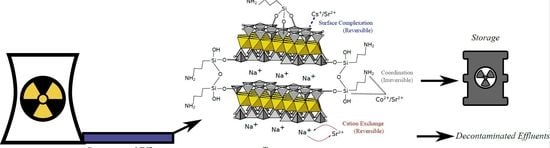
Laponites® for the Recovery of 133Cs, 59Co, and 88Sr from Aqueous Solutions and Subsequent Storage: Impact of Grafted Silane Loads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Synthesis of Grafted-LAP
2.3. Experimental Techniques
2.4. Adsorption Experiments
2.5. Desorption Experiments
2.6. Sorption Modeling
3. Results and Discussion
3.1. Characterization of LAP-APTES
3.2. Adsorption Experiments
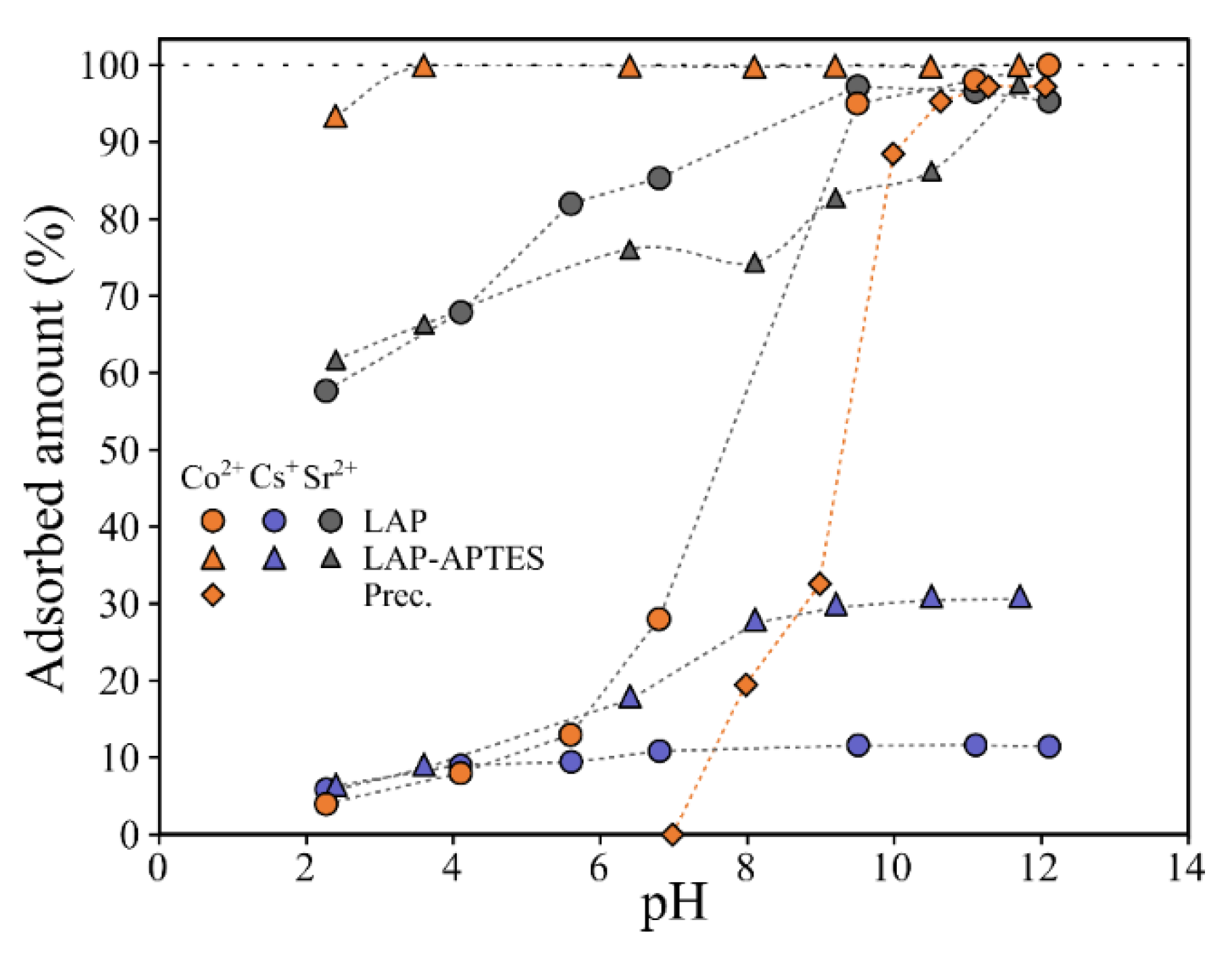
3.2.1. Impact of pH
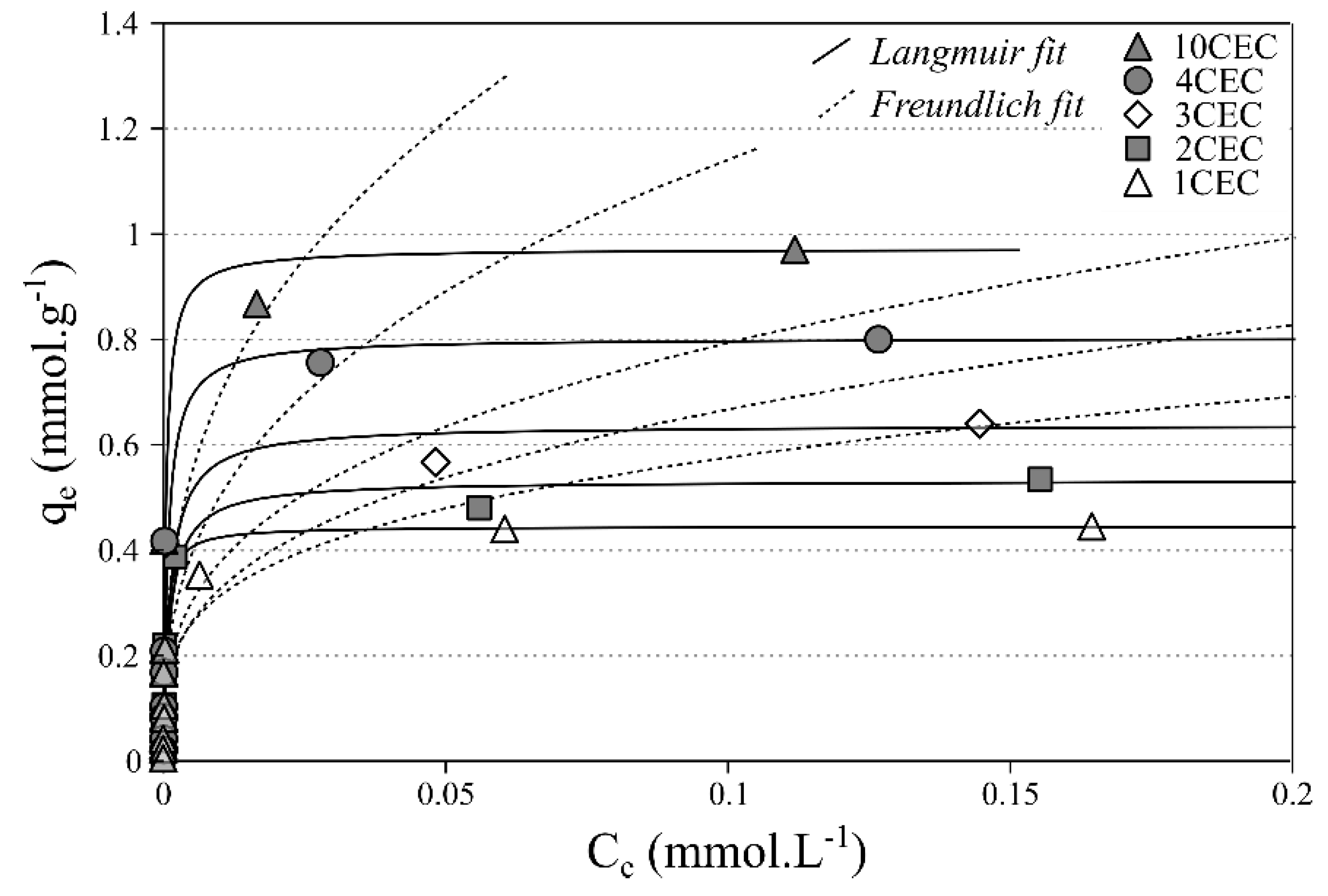
3.2.2. Single-Solute Adsorption Isotherms
3.2.3. Competitive Adsorption Isotherms
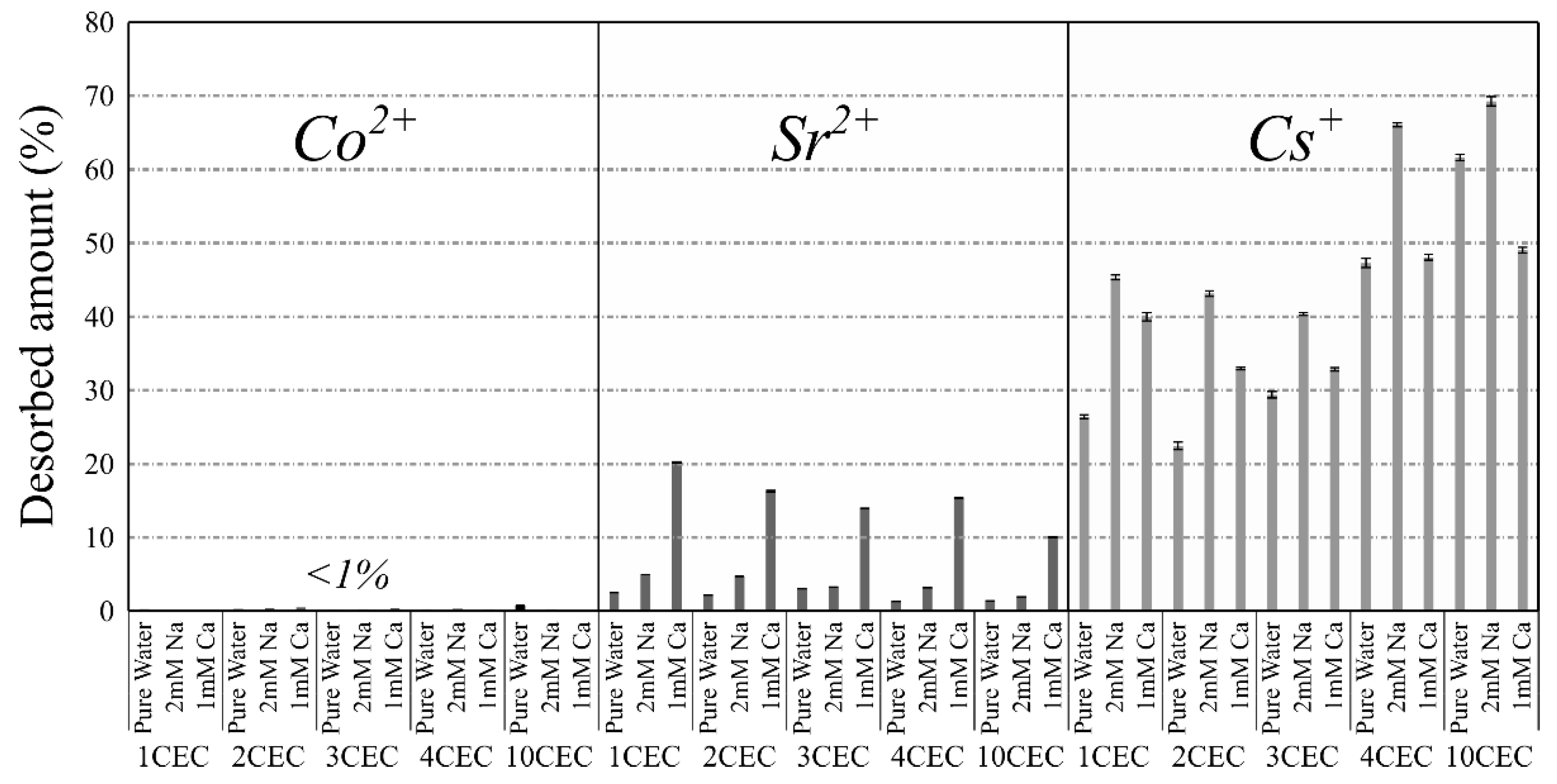
3.3. Desorption Experiments
3.4. Sorption Mechanisms
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gershey, E.L.; Klein, R.C.; Party, E.; Wilkerson, A. Low-Level Radioactive Waste; Van Nostrand Reinhold Co.: New York, NY, USA, 1990; ISBN 0-442-23958-0. [Google Scholar]
- Salbu, B. Fractionation of radionuclide species in the environment. J. Environ. Radioact. 2009, 100, 283–289. [Google Scholar] [CrossRef]
- Salbu, B.; Lind, O.C.; Skipperud, L. Radionuclide speciation and its relevance in environmental impact assessments. J. Environ. Radioact. 2004, 74, 233–242. [Google Scholar] [CrossRef]
- Periáñez, R.; Bezhenar, R.; Brovchenko, I.; Duffa, C.; Iosjpe, M.; Jung, K.T.; Kobayashi, T.; Lamego, F.; Maderich, V.; Min, B.I.; et al. Modelling of marine radionuclide dispersion in IAEA MODARIA program: Lessons learnt from the Baltic Sea and Fukushima scenarios. Sci. Total Environ. 2016, 569–570, 594–602. [Google Scholar]
- Rosenberg, B.L.; Ball, J.E.; Shozugawa, K.; Korschinek, G.; Hori, M.; Nanba, K.; Johnson, T.E.; Brandl, A.; Steinhauser, G. Radionuclide pollution inside the Fukushima Daiichi exclusion zone, part 1: Depth profiles of radiocesium and strontium-90 in soil. Appl. Geochem. 2017, 85, 201–208. [Google Scholar] [CrossRef]
- Dang, T.T.H.; Li, C.-W.; Choo, K.-H. Comparison of low-pressure reverse osmosis filtration and polyelectrolyte-enhanced ultrafiltration for the removal of Co and Sr from nuclear plant wastewater. Sep. Purif. Technol. 2016, 157, 209–214. [Google Scholar] [CrossRef]
