Controlling the Electronic Properties of a Nanoporous Carbon Surface by Modifying the Pores with Alkali Metal Atoms
Abstract
:1. Introduction
2. Atomistic Model of the Nanoporous Carbon Surface
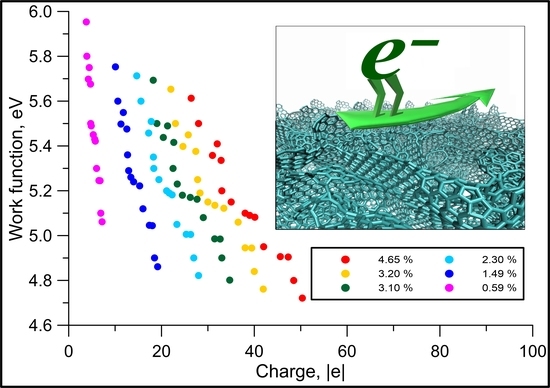
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Gay, S.; Orlanducci, S.; Passeri, D.; Rossic, M.; Terranova, M.L. Nanoshaping field emitters from glassy carbon sheets: A new functionality induced by H-plasma etching. Phys. Chem. Chem. Phys. 2016, 18, 25364–25372. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.C.; Hunt, C.E.; Brodiec, I.; Carpenter, A.C. High-performance field-emission electron gun using a reticulated vitreous carbon cathode. J. Vac. Sci. Technol. B 2011, 29, 02B108. [Google Scholar] [CrossRef]
- Cao, M.M.; Chacon, R.J.; Hunt, C.E. A Field Emission Light Source Using a Reticulated Vitreous Carbon (RVC) Cathode and Cathodoluminescent Phosphors. J. Disp. Technol. 2011, 7, 467–472. [Google Scholar] [CrossRef]
- Matsubara, E.Y.; Rosolen, J.M.; Silva, S.R.P. Composite electrode of carbon nanotubes and vitreous carbon for electron field emission. J. Appl. Phys. 2008, 104, 054303. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, A.C.; Hunt, C.E. High-current, low-cost field emission triode using a reticulated vitreous carbon cathode. J. Vac. Sci. Technol. B 2010, 28, C2C37–C2C40. [Google Scholar] [CrossRef]
- Chakhovskoi, A.G.; Hunt, C.E. Reticulated vitreous carbon field emission cathodes for light source applications. J. Vac. Sci. Technol. B 2003, 21, 571–575. [Google Scholar] [CrossRef]
- Egorov, N.; Sheshin, E. Carbon-Based Field-Emission Cathodes. In Field Emission Electronics, 1st ed.; Springer: Berlin, Germany, 2017; Volume 60, pp. 295–367. [Google Scholar]
- Hunt, C.E.; Chakhovskoi, A.G.; Wang, Y. Ion-beam morphological conditioning of carbon field emission cathode surfaces. J. Vac. Sci. Tech. B 2005, 23, 731–734. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Kalijadis, A.; Vasiljevic-Radovic, D.; Eric, M.; Lausevic, M.; Mentus, S.; Lausevic, Z. Modification of glassy carbon properties under low energy proton irradiation. Carbon 2011, 49, 3737–3746. [Google Scholar] [CrossRef]
- Serp, P.; Machado, B. Nanostructured Carbon Materials for Catalysis, 1st ed.; Royal Society of Chemistry: London, UK, 2015; pp. 1–45. [Google Scholar]
- Arkhipov, A.; Davydov, S.; Gabdullin, P.; Gnuchev, N.; Kravchik, A.; Krel, S. Field-induced electron emission from nanoporous carbons. J. Nanomater. 2014, 2014, 190232. [Google Scholar] [CrossRef]
- Kravchik, A.E.; Kukushkina, J.A.; Sokolov, V.V.; Tereshchenko, G.F. Structure of nanoporous carbon produced from boron carbide. Carbon 2006, 44, 3263–3268. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Long, C.; Jiang, L.; Wu, X.; Jiang, Y.; Yang, D.; Wang, C.; Wei, T.; Fan, Z. Facile synthesis of functionalized porous carbon with three-dimensional interconnected pore structure for high volumetric performance supercapacitors. Carbon 2015, 93, 412–420. [Google Scholar] [CrossRef]
- Pykal, M.; Jurečka, P.; Karlický, F.; Otyepka, M. Modelling of graphene functionalization. Phys. Chem. Chem. Phys. 2016, 18, 6351–6372. [Google Scholar] [CrossRef] [Green Version]
- Jurkiewicz, K.; Duber, S.; Fischer, H.E.; Burian, A. Modelling of glass-like carbon structure and its experimental verification by neutron and X-ray diffraction. J. Appl. Cryst. 2017, 50, 36–48. [Google Scholar] [CrossRef]
