, G.; Lehmkühler, F. Coexistence of hcp and bct Phases during In Situ Superlattice Assembly from Faceted Colloidal Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 6331–6338. [Google Scholar] [CrossRef]
- Cabane, B.; Li, J.; Artzner, F.; Botet, R.; Labbez, C.; Bareigts, G.; Sztucki, M.; Goehring, L. Hiding in plain view: Colloidal self-assembly from polydisperse populations. Phys. Rev. Lett. 2016, 116, 208001. [Google Scholar] [CrossRef]
- Zhang, F. Nonclassical nucleation pathways in protein crystallization. J. Phys. Condens. Matter 2017, 29, 443002. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Roosen-Runge, F.; Zhang, F.; Lotze, G.; Jacobs, R.M.; Schreiber, F. Real-time observation of nonclassical protein crystallization kinetics. J. Am. Chem. Soc. 2015, 137, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Keidel, R.; Ghavami, A.; Lugo, D.M.; Lotze, G.; Virtanen, O.; Beumers, P.; Pedersen, J.S.; Bardow, A.; Winkler, R.G.; Richtering, W. Time-resolved structural evolution during the collapse of responsive hydrogels: The microgel-to-particle transition. Sci. Adv. 2018, 4, eaao7086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
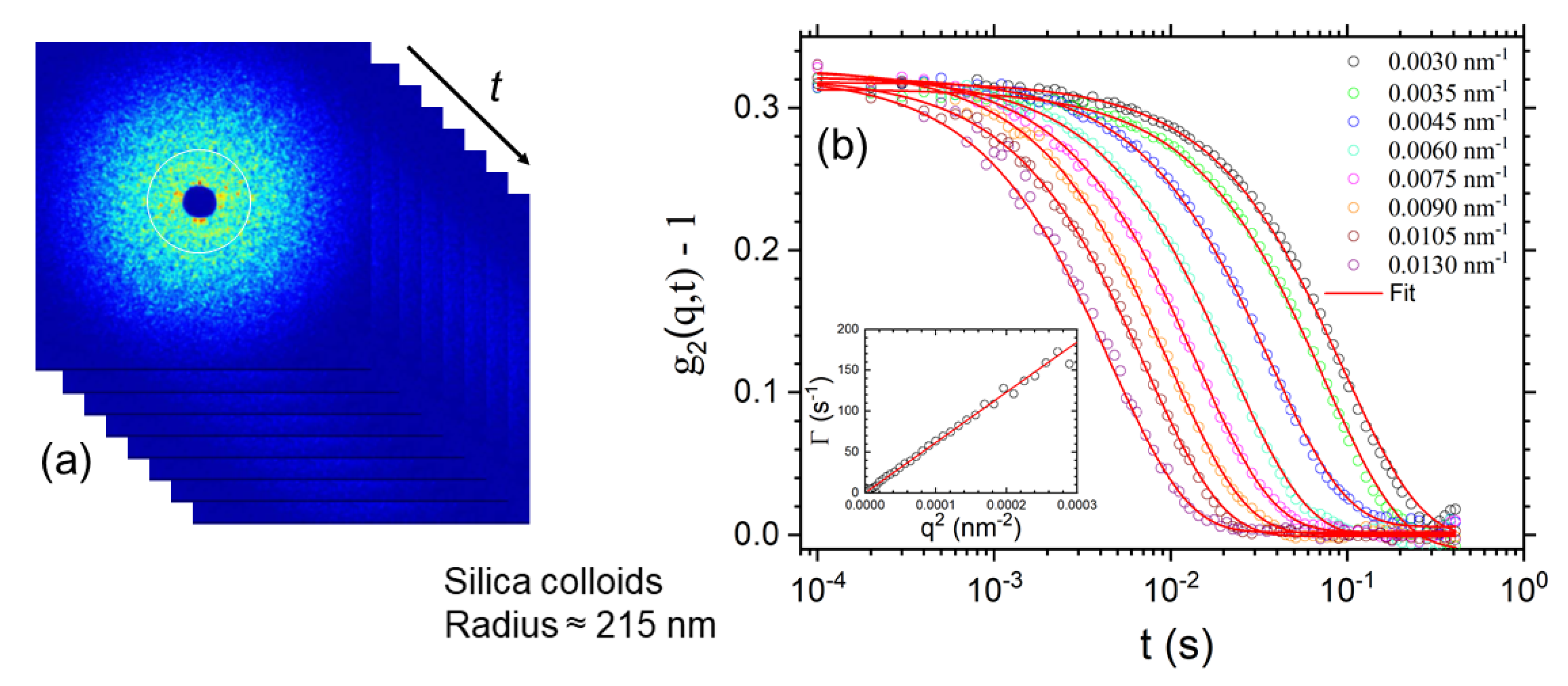
- Leheny, R.L.; Rogers, M.C.; Chen, K.; Narayanan, S.; Harden, J.L. Rheo-XPCS. Curr. Opin. Colloid Interface Sci. 2015, 20, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Bikondoa, O. X-ray Photon Correlation Spectroscopy for the Characterization of Soft and Hard Condensed Matter. In X-ray and Neutron Techniques for Nanomaterials Characterization; Springer: Berlin, Germany, 2016; pp. 95–156. [Google Scholar]
- Madsen, A.; Fluerasu, A.; Ruta, B. Structural Dynamics of Materials Probed by X-ray Photon Correlation Spectroscopy; Springer: Berlin, Germany, 2016; pp. 1617–1641. [Google Scholar]
- Wagner, J.; Märkert, C.; Fischer, B.; Müller, L. Direction dependent diffusion of aligned magnetic rods by means of X-ray photon correlation spectroscopy. Phys. Rev. Lett. 2013, 110, 048301. [Google Scholar] [CrossRef] [Green Version]
- Lüttich, M.; Giordano, V.M.; Le Floch, S.; Pineda, E.; Zontone, F.; Luo, Y.; Samwer, K.; Ruta, B. Anti-Aging in ultrastable metallic glasses. Phys. Rev. Lett. 2018, 120, 135504. [Google Scholar] [CrossRef] [Green Version]
- Ruta, B.; Zontone, F.; Chushkin, Y.; Baldi, G.; Pintori, G.; Monaco, G.; Ruffle, B.; Kob, W. Hard X-rays as pump and probe of atomic motion in oxide glasses. Sci. Rep. 2017, 7, 3962. [Google Scholar] [CrossRef]
- Pal, A.; Zinn, T.; Kamal, M.A.; Narayanan, T.; Schurtenberger, P. Anomalous Dynamics of Magnetic Anisotropic Colloids Studied by XPCS. Small 2018, 14, 1802233. [Google Scholar] [CrossRef]
- Möller, J.; Narayanan, T. Velocity fluctuations in sedimenting brownian particles. Phys. Rev. Lett. 2017, 118, 198001. [Google Scholar] [CrossRef] [PubMed]
- Nygård, K.; Buitenhuis, J.; Kagias, M.; Jefimovs, K.; Zontone, F.; Chushkin, Y. Anisotropic de Gennes narrowing in confined fluids. Phys. Rev. Lett. 2016, 116, 167801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dattani, R.; Semeraro, E.F.; Narayanan, T. Phoretic motion of colloids in a phase separating medium. Soft Matter 2017, 13, 2817–2822. [Google Scholar] [CrossRef]
- Semeraro, E.F.; Dattani, R.; Narayanan, T. Microstructure and dynamics of janus particles in a phase separating medium. J. Chem. Phys. 2018, 148, 014904. [Google Scholar] [CrossRef]
- Zinn, T.; Homs, A.; Sharpnack, L.; Tinti, G.; Fröjdh, E.; Douissard, P.A.; Kocsis, M.; Möller, J.; Chushkin, Y.; Narayanan, T. Ultra-small-angle X-ray photon correlation spectroscopy using the Eiger detector. J. Synchrotron Radiat. 2018, 25, 1753–1759. [Google Scholar] [CrossRef]
- Orsi, D.; Guzmán, E.; Liggieri, L.; Ravera, F.; Ruta, B.; Chushkin, Y.; Rimoldi, T.; Cristofolini, L. 2D dynamical arrest transition in a mixed nanoparticle-phospholipid layer studied in real and momentum spaces. Sci. Rep. 2015, 5, 17930. [Google Scholar] [CrossRef] [Green Version]
- Orsi, D.; Cristofolini, L.; Baldi, G.; Madsen, A. Heterogeneous and anisotropic dynamics of a 2D gel. Phys. Rev. Lett. 2012, 108, 105701. [Google Scholar] [CrossRef] [Green Version]
- Perakis, F.; Camisasca, G.; Lane, T.J.; Späh, A.; Wikfeldt, K.T.; Sellberg, J.A.; Lehmkühler, F.; Pathak, H.; Kim, K.H.; Amann-Winkel, K.; et al. Coherent x-rays reveal the influence of cage effects on ultrafast water dynamics. Nat. Commun. 2018, 9, 1917. [Google Scholar] [CrossRef]
- Kato, T. Shear-Induced Lamellar/Onion Transition in Surfactant Systems. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 187–222. [Google Scholar]
- Lee, J.; Jiang, Z.; Wang, J.; Sandy, A.R.; Narayanan, S.; Lin, X.M. Unraveling the role of order-to-disorder transition in shear thickening suspensions. Phys. Rev. Lett. 2018, 120, 028002. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.C.; Chen, K.; Pagenkopp, M.J.; Mason, T.G.; Narayanan, S.; Harden, J.L.; Leheny, R.L. Microscopic signatures of yielding in concentrated nanoemulsions under large-amplitude oscillatory shear. Phys. Rev. Mater. 2018, 2, 095601. [Google Scholar] [CrossRef]