- Bors, J.; Dultz, S.; Riebe, B. Organophilic bentonites as adsorbents for radionuclides. Appl. Clay Sci. 2000, 16, 1–13. [Google Scholar] [CrossRef]
- Fang, X.-H.; Fang, F.; Lu, C.-H.; Zheng, L. Removal of Cs+, Sr2+, and Co2+ ions from the mixture of organics and suspended solids aqueous solutions by zeolites. Nucl. Eng. Technol. 2017, 49, 556–561. [Google Scholar] [CrossRef]
- Rahman, R.O.A.; Ibrahium, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wei, J.; Zhao, X.; Li, F.; Jiang, F.; Zhang, M.; Cheng, X. Removal of strontium(II) and cobalt(II) from acidic solution by manganese antimonate. Chem. Eng. J. 2016, 302, 733–743. [Google Scholar] [CrossRef]
- Claverie, M.; Garcia, J.; Prevost, T.; Brendlé, J.; Limousy, L. Inorganic and hybrid (organic–inorganic) lamellar materials for heavy metals and radionuclides capture in energy wastes management—A review. Materials 2019, 12, 1399. [Google Scholar] [CrossRef] [Green Version]
- Thiebault, T.; Brendlé, J.; Augé, G.; Limousy, L. Zwitterionic-surfactant modified LAPONITE®s for removal of ions (Cs+, Sr2+ and Co2+) from aqueous solutions as a sustainable recovery method for radionuclides from aqueous wastes. Green Chem. 2019, 21, 5118–5127. [Google Scholar] [CrossRef]
- Moyo, F.; Tandlich, R.; Wilhelmi, B.S.; Balaz, S. Sorption of hydrophobic organic compounds on natural sorbents and organoclays from aqueous and non-aqueous solutions: A mini-review. Int. J. Environ. Res. Public Health 2014, 11, 5020–5048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.A.d.S.; Figueiras, A.; Veiga, F.; de Freitas, R.M.; Nunes, L.C.C.; da Silva Filho, E.C.; da Silva Leite, C.M. The systems containing clays and clay minerals from modified drug release: A review. Colloids Surf. B Biointerfaces 2013, 103, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Thiebault, T.; Guégan, R.; Boussafir, M. Adsorption mechanisms of emerging micro-pollutants with a clay mineral: Case of tramadol and doxepine pharmaceutical products. J. Colloid Interface Sci. 2015, 453, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef] [Green Version]
