TiO2 and N-TiO2 Sepiolite and Zeolite Composites for Photocatalytic Removal of Ofloxacin from Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Morphostructural Characterization of the Composite Catalysts
2.2.1. Zeolite–TiO2 Synthesis via Sol–Gel Route
2.2.2. Sepiolite–TiO2 Synthesis via Sol–Gel Route
2.2.3. Morphostructural Characterization
2.3. Adsorption Experiments
2.4. Irradiation Experiments
2.5. Analytical Determinations
3. Results and Discussion
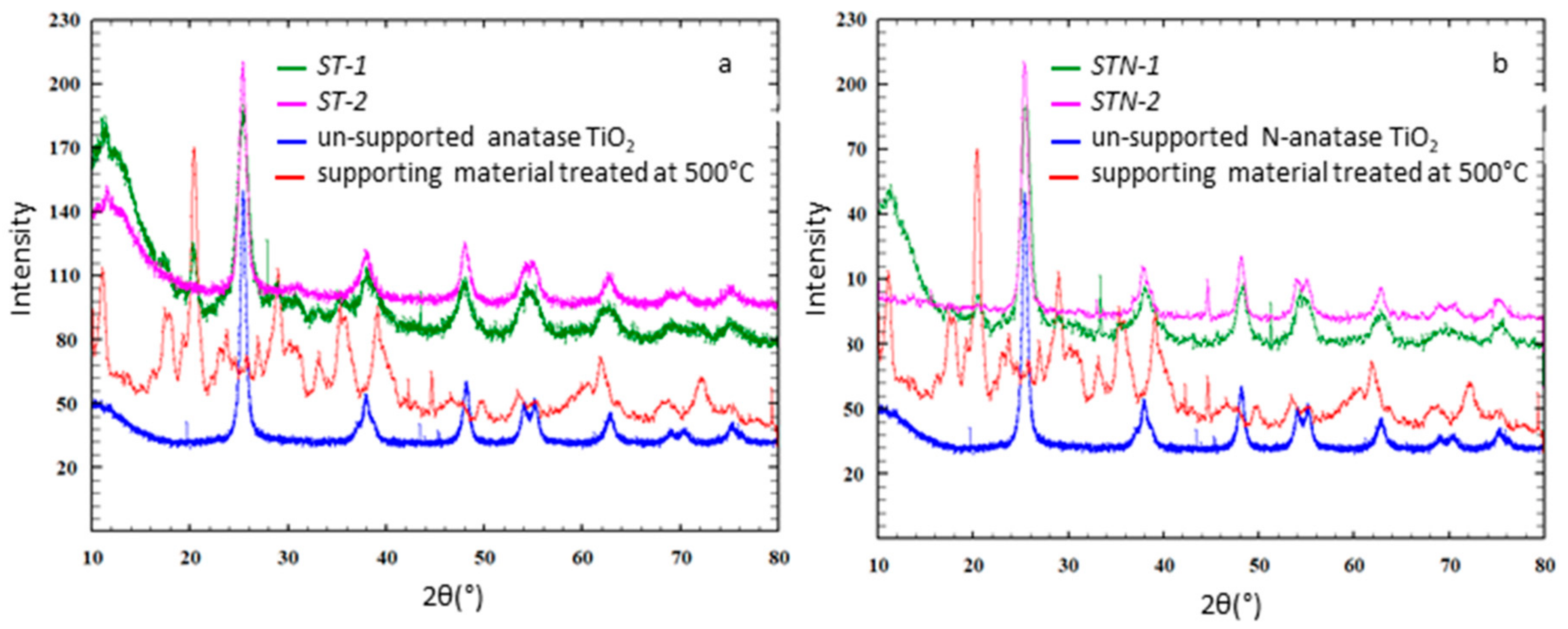
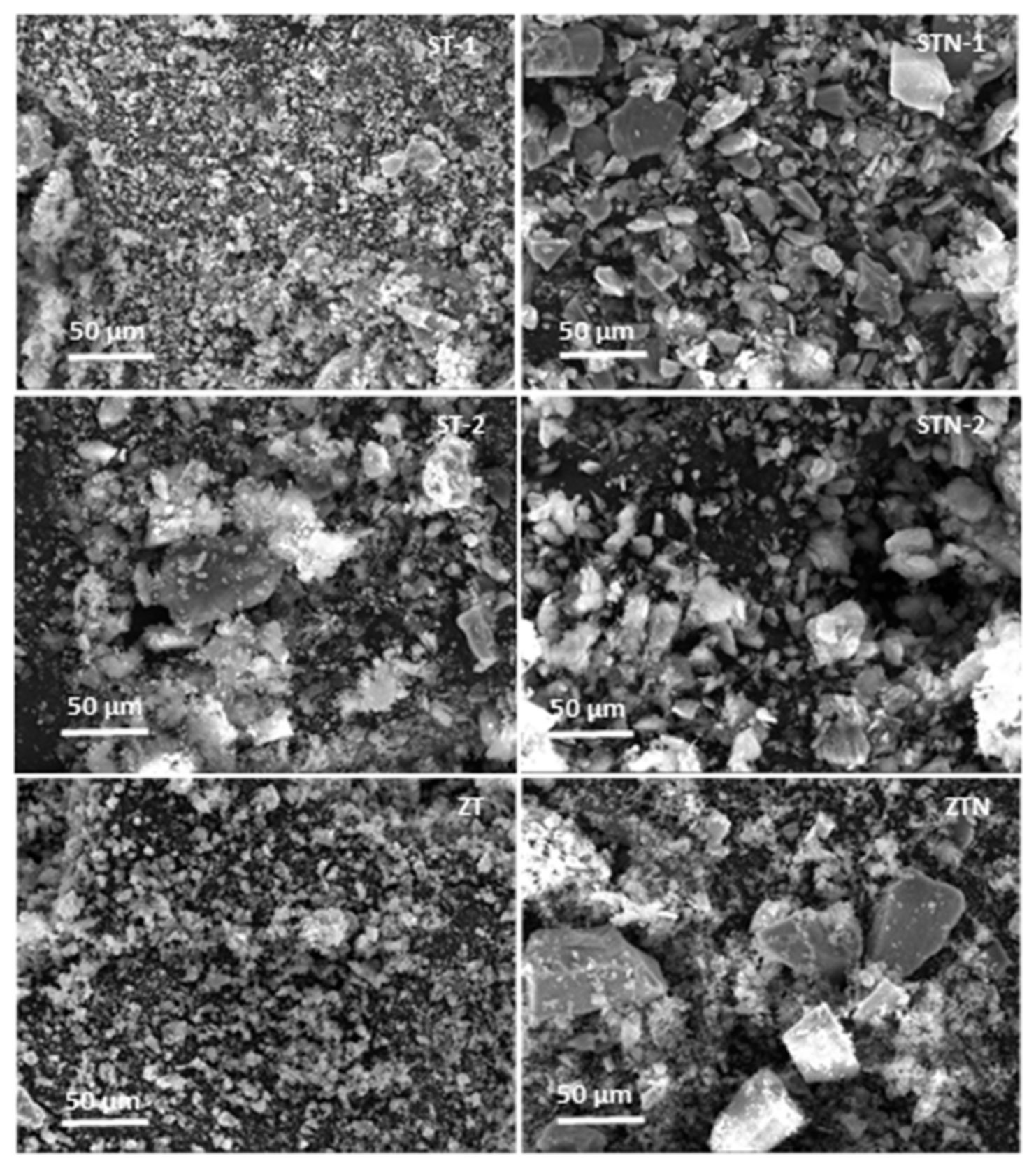
3.1. Morphostructural Characterization
3.2. Adsorption Isotherms
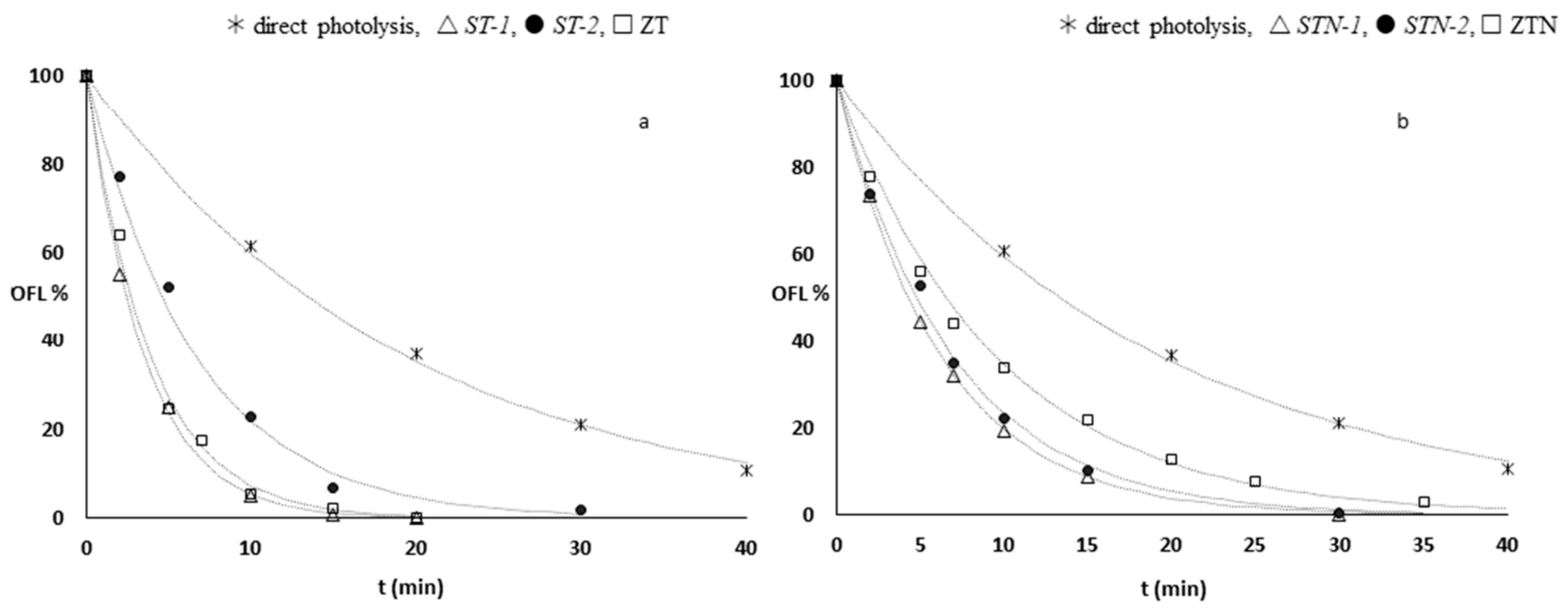
3.3. Photodegradation Kinetics
3.4. Photoproducts Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Data on occurrence and fate of emerging contaminants in a urbanised area. Data Brief 2018, 17, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- European Union Strategic Approach to Pharmaceuticals in the Environment–Communication from the Commission to the European Parliament, the Council and the European Economic and Social Commettee. Available online: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:52019DC0128 (accessed on 18 December 2019).