- Harris, P.J.F. Fullerene-related structure of commercial glassy carbons. Philos. Mag. 2004, 84, 3159–3167. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, E.F.; Yan, H.; Kono, Y.; Wen, B.; Bai, L.; Shi, F.; Zhang, J.; Kenney-Benson, C.; Park, C.; et al. Nanoarchitectured materials composed of fullerene-like spheroids and disordered grapheme layers with tunable mechanical properties. Nat. Commun. 2015, 6, 6212. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.J.F. Fullerene-like models for microporous carbon. J. Mater Sci. 2013, 48, 565–577. [Google Scholar] [CrossRef]
- Liang, H.; Ma, X.; Yang, Z.; Wang, P.; Zhang, X.; Ren, Z.; Xue, M.; Chen, G. Emergence of superconductivity in doped glassy-carbon. Carbon 2016, 99, 585–590. [Google Scholar] [CrossRef]
- Bessonov, D.A.; Sokolova, T.N.; Shesterkin, V.I.; Surmenko, E.L.; Popov, I.A.; Chebotarevsky, Y.V. Laser formation of tip emitting structures with high aspect ratio on glass-carbon field-emission cathodes. J. Phys. Conf. Ser. 2016, 741, 012166. [Google Scholar] [CrossRef] [Green Version]
- Araujo, P.T.; Terrones, M.; Dresselhaus, M.S. Defects and impurities in graphene-like materials. Mater. Today 2012, 15, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.L.; Gidcumb, E.; Zhoubc, O.; Sinclair, R. The dissipation of field emitting carbon nanotubes in an oxygen environment as revealed by in situ transmission electron microscopy. Nanoscale 2016, 8, 16405–16415. [Google Scholar] [CrossRef]
- Giubileoa, F.; Di Bartolomeoa, A.; Scarfatoa, A.; Iemmoa, L.; Bobbaa, F.; Passacantandob, M.; Santuccib, S.; Cucoloa, A.M. Local probing of the field emission stability of vertically aligned multi-walled carbon nanotubes. Carbon 2009, 47, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yao, X.H.; Huang, G.; Shao, Q.Y. The enhanced field emission properties of K and Rb doped (5,5) capped single-walled carbon nanotubes. RSC Adv. 2015, 5, 16718–16722. [Google Scholar] [CrossRef]
- Izrael’yants, K.R.; Orlov, A.P.; Ormont, A.B.; Chirkov, E.G. Effect of the cesium and potassium doping of multiwalled carbon nanotubes grown in an electrical arc on their emission characteristics. Phys. Solid State 2017, 59, 838–844. [Google Scholar] [CrossRef]
- Ye, D.; Moussa, S.; Ferguson, J.D.; Baski, A.A.; El-Shall, M.S. Highly Efficient Electron Field Emission from Graphene Oxide Sheets Supported by Nickel Nanotip Arrays. Nano Lett. 2012, 12, 1265–1268. [Google Scholar] [CrossRef]
- Iemmo, L.; Di Bartolomeo, A.; Giubileo, F.; Luongo, G.; Passacantando, M.; Niu, G.; Hatami, F.; Skibitzki, O.; Schroeder, T. Graphene enhanced field emission from InP nanocrystals. Nanotechnology 2017, 28, 495705. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.P.; Chang, H.B.; Kim, B.J.; Park, J.S. Enhancement of electron emission and long-term stability of tip-type carbon nanotube field emitters via lithium coating. Thin Solid Film. 2013, 528, 242–246. [Google Scholar] [CrossRef]
- Giubileo, F.; Di Bartolomeo, A.; Iemmo, L.; Luongo, G.; Urban, F. Field Emission from Carbon Nanostructures. Appl. Sci. 2018, 8, 526. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.K.; Kedawat, G.; Kumar, P.; Singh, S.; Suryawanshi, S.R.; Agrawal (Garg), N.; Gupta, G.; Kim, A.R.; Gupta, R.K.; More, M.A.; et al. Field emission properties of highly ordered low-aspect ratio carbon nanocup arrays. RSC Adv. 2016, 6, 9932–9939. [Google Scholar] [CrossRef]