- Thiebault, T. Raw and modified clays and clay minerals for the removal of pharmaceutical products from aqueous solutions: State of the art and future perspectives. Crit. Rev. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Le Forestier, L.; Le Milbeau, C.; Monnin, L.; Guégan, R. Competitive adsorption of a pool of pharmaceuticals onto a raw clay mineral. RSC Adv. 2016, 6, 65257–65265. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef]
- De Paiva, L.B.; Morales, A.R.; Valenzuela Díaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. Comptes Rendus Chimie 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Jaber, M.; Miehé-Brendlé, J.; Michelin, L.; Delmotte, L. Heavy metal retention by organoclays: Synthesis, applications, and retention mechanism. Chem. Mater. 2005, 17, 5275–5281. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Horváth, E.; Kurdi, R.; Kristof, J.; Frost, R.L. Structural characterisation and environmental application of organoclays for the removal of phenolic compounds. J. Colloid Interface Sci. 2013, 393, 319–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Sheng, G.; Boyd, S.A. Use of organoclays in pollution abatement. Adv. Agron. 1997, 59, 25–62. [Google Scholar]
- Hari, A.C.; Paruchuri, R.A.; Sabatini, D.A.; Kibbey, T.C.G. Effects of pH and cationic and nonionic surfactants on the adsorption of pharmaceuticals to a natural aquifer material. Environ. Sci. Technol. 2005, 39, 2592–2598. [Google Scholar] [CrossRef]
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ion. 1986, 22, 43–51. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Frost, R.L. Application of organoclays for the adsorption of recalcitrant organic molecules from aqueous media. J. Colloid Interface Sci. 2011, 354, 292–305. [Google Scholar] [CrossRef]
- Daniel, L.M.; Frost, R.L.; Zhu, H.Y. Edge-modification of laponite with dimethyl-octylmethoxysilane. J. Colloid Interface Sci. 2008, 321, 302–309. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Tao, Q.; Zhu, J.; Yuan, P.; Shen, W.; Yang, S. Silylation of clay mineral surfaces. Appl. Clay Sci. 2013, 71, 15–20. [Google Scholar] [CrossRef]