- Menz, J.; Baginska, E.; Arrhenius, Å.; Haiß, A.; Backhaus, T.; Kümmerer, K. Antimicrobial activity of pharmaceutical cocktails in sewage treatment plant effluent–An experimental and predictive approach to mixture risk assessment. Environ. Pollut. 2017, 231, 1507–1517. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Albini, A.; Chen, C.; Sturini, M. Use the drugs and respect the environment. Curr. Opin. Green Sustain. Chem. 2017, 6, A1–A4. [Google Scholar] [CrossRef]
- Mahmoud, W.M.; Rastogi, T.; Kümmerer, K. Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Calvete, M.J.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Backhaus, T.; Karlsson, M. Screening level mixture risk assessment of pharmaceuticals in STP effluents. Water Res. 2014, 49, 157–165. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Bish, D.L.; Ming, D.W. (Eds.) Natural zeolites: Occurrence, properties, applications. In Reviews in Mineralogy and Geochemistry; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2000; Volume 45. [Google Scholar]
- Murray, H.H. Applied clay mineralogy today and tomorrow. Clay Miner. 1999, 34, 39–49. [Google Scholar] [CrossRef]
- Maraschi, F.; Sturini, M.; Speltini, A.; Pretali, L.; Profumo, A.; Pastorello, A.; Kumar, V.; Ferretti, M.; Caratto, V. TiO2-modified zeolites for fluoroquinolones removal from wastewaters and reuse after solar light regeneration. J. Environ. Chem. Eng. 2014, 2, 2170–2176. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Tarantino, S.; Gualtieri, A.F.; Zema, M. Removal of fluoroquinolone contaminants from environmental waters on sepiolite and its photo-induced regeneration. Chemosphere 2016, 150, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Stemmler, E.A.; Cho, H.J.; Fan, W.; Leblanc, L.A.; Patterson, H.H.; Amirbahman, A. Photocatalytic degradation of 17α-ethinylestradiol (EE2) in the presence of TiO2-doped zeolite. J. Hazard. Mater. 2014, 279, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Kockler, J.; Motti, C.A.; Glass, B.D.; Oelgemöller, M.; Oelgemoeller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B Environ. 2015, 166, 45–55. [Google Scholar] [CrossRef]
- An, Y.; De Ridder, D.J.; Zhao, C.; Schoutteten, K.; Bussche, J.V.; Zheng, H.; Chen, G.; Vanhaecke, L. Adsorption and photocatalytic degradation of pharmaceuticals and pesticides by carbon doped-TiO2 coated on zeolites under solar light irradiation. Water Sci. Technol. 2016, 73, 2868–2881. [Google Scholar] [CrossRef] [Green Version]
- Eskandarian, M.R.; Fazli, M.; Rasoulifard, M.H.; Choi, H. Decomposition of organic chemicals by zeolite-TiO2 nanocomposite supported onto low density polyethylene film under UV-LED powered by solar radiation. Appl. Catal. B Environ. 2016, 183, 407–416. [Google Scholar] [CrossRef]
- Ihos, M.; Lazau, C.; Manea, F.; Andres, L.; Pode, R. Diclofenac degradation by photocatalytically-assisted electrochemical method. J. Environ. Prot. Ecol. 2016, 17, 307–314. [Google Scholar]
- Kovacic, M.; Salaeh, S.; Kusic, H.; Suligoj, A.; Kete, M.; Fanetti, M.; Stangar, U.L.; Dionysiou, D.D.; Bozic, A.L. Solar-driven photocatalytic treatment of diclofenac using immobilized TiO2-based zeolite composites. Environ. Sci. Pollut. Res. 2016, 23, 17982–17994. [Google Scholar] [CrossRef]
- Malakootian, M.; Pourshaban-Mazandarani, M.; Hossaini, H.; Ehrampoush, M.H. Preparation and characterization of TiO2 incorporated 13X molecular sieves for photocatalytic removal of acetaminophen from aqueous solutions. Process. Saf. Environ. Prot. 2016, 104, 334–345. [Google Scholar] [CrossRef]