- Lim, S.H.; Jang, H.S.; Ha, J.M.; Kim, T.H.; Kwasniewski, P.; Narayanan, T.; Jin, K.S.; Choi, S.M. Highly Ordered and Highly Aligned Two-Dimensional Binary Superlattice of a SWNT/Cylindrical-Micellar System. Angew. Chem. Int. Ed. 2014, 126, 12756–12762. [Google Scholar] [CrossRef]
- Lim, S.H.; Lee, T.; Oh, Y.; Narayanan, T.; Sung, B.J.; Choi, S.M. Hierarchically self-assembled hexagonal honeycomb and kagome superlattices of binary 1D colloids. Nat. Commun. 2017, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Hengl, N.; Baup, S.; Karrouch, M.; Gicquel, E.; Dufresne, A.; Djeridi, H.; Dattani, R.; JIN, Y.; Pignon, F. Structure, Rheological Behavior and in-situ Local Flow Fields of Cellulose Nanocrystal Dispersions during Cross-flow Ultrafiltration. ACS Sustain. Chem. Eng. 2019, 7, 10679–10689. [Google Scholar] [CrossRef]
- Ghazal, A.; Lafleur, J.P.; Mortensen, K.; Kutter, J.P.; Arleth, L.; Jensen, G.V. Recent advances in X-ray compatible microfluidics for applications in soft materials and life sciences. Lab Chip 2016, 16, 4263–4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.F. SAXS on a chip: From dynamics of phase transitions to alignment phenomena at interfaces studied with microfluidic devices. Phys. Chem. Chem. Phys. 2017, 19, 23690–23703. [Google Scholar] [CrossRef] [PubMed]
- Beuvier, T.; Panduro, E.A.C.; Kwaśniewski, P.; Marre, S.; Lecoutre, C.; Garrabos, Y.; Aymonier, C.; Calvignac, B.; Gibaud, A. Implementation of in situ SAXS/WAXS characterization into silicon/glass microreactors. Lab Chip 2015, 15, 2002–2008. [Google Scholar] [CrossRef]
- Merkens, S.; Vakili, M.; Sánchez-Iglesias, A.; Litti, L.; Gao, Y.; Gwozdz, P.V.; Sharpnack, L.; Blick, R.H.; Liz-Marzán, L.M.; Grzelczak, M.; et al. Time-Resolved Analysis of the Structural Dynamics of Assembling Gold Nanoparticles. ACS Nano 2019, 13, 6596–6604. [Google Scholar] [CrossRef]
- Meijer, J.M.; Pal, A.; Ouhajji, S.; Lekkerkerker, H.N.; Philipse, A.P.; Petukhov, A.V. Observation of solid–solid transitions in 3D crystals of colloidal superballs. Nat. Commun. 2017, 8, 14352. [Google Scholar] [CrossRef]
- Davidson, P.; Penisson, C.; Constantin, D.; Gabriel, J.C.P. Isotropic, nematic, and lamellar phases in colloidal suspensions of nanosheets. Proc. Natl. Acad. Sci. USA 2018, 115, 6662–6667. [Google Scholar] [CrossRef] [Green Version]
- Saranathan, V.; Seago, A.E.; Sandy, A.; Narayanan, S.; Mochrie, S.G.; Dufresne, E.R.; Cao, H.; Osuji, C.O.; Prum, R.O. Structural diversity of arthropod biophotonic nanostructures spans amphiphilic phase-space. Nano Lett. 2015, 15, 3735–3742. [Google Scholar] [CrossRef]
- Parnell, A.J.; Washington, A.L.; Mykhaylyk, O.O.; Hill, C.J.; Bianco, A.; Burg, S.L.; Dennison, A.J.; Snape, M.; Cadby, A.J.; Smith, A.; et al. Spatially modulated structural colour in bird feathers. Sci. Rep. 2015, 5, 18317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bershitsky, S.Y.; Koubassova, N.A.; Ferenczi, M.A.; Kopylova, G.V.; Narayanan, T.; Tsaturyan, A.K. The Closed State of the Thin Filament Is Not Occupied in Fully Activated Skeletal Muscle. Biophys. J. 2017, 112, 1455–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linari, M.; Brunello, E.; Reconditi, M.; Fusi, L.; Caremani, M.; Narayanan, T.; Piazzesi, G.; Lombardi, V.; Irving, M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 2015, 528, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reconditi, M.; Caremani, M.; Pinzauti, F.; Powers, J.D.; Narayanan, T.; Stienen, G.J.; Linari, M.; Lombardi, V.; Piazzesi, G. Myosin filament activation in the heart is tuned to the mechanical task. Proc. Natl. Acad. Sci. USA 2017, 114, 3240–3245. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Prévost, S.F.; Blowes, L.M.; Egertová, M.; Terrill, N.J.; Wang, W.; Elphick, M.R.; Gupta, H.S. Interfibrillar stiffening of echinoderm mutable collagenous tissue demonstrated at the nanoscale. Proc. Natl. Acad. Sci. USA 2016, 113, E6362–E6371. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.A.; Xu, R.; Chen, C.C.; Huang, Z.; Jiang, H.; Chen, A.L.; Raines, K.S.; Pryor, A., Jr.; Nam, D.; Wiegart, L.; et al. Three-dimensional coherent X-ray diffractive imaging of whole frozen-hydrated cells. IUCrJ 2015, 2, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Frank, V.; Chushkin, Y.; Fröhlich, B.; Abuillan, W.; Rieger, H.; Becker, A.S.; Yamamoto, A.; Rossetti, F.F.; Kaufmann, S.; Lanzer, M.; et al. Lensless Tomographic Imaging of Near Surface Structures of Frozen Hydrated Malaria-Infected Human Erythrocytes by Coherent X-ray Diffraction Microscopy. Sci. Rep. 2017, 7, 14081. [Google Scholar] [CrossRef] [Green Version]
- Cherkas, O.; Beuvier, T.; Breiby, D.W.; Chushkin, Y.; Zontone, F.; Gibaud, A. Direct observation of microparticle porosity changes in solid-state vaterite to calcite transformation by coherent X-ray diffraction imaging. Cryst. Growth Des. 2017, 17, 4183–4188. [Google Scholar] [CrossRef]