- Park, M.; Shim, I.-K.; Jung, E.-Y.; Choy, J.-H. Modification of external surface of laponite by silane grafting. J. Phys. Chem. Solids 2004, 65, 499–501. [Google Scholar] [CrossRef]
- He, H.; Zhou, Q.; Martens, W.N.; Kloprogge, T.J.; Yuan, P.; Xi, Y.; Zhu, J.; Frost, R.L. Microstructure of HDTMA+-modified montmorillonite and its influence on sorption characteristics. Clays Clay Miner. 2006, 54, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Z.; Sparks, D.L.; Scrivner, N.C. Sorption and desorption of quaternary amine cations on clays. Environ. Sci. Technol. 1993, 27, 1625–1631. [Google Scholar] [CrossRef]
- De Mello Ferreira Guimarães, A.; Ciminelli, V.S.T.; Vasconcelos, W.L. Smectite organofunctionalized with thiol groups for adsorption of heavy metal ions. Appl. Clay Sci. 2009, 42, 410–414. [Google Scholar] [CrossRef]
- Guerra, D.L.; Oliveira, S.P.; Silva, R.A.S.; Silva, E.M.; Batista, A.C. Dielectric properties of organofunctionalized kaolinite clay and application in adsorption mercury cation. Ceram. Int. 2012, 38, 1687–1696. [Google Scholar] [CrossRef]
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.G.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Parolo, M.E.; Pettinari, G.R.; Musso, T.B.; Sánchez-Izquierdo, M.P.; Fernández, L.G. Characterization of organo-modified bentonite sorbents: The effect of modification conditions on adsorption performance. Appl. Surf. Sci. 2014, 320, 356–363. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Wu, P.; Dai, Y.; Tran, L.; Zhu, N.; Guo, C.; Sohoo, I. Enhancing the adsorption behavior and mechanism of Sr(II) by functionalized montmorillonite with different 3-aminopropyltriethoxysilane (APTES) ratios. RSC Adv. 2016, 6, 83288–83295. [Google Scholar] [CrossRef]
- Lima, V.V.C.; Dalla Nora, F.B.; Peres, E.C.; Reis, G.S.; Lima, É.C.; Oliveira, M.L.S.; Dotto, G.L. Synthesis and characterization of biopolymers functionalized with APTES (3–aminopropyltriethoxysilane) for the adsorption of sunset yellow dye. J. Environ. Chem. Eng. 2019, 7, 103410. [Google Scholar] [CrossRef]