- Salaeh, S.; Perisic, D.J.; Biosic, M.; Kusic, H.; Babic, S.; Stangar, U.L.; Dionysiou, D.D.; Bozic, A.L. Diclofenac removal by simulated solar assisted photocatalysis using TiO2-based zeolite catalyst; mechanisms, pathways and environmental aspects. Chem. Eng. J. 2016, 304, 289–302. [Google Scholar] [CrossRef]
- Gou, J.; Ma, Q.; Deng, X.; Cui, Y.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q. Fabrication of Ag2O/TiO2-Zeolite composite and its enhanced solar light photocatalytic performance and mechanism for degradation of norfloxacin. Chem. Eng. J. 2017, 308, 818–826. [Google Scholar] [CrossRef]
- Hassaninejad-Darzi, S.K.; Shajie, F. Simultaneous determination of acetaminophen, pramipexole and carbamazepine by ZSM-5 nanozeolite and TiO2 nanoparticles modified carbon paste electrode. Mater. Sci. Eng. C 2018, 91, 64–77. [Google Scholar] [CrossRef]
- Ihos, M.; Bogatu, C.; Lazau, C.; Manea, F.; Pode, R. Pharmaceutically active compounds degradation using doped TiO2 functionalized zeolite photocatalyst. Rev. Chim. 2018, 69, 34–37. [Google Scholar]
- Kolosov, P.; Peyot, M.-L.; Yargeau, V. Novel materials for catalytic ozonation of wastewater for disinfection and removal of micropollutants. Sci. Total Environ. 2018, 644, 1207–1218. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Fasani, E.; Albini, A. Photochemical Degradation of Marbofloxacin and Enrofloxacin in Natural Waters. Environ. Sci. Technol. 2010, 44, 4564–4569. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.A.; Fasani, E.; Albini, A. Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl. Catal. B Environ. 2012, 119, 32–39. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Profumo, A.; Fasani, E.; Albini, A.; Migliavacca, R.; Nucleo, E. Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water Res. 2012, 46, 5575–5582. [Google Scholar] [CrossRef]
- Caratto, V.; Setti, L.; Campodonico, S.; Carnasciali, M.M.; Botter, R.; Ferretti, M. Synthesis and characterization of nitrogen-doped TiO2 nanoparticles prepared by sol–gel method. J. Sol Gel Sci. Technol. 2012, 63, 16–22. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why Dyes Should Not Be Used to Test the Photocatalytic Activity of Semiconductor Oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef] [PubMed]
- Balcerski, W.; Ryu, S.Y.; Hoffmann, M.R. Visible-Light Photoactivity of Nitrogen-Doped TiO2: Photo-oxidation of HCO2H to CO2 and H2O. J. Phys. Chem. C 2007, 111, 15357–15362. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced photocatalytic activity of TiO2/zeolite composite for abatement of pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Barreca, S.; Orecchio, S.; Pace, A. The effect of montmorillonite clay in alginate gel beads for polychlorinated biphenyl adsorption: Isothermal and kinetic studies. Appl. Clay Sci. 2014, 99, 220–228. [Google Scholar] [CrossRef]
- Rivagli, E.; Pastorello, A.; Sturini, M.; Maraschi, F.; Speltini, A.; Zampori, L.; Setti, M.; Malavasi, L.; Profumo, A. Clay minerals for adsorption of veterinary FQs: Behavior and modeling. J. Environ. Chem. Eng. 2014, 2, 738–744. [Google Scholar] [CrossRef]
- Diwald, O.; Thompson, T.L.; Zubkov, T.; Walck, S.D.; Yates, J.T. Photochemical Activity of Nitrogen-Doped Rutile TiO2 (110) in Visible Light. J. Phys. Chem. B 2004, 108, 6004–6008. [Google Scholar] [CrossRef]