- Chushkin, Y.; Zontone, F.; Cherkas, O.; Gibaud, A. Quantitative nanotomography of amorphous and polycrystalline samples using coherent X-ray diffraction. J. Appl. Crystallogr. 2019, 52, 571–578. [Google Scholar] [CrossRef]
- Sanzaro, S.; Zontone, F.; Grosso, D.; Bottein, T.; Neri, F.; Smecca, E.; Mannino, G.; Bongiorno, C.; Spinella, C.; La Magna, A.; et al. Bimodal Porosity and Stability of a TiO2 Gig-Lox Sponge Infiltrated with Methyl-Ammonium Lead Iodide Perovskite. Nanomaterials 2019, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Erokhina, S.; Pastorino, L.; Di Lisa, D.; Kiiamov, A.G.; Faizullina, A.; Tayurskii, D.A.; Iannotta, S.; Erokhin, V. Coherent X-ray diffraction imaging of nanoengineered polymeric capsules. JETP Lett. 2017, 106, 540–543. [Google Scholar] [CrossRef]
- Beuvier, T.; Probert, I.; Beaufort, L.; Suchéras-Marx, B.; Chushkin, Y.; Zontone, F.; Gibaud, A. X-ray nanotomography of coccolithophores reveals that coccolith mass and segment number correlate with grid size. Nat. Commun. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.H.; Zhao, L.; Gallagher-Jones, M.; Rana, A.; Lodico, J.J.; Xiao, W.; Regan, B.; Miao, J. In situ coherent diffractive imaging. Nat. Commun. 2018, 9, 1826. [Google Scholar] [CrossRef] [PubMed]
- Skoda, M.W. Recent developments in the application of X-ray and neutron reflectivity to soft matter systems. Curr. Opin. Colloid Interface Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Calzolari, D.C.; Pontoni, D.; Deutsch, M.; Reichert, H.; Daillant, J. Nanoscale structure of surfactant-induced nanoparticle monolayers at the oil–water interface. Soft Matter 2012, 8, 11478–11483. [Google Scholar] [CrossRef]
- Isa, L.; Calzolari, D.C.; Pontoni, D.; Gillich, T.; Nelson, A.; Zirbs, R.; Sánchez-Ferrer, A.; Mezzenga, R.; Reimhult, E. Core–shell nanoparticle monolayers at planar liquid–liquid interfaces: Effects of polymer architecture on the interface microstructure. Soft Matter 2013, 9, 3789–3797. [Google Scholar] [CrossRef] [Green Version]
- Pietra, F.; Rabouw, F.T.; Evers, W.H.; Byelov, D.V.; Petukhov, A.V.; de Mello Donega, C.; Vanmaekelbergh, D. Semiconductor nanorod self-assembly at the liquid/air interface studied by in situ GISAXS and ex situ TEM. Nano Lett. 2012, 12, 5515–5523. [Google Scholar] [CrossRef] [Green Version]
- Van Der Stam, W.; Rabouw, F.T.; Vonk, S.J.; Geuchies, J.J.; Ligthart, H.; Petukhov, A.V.; de Mello Donega, C. Oleic acid-induced atomic alignment of ZnS polyhedral nanocrystals. Nano Lett. 2016, 16, 2608–2614. [Google Scholar] [CrossRef]
- Geuchies, J.J.; Van Overbeek, C.; Evers, W.H.; Goris, B.; De Backer, A.; Gantapara, A.P.; Rabouw, F.T.; Hilhorst, J.; Peters, J.L.; Konovalov, O.; et al. In situ study of the formation mechanism of two-dimensional superlattices from PbSe nanocrystals. Nat. Mater. 2016, 15, 1248. [Google Scholar] [CrossRef]
- Maiti, S.; André, A.; Banerjee, R.; Hagenlocher, J.; Konovalov, O.; Schreiber, F.; Scheele, M. Monitoring self-assembly and ligand exchange of PbS nanocrystal superlattices at the liquid/air interface in real-time. J. Phys. Chem. Lett. 2018, 9, 739–744. [Google Scholar] [CrossRef]
- Vorobiev, A.; Khassanov, A.; Ukleev, V.; Snigireva, I.; Konovalov, O. Substantial difference in ordering of 10, 15, and 20 nm iron oxide nanoparticles on a water surface: In situ characterization by the grazing incidence X-ray scattering. Langmuir 2015, 31, 11639–11648. [Google Scholar] [CrossRef] [PubMed]
- Ukleev, V.; Khassanov, A.; Snigireva, I.; Konovalov, O.; Vorobiev, A. X-ray scattering characterization of iron oxide nanoparticles Langmuir film on water surface and on a solid substrate. Thin Solid Films 2016, 616, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Ukleev, V.; Khassanov, A.; Snigireva, I.; Konovalov, O.; Dudnik, M.; Dubitskiy, I.; Vorobiev, A. Self-assembly of a binary mixture of iron oxide nanoparticles in Langmuir film: X-ray scattering study. Mater. Chem. Phys. 2017, 202, 31–39. [Google Scholar] [CrossRef]
- Das, K.; Kundu, S. Variation in surface plasmonic response due to the reorganization of Au nanoparticles in Langmuir-Blodgett film. J. Appl. Phys. 2014, 116, 024316. [Google Scholar] [CrossRef]
- Costa, L.; Li-Destri, G.; Thomson, N.H.; Konovalov, O.; Pontoni, D. Real Space Imaging of Nanoparticle Assembly at Liquid–Liquid Interfaces with Nanoscale Resolution. Nano Lett. 2016, 16, 5463–5468. [Google Scholar] [CrossRef] [PubMed]
- Abuillan, W.; Veschgini, M.; Mielke, S.; Yamamoto, A.; Liu, X.; Konovalov, O.; Krafft, M.P.; Tanaka, M. Long-Range Lateral Correlation between Self-Assembled Domains of Fluorocarbon-Hydrocarbon Tetrablocks by Quantitative GISAXS. ChemPhysChem 2019, 20, 898–904. [Google Scholar] [CrossRef]