- Thiebault, T.; Brendlé, J.; Augé, G.; Limousy, L. Cleaner synthesis of silylated clay minerals for the durable recovery of ions (Co2+ and Sr2+) from aqueous solutions. Ind. Eng. Chem. Res. 2020. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Calvé, S.L.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Serwicka, E.M. Laponite-derived porous clay heterostructures: II. FTIR study of the structure evolution. Microporous Mesoporous Mater. 2010, 127, 237–244. [Google Scholar] [CrossRef]
- Avila, L.R.; de Faria, E.H.; Ciuffi, K.J.; Nassar, E.J.; Calefi, P.S.; Vicente, M.A.; Trujillano, R. New synthesis strategies for effective functionalization of kaolinite and saponite with silylating agents. J. Colloid Interface Sci. 2010, 341, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Negrete Herrera, N.; Letoffe, J.-M.; Reymond, J.-P.; Bourgeat-Lami, E. Silylation of laponite clay particles with monofunctional and trifunctional vinyl alkoxysilanes. J. Mater. Chem. 2005, 15, 863–871. [Google Scholar] [CrossRef]
- Tao, Q.; Fang, Y.; Li, T.; Zhang, D.; Chen, M.; Ji, S.; He, H.; Komarneni, S.; Zhang, H.; Dong, Y.; et al. Silylation of saponite with 3-aminopropyltriethoxysilane. Appl. Clay Sci. 2016, 132–133, 133–139. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Z.; Liu, K.; Pan, W.-P.; Vaia, R.; Hunter, D.; Singh, A. Thermal characterization of organically modified montmorillonite. Thermochim. Acta 2001, 367–368, 339–350. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, P.; He, H.; Qin, Z.; Zhou, Q.; Zhu, J.; Liu, D. Effect of reaction temperature on grafting of γ-aminopropyl triethoxysilane (APTES) onto kaolinite. Appl. Clay Sci. 2012, 62–63, 8–14. [Google Scholar] [CrossRef]
- Fenero, M.; Palenzuela, J.; Azpitarte, I.; Knez, M.; Rodríguez, J.; Tena-Zaera, R. Laponite-based surfaces with holistic self-cleaning functionality by combining antistatics and omniphobicity. ACS Appl. Mater. Interfaces 2017, 9, 39078–39085. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Wang, J.; Baker, J.; Mathias, L.J. Synthesis and characterization of covalently functionalized laponite clay. Chem. Mater. 2005, 17, 3012–3018. [Google Scholar] [CrossRef]