- Parveen, S.; Husain, S.; Kumar, A.; Ali, J.; Harsh; Husain, H.M. Improved field emission properties of carbon nanotubes by dual layer deposition. J. Exp. Nanosci. 2015, 10, 499–510. [Google Scholar] [CrossRef]
- Yu, J.; Chua, D.H.C. Enhanced field emission properties of hydrogenated tetrahedral amorphous carbon/carbon nanotubes nanostructures electrochem. Solid-State Lett. 2010, 13, K80–K82. [Google Scholar] [CrossRef]
- Varshney, D.; Makarov, V.I.; Saxena, P.; González-Berríos, A.; Scott, J.F.; Weiner, B.R.; Morell, G. Fabrication and field emission study of novel rod-shaped diamond-like carbon nanostructures. Nanotechnology 2010, 21, 285301. [Google Scholar] [CrossRef]
- Glukhova, O.E.; Slepchenkov, M.M. Electronic Properties of the Functionalized Porous Glass-like Carbon. J. Phys. Chem. C 2016, 120, 17753–17758. [Google Scholar] [CrossRef]
- Elstner, M.; Seifert, G. Density functional tight binding. Phil. Trans. R. Soc. A 2014, 372, 20120483. [Google Scholar] [CrossRef] [Green Version]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a sparse matrix-based implementation of the DFTB method. J. Phys. Chem. A 2007, 111, 5678–5684. [Google Scholar] [CrossRef]
- Zobelli, A.; Ivanovskaya, V.; Wagner, P.; Suarez-Martinez, I.; Yaya, A.; Ewels, C.P. A comparative study of density functional and density functional tight binding calculations of defects in graphene. Phys. Status Solidi B 2012, 249, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Shunaev, V.V.; Savostyanov, G.V.; Slepchenkov, M.M.; Glukhova, O.E. Phenomenon of current occurrence during the motion of a C60 fullerene on substrate-supported graphene. RSC Adv. 2015, 5, 86337–86346. [Google Scholar] [CrossRef]
- Glukhova, O.E. Molecular Dynamics as the Tool for Investigation of Carbon Nanostructures Properties. In Thermal Transport in Carbon-Based Nanomaterials, 1st ed.; Zhang, G., Ed.; Elsevier: Oxford, UK, 2017; pp. 267–289. [Google Scholar]
- Nakao, A.; Iwaki, M.; Yokoyama, Y. Potassium ion implantation into glassy carbon. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 206, 211–214. [Google Scholar] [CrossRef]





| N/N atoms, % | Charge, |e| | ||||
|---|---|---|---|---|---|
| K | H | O | OH | Carbon | |
| 3.56% H | +39.95 | +10.39 | - | - | −50.34 |
| 2.22% H | +40.20 | +7.10 | - | - | −47.30 |
| 1.55% H | +40.46 | +5.14 | - | - | −45.60 |
| 0.81% O | +41.28 | - | −10.38 | - | −30.90 |
| 1.12% O | +41.45 | - | −15.04 | - | −26.41 |
| 2.58% OH | +40.10 | - | - | −1.20 | −38.90 |
| 1.12% O, 1.12% H | +40.75 | +1.60 | −8.42 | - | −33.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepchenkov, M.M.; Nefedov, I.S.; Glukhova, O.E. Controlling the Electronic Properties of a Nanoporous Carbon Surface by Modifying the Pores with Alkali Metal Atoms. Materials 2020, 13, 610. https://doi.org/10.3390/ma13030610
Slepchenkov MM, Nefedov IS, Glukhova OE. Controlling the Electronic Properties of a Nanoporous Carbon Surface by Modifying the Pores with Alkali Metal Atoms. Materials. 2020; 13(3):610. https://doi.org/10.3390/ma13030610
Chicago/Turabian StyleSlepchenkov, Michael M., Igor S. Nefedov, and Olga E. Glukhova. 2020. "Controlling the Electronic Properties of a Nanoporous Carbon Surface by Modifying the Pores with Alkali Metal Atoms" Materials 13, no. 3: 610. https://doi.org/10.3390/ma13030610