- Veschgini, M.; Abuillan, W.; Inoue, S.; Yamamoto, A.; Mielke, S.; Liu, X.; Konovalov, O.; Krafft, M.P.; Tanaka, M. Size, Shape, and Lateral Correlation of Highly Uniform, Mesoscopic, Self-Assembled Domains of Fluorocarbon–Hydrocarbon Diblocks at the Air/Water Interface: A GISAXS Study. ChemPhysChem 2017, 18, 2791–2798. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, P.; Fauré, M.C.; Bardin, L.; Filipe, E.J.; Goldmann, M. Evidence for Interaction with the Water Subphase As the Origin and Stabilization of Nano-Domain in Semi-Fluorinated Alkanes Monolayer at the Air/Water Interface. Langmuir 2014, 30, 15193–15199. [Google Scholar] [CrossRef]
- Fontaine, P.; Bardin, L.; Fauré, M.C.; Filipe, E.J.; Goldmann, M. Evidence of lying molecules in the structure of the most condensed phase of semi-fluorinated alkane monolayers. Nanoscale 2018, 10, 2310–2316. [Google Scholar] [CrossRef]
- Dyadkina, E.; Vorobiev, A.; Ukleev, V.; Lott, D.; Sitnikov, A.; Kalinin, Y.E.; Gerashchenko, O.; Grigoriev, S. Morphology and the magnetic and conducting properties of heterogeneous layered magnetic structures [(Co45Fe45Zr10)35(Al2O3)65/a-Si:H]36. J. Exp. Theor. Phys. 2014, 118, 410–416. [Google Scholar] [CrossRef]
- Ukleev, V.; Dyadkina, E.; Vorobiev, A.; Gerashchenko, O.; Caron, L.; Sitnikov, A.; Kalinin, Y.E.; Grigoriev, S. Morphology and magnetic properties of nanocomposite magnetic multilayers [(Co40Fe40B20)34(SiO2)66]/[C]47. J. Non-Cryst. Solids 2016, 432, 499–504. [Google Scholar] [CrossRef]
- Panduro, E.A.C.; Granlund, H.; Sztucki, M.; Konovalov, O.; Breiby, D.W.; Gibaud, A. Using three-dimensional 3D grazing-incidence small-angle X-ray scattering (GISAXS) analysis to probe pore deformation in mesoporous silica films. ACS Appl. Mater. Interfaces 2014, 6, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Konovalov, O. Measuring elastic properties of a protein monolayer at water surface by lateral compression. Soft Matter 2013, 9, 2845–2851. [Google Scholar] [CrossRef]
- Singh, A.; Konovalov, O. Elastic response of a protein monolayer adsorbed at decorated water surface. AIP Conf. Proc. 2015, 1661, 110020. [Google Scholar]
- Wieland, D.F.; Degen, P.; Zander, T.; Gayer, S.; Raj, A.; An, J.; Dėdinaitė, A.; Claesson, P.; Willumeit-Römer, R. Structure of DPPC–hyaluronan interfacial layers–effects of molecular weight and ion composition. Soft Matter 2016, 12, 729–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, A.; Abuillan, W.; Deichmann, C.; Rossetti, F.F.; Köhler, A.; Konovalov, O.V.; Wedlich, D.; Tanaka, M. Quantitative determination of lateral concentration and depth profile of histidine-tagged recombinant proteins probed by grazing incidence X-ray fluorescence. J. Phys. Chem. B 2013, 117, 5002–5008. [Google Scholar] [CrossRef]
- Stefaniu, C.; Brezesinski, G. X-ray investigation of monolayers formed at the soft air/water interface. Curr. Opin. Colloid Interface Sci. 2014, 19, 216–227. [Google Scholar] [CrossRef]
- Korytowski, A.; Abuillan, W.; Makky, A.; Konovalov, O.; Tanaka, M. Impact of lipid oxidization on vertical structures and electrostatics of phospholipid monolayers revealed by combination of specular X-ray reflectivity and grazing-incidence X-ray fluorescence. J. Phys. Chem. B 2015, 119, 9787–9794. [Google Scholar] [CrossRef]
- Hemmerle, A.; Fragneto, G.; Daillant, J.; Charitat, T. Reduction in tension and stiffening of lipid membranes in an electric field revealed by X-ray scattering. Phys. Rev. Lett. 2016, 116, 228101. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, F.F.; Schneck, E.; Fragneto, G.; Konovalov, O.V.; Tanaka, M. Generic role of polymer supports in the fine adjustment of interfacial interactions between solid substrates and model cell membranes. Langmuir 2015, 31, 4473–4480. [Google Scholar] [CrossRef]
- Massiot, J.; Rosilio, V.; Ibrahim, N.; Yamamoto, A.; Nicolas, V.; Konovalov, O.; Tanaka, M.; Makky, A. Newly Synthesized Lipid–Porphyrin Conjugates: Evaluation of Their Self-Assembling Properties, Their Miscibility with Phospholipids and Their Photodynamic Activity In Vitro. Chem. Eur. J. 2018, 24, 19179–19194. [Google Scholar] [CrossRef] [PubMed]
- Novikova, N.; Kovalchuk, M.; Yakunin, S.; Konovalov, O.; Stepina, N.; Rogachev, A.; Yurieva, E.; Marchenko, I.; Bukreeva, T.; Ivanova, O.; et al. Possibilities of surface-sensitive X-ray methods for studying the molecular mechanisms of interaction of nanoparticles with model membranes. Crystallogr. Rep. 2016, 61, 857–865. [Google Scholar] [CrossRef]
- Schneck, E.; Rodriguez-Loureiro, I.; Bertinetti, L.; Marin, E.; Novikov, D.; Konovalov, O.; Gochev, G. Element-specific density profiles in interacting biomembrane models. J. Phys. Appl. Phys. 2017, 50, 104001. [Google Scholar] [CrossRef] [Green Version]
- Degen, P.; Wieland, D.F.; Strötges, C. Mixed layers of nonionic dendritic amphiphiles and DPPC at the water surface. Langmuir 2015, 31, 11851–11857. [Google Scholar] [CrossRef] [PubMed]
- Nervo, R.; Khokhlova, M.A.; Konovalov, O.; Rinaudo, M. Colloid Stabilization by an Oppositely Charged Polysaccharide: Mechanism of Interaction and Interface Studied with Synchrotron X-rays. Int. J. Polym. Anal. Charact. 2014, 19, 1–9. [Google Scholar] [CrossRef]