- Brendlé, J. Organic–inorganic hybrids having a talc-like structure as suitable hosts to guest a wide range of species. Dalton Trans. 2018, 47, 2925–2932. [Google Scholar] [CrossRef]
- Borsacchi, S.; Geppi, M.; Ricci, L.; Ruggeri, G.; Veracini, C.A. Interactions at the surface of organophilic-modified laponites: A multinuclear solid-state NMR study. Langmuir 2007, 23, 3953–3960. [Google Scholar] [CrossRef]
- Negrete Herrera, N.; Letoffe, J.-M.; Putaux, J.-L.; David, L.; Bourgeat-Lami, E. Aqueous dispersions of silane-functionalized laponite clay platelets. A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir 2004, 20, 1564–1571. [Google Scholar] [CrossRef]
- Alba, M.D.; Becerro, A.I.; Castro, M.A.; Perdigón, A.C. High-resolution 1H MAS NMR spectra of 2:1 phyllosilicates. Chem. Commun. 2000, 37–38. [Google Scholar] [CrossRef]
- Kaya, A.; Yukselen, Y. Zeta potential of clay minerals and quartz contaminated by heavy metals. Can. Geotech. J. 2005, 42, 1280–1289. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Jatav, S.; Joshi, Y.M. Chemical stability of laponite in aqueous media. Appl. Clay Sci. 2014, 97–98, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Mongondry, P.; Nicolai, T.; Tassin, J.-F. Influence of pyrophosphate or polyethylene oxide on the aggregation and gelation of aqueous laponite dispersions. J. Colloid Interface Sci. 2004, 275, 191–196. [Google Scholar] [CrossRef]
- Janik, P.; Zawisza, B.; Talik, E.; Sitko, R. Selective adsorption and determination of hexavalent chromium ions using graphene oxide modified with amino silanes. Microchim. Acta 2018, 185, 117. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Oh, S.; Shin, W.S.; Choi, S.-J. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM). Desalination 2011, 276, 336–346. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Removal of cadmium(II) and zinc(II) metal ions from binary aqueous solution by rice husk ash. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 172–184. [Google Scholar] [CrossRef]