- Liu, G.; Li, F.; Wang, D.W.; Tang, D.M.; Liu, C.; Ma, X.; Lu, G.Q.; Cheng, H.M. Electron field emission of a nitrogen-doped TiO2 nanotube array. Nanotechnology 2008, 19, 025606. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Pan, J.; Lu, G.Q., (Max); Cheng, H.-M. Visible Light Responsive Nitrogen Doped Anatase TiO2 Sheets with Dominant {001} Facets Derived from TiN. J. Am. Chem. Soc. 2009, 131, 12868–12869. [Google Scholar] [CrossRef]
- Karaoğlu, M.H.; Uğurlu, M. Studies on UV/NaOCl/TiO2/Sep photocatalysed degradation of Reactive Red 195. J. Hazard. Mater. 2010, 174, 864–871. [Google Scholar] [CrossRef]
- Albini, A.; Monti, S. Photophysics and photochemistry of fluoroquinolones. Chem. Soc. Rev. 2003, 32, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gao, N.; Yang, Y.; Zhang, Y. Kinetics and transformation pathways on oxidation of fluoroquinolones with thermally activated persulfate. Chem. Eng. J. 2016, 292, 82–91. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Pretali, L.; Profumo, A.; Fasani, E.; Albini, A. Environmental photochemistry of fluoroquinolones in soil and in aqueous soil suspensions under solar light. Environ. Sci. Pollut. Res. 2014, 21, 13215–13221. [Google Scholar] [CrossRef] [PubMed]
- Sturini, M.; Speltini, A.; Maraschi, F.; Vinci, G.; Profumo, A.; Pretali, L.; Albini, A.; Malavasi, L. g-C3N4-promoted degradation of Ofloxacin antibiotic in natural waters under simulated sunlight. Environ. Sci. Pollut. Res. 2017, 24, 4153–4161. [Google Scholar] [CrossRef]



| Photocatalysts | Type of Support | Crystallization Temperature (°C) | Treatment Time (h) |
|---|---|---|---|
| ST-1 | Sepiolite | 500 | 1 |
| STN-11 | Sepiolite | 500 | 1 |
| ST-22 | Sepiolite | 500 | 1 |
| STN-21,2 | Sepiolite | 500 | 1 |
| ZT | Zeolite | 350 | 1 |
| ZTN1 | Zeolite | 350 | 1 |
| TiO2 Anatase | — | 350 | 1 |
| Photocatalysts | BET (m2 g−1) | Band Gap (eV) |
|---|---|---|
| ST-1 | 262 | 3.18 |
| STN-1 | 166 | 3.03 |
| ST-2 | 159 | 3.20 |
| STN-2 | 153 | 3.18 |
| ZT | 228 | 3.16 |
| ZTN | 108 | 3.03 |
| TiO2 Anatase | 120 | 3.21 |
| Photocatalysts | Csmax (mg g−1) | F (mg L−1) | A | R2 |
|---|---|---|---|---|
| ST-1 | 34 (2) | 135 (8) | 0.027 (4) | 0.98 |
| STN-1 | 30 (2) | 122 (14) | 0.019 (3) | 0.97 |
| ST-2 | 20 (1) | 62 (3) | 0.029 (3) | 0.99 |
| STN-2 | 23 (1) | 82 (6) | 0.023 (3) | 0.98 |
| ZT | 16 (1) | 84 (4) | 0.05 (1) | 0.98 |
| ZTN | 14 (1) | 66 (5) | 0.06 (1) | 0.97 |
| TiO2 Anatase | 19 (1) | 118 (9) | 0.025 (4) | 0.98 |
| Photocatalysts | % OFL (20 min, Dark) | kdeg (min−1) |
|---|---|---|
| ST-1 | 17 | 0.289 (1) |
| STN-1 | 22 | 0.162 (2) |
| ST-2 | 43 | 0.145 (6) |
| STN-2 | 43 | 0.131 (8) |
| ZT | 25 | 0.26 (1) |
| ZTN | 31 | 0.106 (3) |
| TiO2 Anatase | 20 | 0.31 (1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturini, M.; Maraschi, F.; Cantalupi, A.; Pretali, L.; Nicolis, S.; Dondi, D.; Profumo, A.; Caratto, V.; Sanguineti, E.; Ferretti, M.; et al. TiO2 and N-TiO2 Sepiolite and Zeolite Composites for Photocatalytic Removal of Ofloxacin from Polluted Water. Materials 2020, 13, 537. https://doi.org/10.3390/ma13030537
Sturini M, Maraschi F, Cantalupi A, Pretali L, Nicolis S, Dondi D, Profumo A, Caratto V, Sanguineti E, Ferretti M, et al. TiO2 and N-TiO2 Sepiolite and Zeolite Composites for Photocatalytic Removal of Ofloxacin from Polluted Water. Materials. 2020; 13(3):537. https://doi.org/10.3390/ma13030537
Chicago/Turabian StyleSturini, Michela, Federica Maraschi, Alice Cantalupi, Luca Pretali, Stefania Nicolis, Daniele Dondi, Antonella Profumo, Valentina Caratto, Elisa Sanguineti, Maurizio Ferretti, and et al. 2020. "TiO2 and N-TiO2 Sepiolite and Zeolite Composites for Photocatalytic Removal of Ofloxacin from Polluted Water" Materials 13, no. 3: 537. https://doi.org/10.3390/ma13030537