- Reischl, D.; Röthel, C.; Christian, P.; Roblegg, E.; Ehmann, H.M.; Salzmann, I.; Werzer, O. Surface-induced polymorphism as a tool for enhanced dissolution: The example of phenytoin. Cryst. Growth Des. 2015, 15, 4687–4693. [Google Scholar] [CrossRef] [PubMed]
- Bu, W.; Schlossman, M.L. Synchrotron X-ray Scattering from Liquid Surfaces and Interfaces; Springer: Berlin, Germany, 2016; pp. 1579–1616. [Google Scholar]
- Takiue, T.; Tottori, T.; Tatsuta, K.; Matsubara, H.; Tanida, H.; Nitta, K.; Uruga, T.; Aratono, M. Multilayer Formation of the Fluoroalkanol-ω-Hydrogenated Fluorocarbon Mixture at the Hexane/Water Interface Studied by Interfacial Tensiometry and X-ray Reflection. J. Phys. Chem. B 2012, 116, 13739–13748. [Google Scholar] [CrossRef]
- Duval, J.F.; Bera, S.; Michot, L.J.; Daillant, J.; Belloni, L.; Konovalov, O.; Pontoni, D. X-ray reflectivity at polarized liquid-Hg–Aqueous-Electrolyte interface: Challenging macroscopic approaches for ion-specificity issues. Phys. Rev. Lett. 2012, 108, 206102. [Google Scholar] [CrossRef]
- Scoppola, E.; Watkins, E.; Destri, G.L.; Porcar, L.; Campbell, R.; Konovalov, O.; Fragneto, G.; Diat, O. Structure of a liquid/liquid interface during solvent extraction combining X-ray and neutron reflectivity measurements. Phys. Chem. Chem. Phys. 2015, 17, 15093–15097. [Google Scholar] [CrossRef] [Green Version]
- Scoppola, E.; Watkins, E.B.; Campbell, R.A.; Konovalov, O.; Girard, L.; Dufrêche, J.F.; Ferru, G.; Fragneto, G.; Diat, O. Solvent extraction: Structure of the liquid–liquid interface containing a diamide ligand. Angew. Chem. Int. 2016, 128, 9472–9476. [Google Scholar] [CrossRef]
- Elfassy, E.; Mastai, Y.; Pontoni, D.; Deutsch, M. Liquid-mercury-supported Langmuir films of ionic liquids: Isotherms, structure, and time evolution. Langmuir 2016, 32, 3164–3173. [Google Scholar] [CrossRef] [PubMed]
- Mars, J.; Hou, B.; Weiss, H.; Li, H.; Konovalov, O.; Festersen, S.; Murphy, B.M.; Rütt, U.; Bier, M.; Mezger, M. Surface induced smectic order in ionic liquids—An X-ray reflectivity study of [C22C1im]+[NTf2]-. Phys. Chem. Chem. Phys. 2017, 19, 26651–26661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontoni, D.; Haddad, J.; Murphy, B.M.; Festersen, S.; Konovalov, O.; Ocko, B.M.; Deutsch, M. Surface Phases and Surface Freezing in an Ionic Liquid. J. Phys. Chem. C 2019, 123, 3058–3066. [Google Scholar] [CrossRef] [Green Version]
- Haddad, J.; Pontoni, D.; Murphy, B.M.; Festersen, S.; Runge, B.; Magnussen, O.M.; Steinrück, H.G.; Reichert, H.; Ocko, B.M.; Deutsch, M. Surface structure evolution in a homologous series of ionic liquids. Proc. Natl. Acad. Sci. USA 2018, 115, E1100–E1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinrück, H.G.; Cao, C.; Tsao, Y.; Takacs, C.J.; Konovalov, O.; Vatamanu, J.; Borodin, O.; Toney, M.F. The nanoscale structure of the electrolyte–metal oxide interface. Energy Environ. Sci. 2018, 11, 594–602. [Google Scholar] [CrossRef]
- Witala, M.; Nervo, R.; Konovalov, O.; Nygård, K. Microscopic segregation of hydrophilic ions in critical binary aqueous solvents. Soft Matter 2015, 11, 5883–5888. [Google Scholar] [CrossRef] [Green Version]
- Witala, M.; Konovalov, O.; Nygård, K. Relative adsorption excess of ions in binary solvents determined by grazing-incidence X-ray fluorescence. J. Colloid Interface Sci. 2016, 484, 249–253. [Google Scholar] [CrossRef] [Green Version]
- Makiura, R.; Tsuchiyama, K.; Pohl, E.; Prassides, K.; Sakata, O.; Tajiri, H.; Konovalov, O. Air/Liquid Interfacial Nanoassembly of Molecular Building Blocks into Preferentially Oriented Porous Organic Nanosheet Crystals via Hydrogen Bonding. ACS Nano 2017, 11, 10875–10882. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Lee, L.T.; Schütz, A.; Zelenay, B.; Zheng, Z.; Borgschulte, A.; Döbeli, M.; Abuillan, W.; Konovalov, O.V.; Tanaka, M.; et al. Three-Legged 2,2′-Bipyridine Monomer at the Air/Water Interface: Monolayer Structure and Reactions with Ni (II) Ions from the Subphase. Langmuir 2017, 33, 1646–1654. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Chumakov, A.; Kan, A.S.; Lebedev, V.A.; Eliseev, A.A.; Konovalov, O.; Eliseev, A.A. Spontaneous MXene monolayer assembly at the liquid–air interface. Nanoscale 2019, 11, 9980. [Google Scholar] [CrossRef]
- Bonatout, N.; Muller, F.; Fontaine, P.; Gascon, I.; Konovalov, O.; Goldmann, M. How exfoliated graphene oxide nanosheets organize at the water interface: Evidence for a spontaneous bilayer self-assembly. Nanoscale 2017, 9, 12543–12548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliseev, A.A.; Poyarkov, A.; Chernova, E.; Eliseev, A.A.; Chumakov, A.; Konovalov, O.; Petukhov, D. Operando study of water vapor transport through ultra-thin graphene oxide membranes. 2D Mater. 2019, 6, 035039. [Google Scholar] [CrossRef]