- Augé, G.; Brendlé, J.; Limousy, L.; Thiebault, T. Matériau Hybride Organique-Inorganique apte à Adsorber des Cations Métalliques. Patent Number FR3078268, 30 August 2019. [Google Scholar]





| Adsorbent | Q3/Q2 | T3/T2 | ΣT | ΣQ/ΣT |
|---|---|---|---|---|
| LAP | 10.2 | - | 0 | - |
| 1CEC | 14.9 | - | 5.1 | 18.6 |
| 2CEC | 20.9 | - | 7.2 | 12.8 |
| 3CEC | 24.9 | 0.31 | 10.2 | 8.7 |
| 4CEC | 29.4 | 0.48 | 14.1 | 5.3 |
| 10CEC | 51.2 | 0.92 | 16.1 | 5.2 |
| Langmuir | Freundlich | Dubinin–Radushkevich | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Load | Qmax mmol g−1 | KL L mmol−1 | ΔG° kJ mol−1 | r2 | n | KF L g−1 | r2 | Qm mmol g−1 | E kJ mol−1 | R2 | ||
| Cs+ | 1CEC | SS | 0.168 | 5.8 | −21.13 | 0.839 | 2.17 | 0.17 | 0.973 | 0.100 | 6.108 | 0.850 |
| Comp | 0.046 | 27.9 | −24.95 | 0.990 | 5.58 | 0.05 | 0.974 | 0.041 | 7.143 | 0.945 | ||
| 2CEC | SS | 0.165 | 4.9 | −20.75 | 0.800 | 2.08 | 0.16 | 0.943 | 0.093 | 6.063 | 0.804 | |
| Comp | 0.048 | 13.6 | −23.20 | 0.949 | 4.06 | 0.05 | 0.948 | 0.038 | 7.809 | 0.872 | ||
| 3CEC | SS | 0.214 | 3.0 | −19.53 | 0.628 | 1.83 | 0.17 | 0.926 | 0.094 | 5.590 | 0.866 | |
| Comp | 0.041 | 13.6 | −23.20 | 0.973 | 3.54 | 0.04 | 0.959 | 0.034 | 7.001 | 0.922 | ||
| 4CEC | SS | 0.184 | 6.0 | −21.22 | 0.826 | 2.16 | 0.18 | 0.975 | 0.110 | 6.154 | 0.861 | |
| Comp | 0.043 | 20.8 | −24.24 | 0.995 | 3.55 | 0.05 | 0.951 | 0.041 | 6.967 | 0.987 | ||
| 10CEC | SS | 0.156 | 3.9 | −20.14 | 0.684 | 1.80 | 0.14 | 0.969 | 0.064 | 6.537 | 0.831 | |
| Comp | 0.054 | 12.1 | −22.92 | 0.981 | 2.16 | 0.07 | 0.952 | 0.051 | 5.376 | 0.950 | ||
| Sr2+ | 1CEC | SS | 0.194 | 114.2 | −28.38 | 0.977 | 3.96 | 0.29 | 0.981 | 0.162 | 12.127 | 0.969 |
| Comp | 0.168 | 106.9 | −28.22 | 0.978 | 4.13 | 0.25 | 0.974 | 0.145 | 11.952 | 0.973 | ||
| 2CEC | SS | 0.201 | 115.8 | −28.42 | 0.977 | 4.01 | 0.30 | 0.988 | 0.162 | 12.500 | 0.967 | |
| Comp | 0.163 | 70.2 | −27.20 | 0.974 | 3.83 | 0.23 | 0.998 | 0.127 | 11.625 | 0.942 | ||
| 3CEC | SS | 0.234 | 118.5 | −28.47 | 0.970 | 3.93 | 0.35 | 0.994 | 0.175 | 12.700 | 0.952 | |