- Petukhov, D.; Chernova, E.; Kapitanova, O.; Boytsova, O.; Valeev, R.; Chumakov, A.; Konovalov, O.; Eliseev, A. Thin graphene oxide membranes for gas dehumidification. J. Membr. Sci. 2019, 577, 184–194. [Google Scholar] [CrossRef]
- Malakhova, Y.N.; Korovin, A.N.; Lapkin, D.A.; Malakhov, S.N.; Shcherban, V.V.; Pichkur, E.B.; Yakunin, S.N.; Demin, V.A.; Chvalun, S.N.; Erokhin, V. Planar and 3D fibrous polyaniline-based materials for memristive elements. Soft Matter 2017, 13, 7300–7306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinrück, H.G.; Schiener, A.; Schindler, T.; Will, J.; Magerl, A.; Konovalov, O.; Li Destri, G.; Seeck, O.H.; Mezger, M.; Haddad, J.; et al. Nanoscale structure of Si/SiO2/organics interfaces. ACS Nano 2014, 8, 12676–12681. [Google Scholar] [CrossRef] [PubMed]
- Steinrück, H.G.; Will, J.; Magerl, A.; Ocko, B. Structure of n-Alkyltrichlorosilane Monolayers on Si (100)/SiO2. Langmuir 2015, 31, 11774–11780. [Google Scholar] [CrossRef] [PubMed]
- Schumann, T.; Dubslaff, M.; Oliveira Jr, M.H.; Hanke, M.; Lopes, J.M.J.; Riechert, H. Effect of buffer layer coupling on the lattice parameter of epitaxial graphene on SiC (0001). Phys. Rev. B 2014, 90, 041403. [Google Scholar] [CrossRef] [Green Version]
- Vignaud, G.; S Chebil, M.; Bal, J.; Delorm, N.; Beuvier, T.; Grohens, Y.; Gibaud, A. Densification and depression in glass transition temperature in polystyrene thin films. Langmuir 2014, 30, 11599–11608. [Google Scholar] [CrossRef]
- Chebil, M.S.; Vignaud, G.; Grohens, Y.; Konovalov, O.; Sanyal, M.; Beuvier, T.; Gibaud, A. In situ X-ray reflectivity study of polystyrene ultrathin films swollen in carbon dioxide. Macromolecules 2012, 45, 6611–6617. [Google Scholar] [CrossRef]
- Chavez Panduro, E.A.; Assaker, K.; Beuvier, T.; Blin, J.L.; Stébé, M.J.; Konovalov, O.; Gibaud, A. Nonionic Fluorinated Surfactant Removal from Mesoporous Film Using sc-CO2. ACS Appl. Mater. Interfaces 2017, 9, 3093–3101. [Google Scholar] [CrossRef]
- Li-Destri, G.; Tummino, A.; Gasperini, A.M.; Monreal, L.P.; Messina, G.; Spampinato, V.; Ceccone, G.; Konovalov, O. Filling nanoporous polymer thin films: An easy route toward the full control of the 3D nanostructure. RSC Adv. 2016, 6, 9175–9179. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Hamplová, V.; Bubnov, A.; Novotná, V.; Kašpar, M.; Piryazev, A.; Anokhin, D.; Ivanov, D. Photo-optical properties of amorphous and crystalline films of azobenzene-containing photochromes with bent-shaped molecular structure. J. Photochem. Photobiol. A Chem. 2016, 316, 75–87. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Piryazev, A.; Anokhin, D.V.; Ivanov, D.A.; Sinitsyna, O.; Hamplova, V.; Kaspar, M.; Bubnov, A. Photo-Orientation Phenomena in Photochromic Liquid Crystalline Azobenzene-Containing Polymethacrylates with Different Spacer Length. Macromol. Chem. Phys. 2017, 218, 1700127. [Google Scholar] [CrossRef]
- Grafskaia, K.; Anokhin, D.; Zimka, B.; Izdelieva, I.; Zhu, X.; Ivanov, D. An on–off switchable cubic phase with exceptional thermal stability and water sorption capacity. Chem. Commun. 2017, 53, 13217–13220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowd, E.B.; Koga, T.; Endoh, M.K.; Kumar, K.; Stamm, M. Pathways of cylindrical orientations in PS-b-P4VP diblock copolymer thin films upon solvent vapor annealing. Soft Matter 2014, 10, 7753–7761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li Destri, G.; Gasperini, A.A.M.; Konovalov, O. The link between self-assembly and molecular conformation of amphiphilic block copolymers monolayers at the air/water interface: The spreading parameter. Langmuir 2015, 31, 8856–8864. [Google Scholar] [CrossRef] [PubMed]
- Guennouni, Z.; Cousin, F.; Fauré, M.C.; Perrin, P.; Limagne, D.; Konovalov, O.; Goldmann, M. Self-organization of polystyrene-b-polyacrylic acid (PS-b-PAA) monolayer at the air/water interface: A process driven by the release of the solvent spreading. Langmuir 2016, 32, 1971–1980. [Google Scholar] [CrossRef]
- Guennouni, Z.; Goldmann, M.; Fauré, M.C.; Fontaine, P.; Perrin, P.; Limagne, D.; Cousin, F. Coupled Effects of Spreading Solvent Molecules and Electrostatic Repulsions on the Behavior of PS-b-PAA Monolayers at the Air–Water Interface. Langmuir 2017, 33, 12525–12534. [Google Scholar] [CrossRef] [Green Version]
- Busse, K.; Fuchs, C.; Hasan, N.; Pulst, M.; Kressler, J. Crystallization of Poly (ethylene oxide) on the Surface of Aqueous Salt Solutions Studied by Grazing Incidence Wide-Angle X-ray Scattering. Langmuir 2018, 34, 12759–12763. [Google Scholar] [CrossRef]
- Khassanov, A.; Steinrück, H.G.; Schmaltz, T.; Magerl, A.; Halik, M. Structural investigations of self-assembled monolayers for organic electronics: Results from X-ray reflectivity. Acc. Chem. Res. 2015, 48, 1901–1908. [Google Scholar] [CrossRef]
- Liscio, F.; Albonetti, C.; Broch, K.; Shehu, A.; Quiroga, S.D.; Ferlauto, L.; Frank, C.; Kowarik, S.; Nervo, R.; Gerlach, A.; et al. Molecular reorganization in organic field-effect transistors and its effect on two-dimensional charge transport pathways. ACS Nano 2013, 7, 1257–1264. [Google Scholar] [CrossRef]