| Comp | 0.192 | 58.8 | −26.77 | 0.976 | 2.48 | 0.39 | 0.990 | 0.179 | 8.333 | 0.981 | ||
| 4CEC | SS | 0.281 | 105.8 | −28.20 | 0.966 | 2.61 | 0.65 | 0.986 | 0.255 | 9.449 | 0.974 | |
| Comp | 0.224 | 74.8 | −27.35 | 0.977 | 2.61 | 0.47 | 0.994 | 0.206 | 8.980 | 0.985 | ||
| 10CEC | SS | 0.313 | 124.2 | −28.59 | 0.957 | 1.71 | 1.35 | 0.868 | 0.336 | 8.771 | 0.990 | |
| Comp | 0.276 | 89.5 | −27.79 | 0.963 | 2.41 | 0.68 | 0.997 | 0.252 | 8.909 | 0.975 | ||
| Co2+ | 1CEC | SS | 0.445 | 204.1 | −29.80 | 0.999 | 3.78 | 0.58 | 0.856 | 0.447 | 10.314 | 0.968 |
| Comp | - | - | - | - | 1.93 | 2.47 | 0.613 | 0.941 | 7.332 | 0.708 | ||
| 2CEC | SS | 0.532 | 84.2 | −27.64 | 0.998 | 3.22 | 0.67 | 0.662 | 0.528 | 10.911 | 0.968 | |
| Comp | - | - | - | - | 1.57 | 5.57 | 0.662 | 1.375 | 6.836 | 0.746 | ||
| 3CEC | SS | 0.638 | 80.0 | −27.52 | 0.998 | 3.09 | 0.79 | 0.626 | 0.647 | 10.426 | 0.958 | |
| Comp | - | - | - | - | 0.54 | 19,256.42 | 0.984 | 48.526 | 4.603 | 0.993 | ||
| 4CEC | SS | 0.803 | 124.5 | −28.59 | 0.999 | 2.80 | 1.14 | 0.685 | 0.841 | 9.901 | 0.930 | |
| Comp | - | - | - | - | 0.54 | 21,504.12 | 0.986 | 50.164 | 4.593 | 0.994 | ||
| 10CEC | SS | 0.972 | 214.3 | −29.92 | 0.999 | 2.87 | 1.55 | 0.774 | 1.011 | 10.314 | 0.933 | |
| Comp | - | - | - | - | 0.94 | 523.06 | 0.993 | 7.514 | 6.682 | 0.995 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiebault, T.; Brendlé, J.; Augé, G.; Limousy, L. Laponites® for the Recovery of 133Cs, 59Co, and 88Sr from Aqueous Solutions and Subsequent Storage: Impact of Grafted Silane Loads. Materials 2020, 13, 572. https://doi.org/10.3390/ma13030572
Thiebault T, Brendlé J, Augé G, Limousy L. Laponites® for the Recovery of 133Cs, 59Co, and 88Sr from Aqueous Solutions and Subsequent Storage: Impact of Grafted Silane Loads. Materials. 2020; 13(3):572. https://doi.org/10.3390/ma13030572
Chicago/Turabian StyleThiebault, Thomas, Jocelyne Brendlé, Grégoire Augé, and Lionel Limousy. 2020. "Laponites® for the Recovery of 133Cs, 59Co, and 88Sr from Aqueous Solutions and Subsequent Storage: Impact of Grafted Silane Loads" Materials 13, no. 3: 572. https://doi.org/10.3390/ma13030572