- Banerjee, R.; Hinderhofer, A.; Weinmann, M.; Reisz, B.; Lorch, C.; Gerlach, A.; Oettel, M.; Schreiber, F. Interrupted Growth to Manipulate Phase Separation in DIP: C60 Organic Semiconductor Blends. J. Phys. Chem. C 2018, 122, 1839–1845. [Google Scholar] [CrossRef]
- Zhang, C.; Langner, S.; Mumyatov, A.V.; Anokhin, D.V.; Min, J.; Perea, J.D.; Gerasimov, K.L.; Osvet, A.; Ivanov, D.A.; Troshin, P.; et al. Understanding the correlation and balance between the miscibility and optoelectronic properties of polymer–fullerene solar cells. J. Mater. Chem. A 2017, 5, 17570–17579. [Google Scholar] [CrossRef] [Green Version]
- Clikeman, T.T.; Schmaltz, T.; Halik, M.; Hirsch, A.; Strauss, S.H.; Boltalina, O.V. Formation of Perfluoroalkyl Fullerene Alkylphosphonic Acid Self-Assembled Monolayers on Aluminum Oxide. ECS J. Solid State Sci. Technol. 2017, 6, M3163–M3167. [Google Scholar] [CrossRef] [Green Version]
- Kassar, T.; Güldal, N.S.; Berlinghof, M.; Ameri, T.; Kratzer, A.; Schroeder, B.C.; Destri, G.L.; Hirsch, A.; Heeney, M.; McCulloch, I.; et al. Real-Time Investigation of Intercalation and Structure Evolution in Printed Polymer: Fullerene Bulk Heterojunction Thin Films. Adv. Energy Mater. 2016, 6, 1502025. [Google Scholar] [CrossRef]
- Schmaltz, T.; Khassanov, A.; Steinrück, H.G.; Magerl, A.; Hirsch, A.; Halik, M. Tuning the molecular order of C 60-based self-assembled monolayers in field-effect transistors. Nanoscale 2014, 6, 13022–13027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güldal, N.S.; Berlinghof, M.; Kassar, T.; Du, X.; Jiao, X.; Meyer, M.; Ameri, T.; Osvet, A.; Li, N.; Destri, G.L.; et al. Controlling additive behavior to reveal an alternative morphology formation mechanism in polymer: Fullerene bulk-heterojunctions. J. Mater. Chem. A 2016, 4, 16136–16147. [Google Scholar] [CrossRef] [Green Version]
- Belova, V.; Wagner, B.; Reisz, B.; Zeiser, C.; Duva, G.; Rozbořil, J.; Novák, J.; Gerlach, A.; Hinderhofer, A.; Schreiber, F. Real-Time Structural and Optical Study of Growth and Packing Behavior of Perylene Diimide Derivative Thin Films: Influence of Side-Chain Modification. J. Phys. Chem. C 2018, 122, 8589–8601. [Google Scholar] [CrossRef]
- Belova, V.; Beyer, P.; Meister, E.; Linderl, T.; Halbich, M.U.; Gerhard, M.; Schmidt, S.; Zechel, T.; Meisel, T.; Generalov, A.V.; et al. Evidence for anisotropic electronic coupling of charge transfer states in weakly interacting organic semiconductor mixtures. J. Am. Chem. Soc. 2017, 139, 8474–8486. [Google Scholar] [CrossRef]
- Kuznetsova, L.I.; Piryazev, A.A.; Anokhin, D.V.; Mumyatov, A.V.; Susarova, D.K.; Ivanov, D.A.; Troshin, P.A. Disubstituted perylene diimides in organic field-effect transistors: Effect of the alkyl side chains and thermal annealing on the device performance. Org. Electron. 2018, 58, 257–262. [Google Scholar] [CrossRef]
- Mrkyvkova, N.; Hodas, M.; Hagara, J.; Nadazdy, P.; Halahovets, Y.; Bodik, M.; Tokar, K.; Chai, J.W.; Wang, S.; Chi, D.; et al. Diindenoperylene thin-film structure on MoS2 monolayer. Appl. Phys. Lett. 2019, 114, 251906. [Google Scholar] [CrossRef]
- Hörmann, U.; Lorch, C.; Hinderhofer, A.; Gerlach, A.; Gruber, M.; Kraus, J.; Sykora, B.; Grob, S.; Linderl, T.; Wilke, A.; et al. V OC from a morphology point of view: The influence of molecular orientation on the open circuit voltage of organic planar heterojunction solar cells. J. Phys. Chem. C 2014, 118, 26462–26470. [Google Scholar] [CrossRef]
- Lorch, C.; Frank, H.; Banerjee, R.; Hinderhofer, A.; Gerlach, A.; Li Destri, G.; Schreiber, F. Controlling length-scales of the phase separation to optimize organic semiconductor blends. Appl. Phys. Lett. 2015, 107, 201903. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.; Novák, J.; Banerjee, R.; Gerlach, A.; Schreiber, F.; Vorobiev, A.; Kowarik, S. Island size evolution and molecular diffusion during growth of organic thin films followed by time-resolved specular and off-specular scattering. Phys. Rev. B 2014, 90, 045410. [Google Scholar] [CrossRef] [Green Version]
- Storzer, T.; Hinderhofer, A.; Zeiser, C.; Novák, J.; Fišer, Z.; Belova, V.; Reisz, B.; Maiti, S.; Duva, G.; Hallani, R.K.; et al. Growth, Structure, and Anisotropic Optical Properties of Difluoro-anthradithiophene Thin Films. J. Phys. Chem. C 2017, 121, 21011–21017. [Google Scholar] [CrossRef]
- Musumeci, C.; Salzmann, I.; Bonacchi, S.; Röthel, C.; Duhm, S.; Koch, N.; Samorì, P. The Relationship between Structural and Electrical Characteristics in Perylenecarboxydiimide-Based Nanoarchitectures. Adv. Funct. Mater. 2015, 25, 2501–2510. [Google Scholar] [CrossRef]
- Truger, M.; Jones, A.O.; Coclite, A.M.; Pachmajer, S.; Kriegner, D.; Röthel, C.; Simbrunner, J.; Salzmann, I.; Resel, R. Crystallization of Tyrian purple (6,6′-dibromoindigo) thin films: The impact of substrate surface modifications. J. Cryst. Growth 2016, 447, 73–79. [Google Scholar] [CrossRef]
- Hodas, M.; Siffalovic, P.; Nadazdy, P.; Mrkyvkova, N.; Bodík, M.; Halahovets, Y.; Duva, G.; Reisz, B.; Konovalov, O.; Ohm, W.; et al. Real-Time Monitoring of Growth and Orientational Alignment of Pentacene on Epitaxial Graphene for Organic Electronics. ACS Appl. Nano Mater. 2018, 1, 2819–2826. [Google Scholar] [CrossRef]
- Pachmajer, S.; Jones, A.O.; Truger, M.; Röthel, C.; Salzmann, I.; Werzer, O.; Resel, R. Self-Limited Growth in Pentacene Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 11977–11984. [Google Scholar] [CrossRef]
- Hosokai, T.; Hinderhofer, A.; Bussolotti, F.; Yonezawa, K.; Lorch, C.; Vorobiev, A.; Hasegawa, Y.; Yamada, Y.; Kubozoro, Y.; Gerlach, A.; et al. Thickness and substrate dependent thin film growth of picene and impact on the electronic structure. J. Phys. Chem. C 2015, 119, 29027–29037. [Google Scholar] [CrossRef]
- Dieterle, J.; Broch, K.; Hinderhofer, A.; Frank, H.; Novák, J.; Gerlach, A.; Breuer, T.; Banerjee, R.; Witte, G.; Schreiber, F. Structural properties of picene–perfluoropentacene and picene–pentacene blends: Superlattice formation versus limited intermixing. J. Phys. Chem. C 2015, 119, 26339–26347. [Google Scholar] [CrossRef]
- Méndez, H.; Heimel, G.; Winkler, S.; Frisch, J.; Opitz, A.; Sauer, K.; Wegner, B.; Oehzelt, M.; Röthel, C.; Duhm, S.; et al. Charge-transfer crystallites as molecular electrical dopants. Nat. Commun. 2015, 6, 8560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, A.; Hinderhofer, A.; Dar, M.I.; Arora, N.; Hagenlocher, J.; Chumakov, A.; Grätzel, M.; Schreiber, F. Kinetics of Ion-Exchange Reactions in Hybrid Organic–Inorganic Perovskite Thin Films Studied by In Situ Real-Time X-ray Scattering. J. Phys. Chem. Lett. 2018, 9, 6750–6754. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Highly efficient all-inorganic planar heterojunction perovskite solar cells produced by thermal coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2016, 8, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [Green Version]
- Schmaltz, T.; Gothe, B.; Krause, A.; Leitherer, S.; Steinrück, H.G.; Thoss, M.; Clark, T.; Halik, M. Effect of structure and disorder on the charge transport in defined self-assembled monolayers of organic semiconductors. ACS Nano 2017, 11, 8747–8757. [Google Scholar] [CrossRef]
- Trul, A.A.; Sizov, A.S.; Chekusova, V.P.; Borshchev, O.V.; Agina, E.V.; Shcherbina, M.A.; Bakirov, A.V.; Chvalun, S.N.; Ponomarenko, S.A. Organosilicon dimer of BTBT as a perspective semiconductor material for toxic gas detection with monolayer organic field-effect transistors. J. Mater. Chem. C 2018, 6, 9649–9659. [Google Scholar] [CrossRef]
- Güldal, N.S.; Kassar, T.; Berlinghof, M.; Ameri, T.; Osvet, A.; Pacios, R.; Destri, G.L.; Unruh, T.; Brabec, C.J. Real-time evaluation of thin film drying kinetics using an advanced, multi-probe optical setup. J. Mater. Chem. C 2016, 4, 2178–2186. [Google Scholar] [CrossRef] [Green Version]
- Buss, F.; Schmidt-Hansberg, B.; Sanyal, M.; Munuera, C.; Scharfer, P.; Schabel, W.; Barrena, E. Gaining further insight into the solvent additive-driven crystallization of bulk-heterojunction solar cells by in situ X-ray scattering and optical reflectometry. Macromolecules 2016, 49, 4867–4874. [Google Scholar] [CrossRef]
- Gothe, B.; De Roo, T.; Will, J.; Unruh, T.; Mecking, S.; Halik, M. Self-assembled monolayer field-effect transistors based on oligo-9, 9’-dioctylfluorene phosphonic acids. Nanoscale 2017, 9, 18584–18589. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.; Singh, C.R.; Lohwasser, R.H.; Himmerlich, M.; Krischok, S.; Müller-Buschbaum, P.; Thelakkat, M.; Hoppe, H.; Thurn-Albrecht, T. Morphology, crystal structure and charge transport in donor–acceptor block copolymer thin films. ACS Appl. Mater. Interfaces 2014, 7, 12309–12318. [Google Scholar] [CrossRef]
- Franke, D.; Jeffries, C.M.; Svergun, D.I. Machine learning methods for X-ray scattering data analysis from biomacromolecular solutions. Biophys. J. 2018, 114, 2485–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, A.; Starostin, V.; Karapanagiotis, C.; Hinderhofer, A.; Gerlach, A.; Pithan, L.; Liehr, S.; Schreiber, F.; Kowarik, S. Fast fitting of reflectivity data of growing thin films using neural networks. J. Appl. Crystallogr. 2019, 52, 1342–1347. [Google Scholar] [CrossRef] [PubMed]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, T.; Konovalov, O. Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research. Materials 2020, 13, 752. https://doi.org/10.3390/ma13030752
Narayanan T, Konovalov O. Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research. Materials. 2020; 13(3):752. https://doi.org/10.3390/ma13030752
Chicago/Turabian StyleNarayanan, Theyencheri, and Oleg Konovalov. 2020. "Synchrotron Scattering Methods for Nanomaterials and Soft Matter Research" Materials 13, no. 3: 752. https://doi.org/10.3390/ma13030752




