A New Preparation Method of Cement with Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimens Preparation
2.3. The Compressive and the Flexural Strength Measurements
2.4. The Initial and the Final Setting Time (Vicat Needle Test)
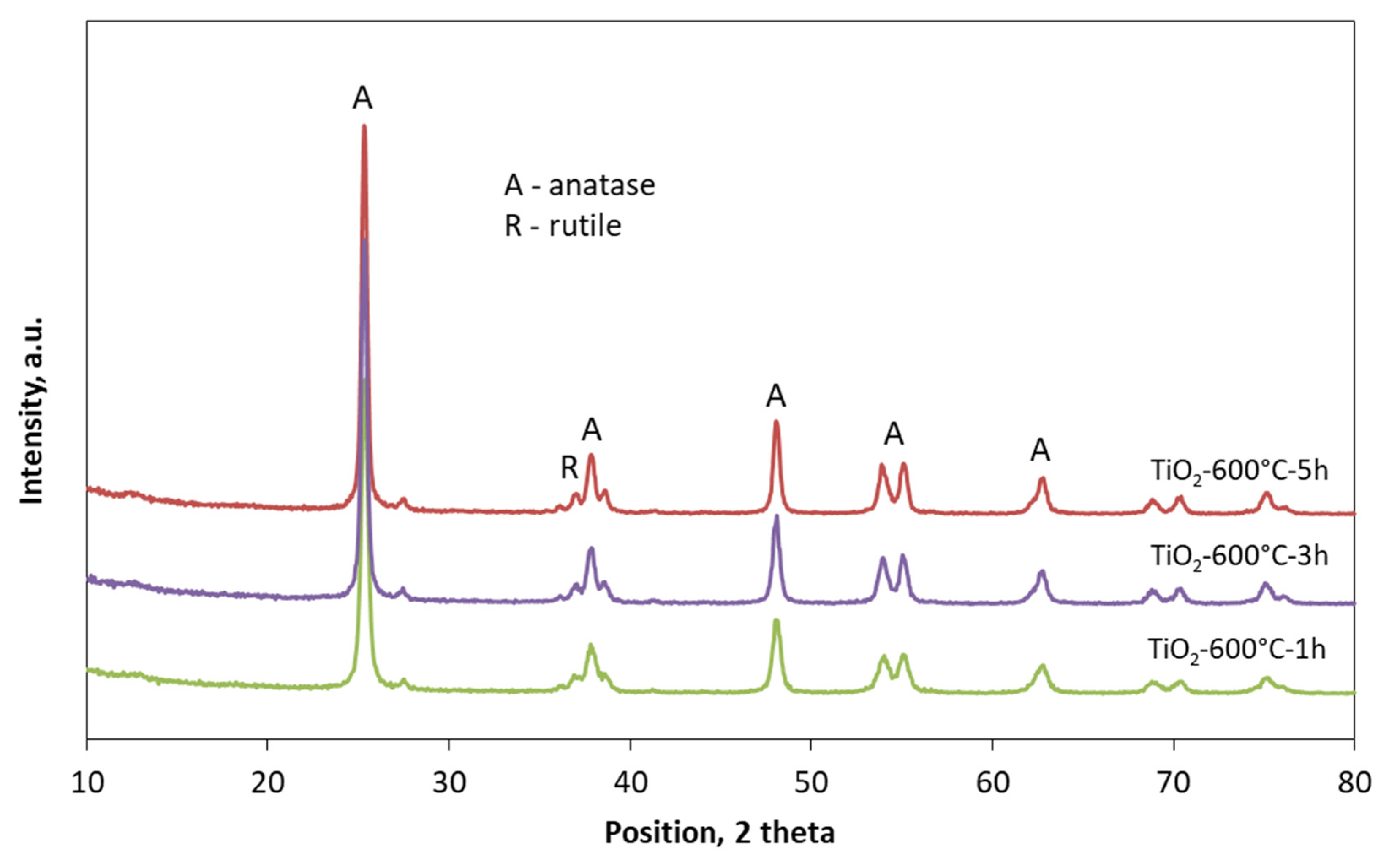
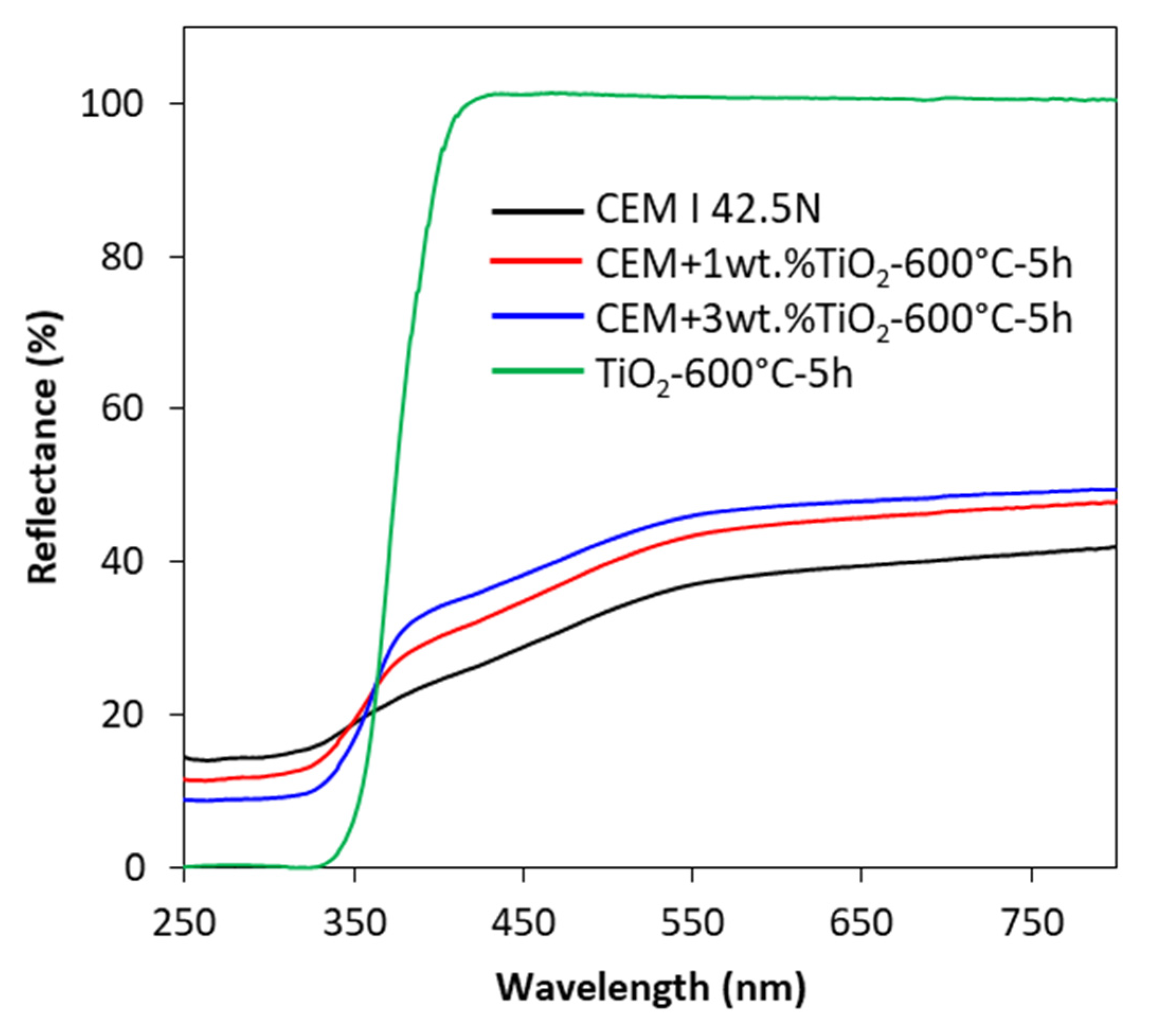
2.5. The Crystalline Structure, UV-Vis/DR Measurments, and Sulphur Content
2.6. NOx Decomposition
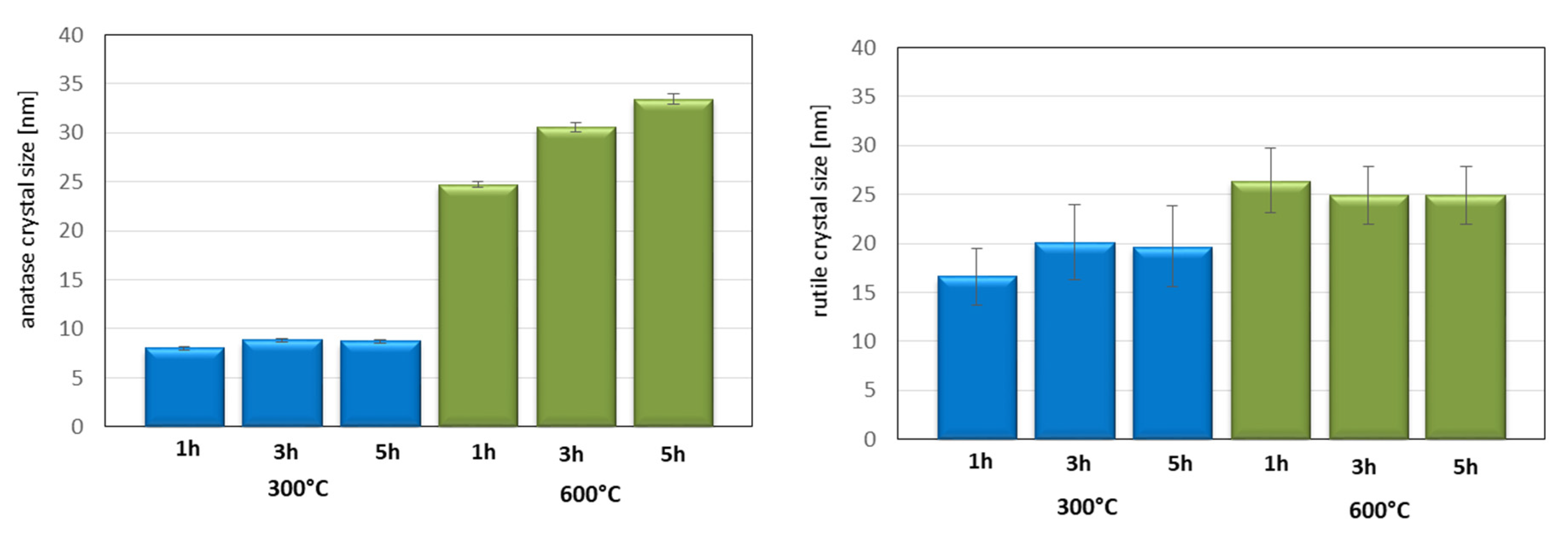
3. Results and Discussion
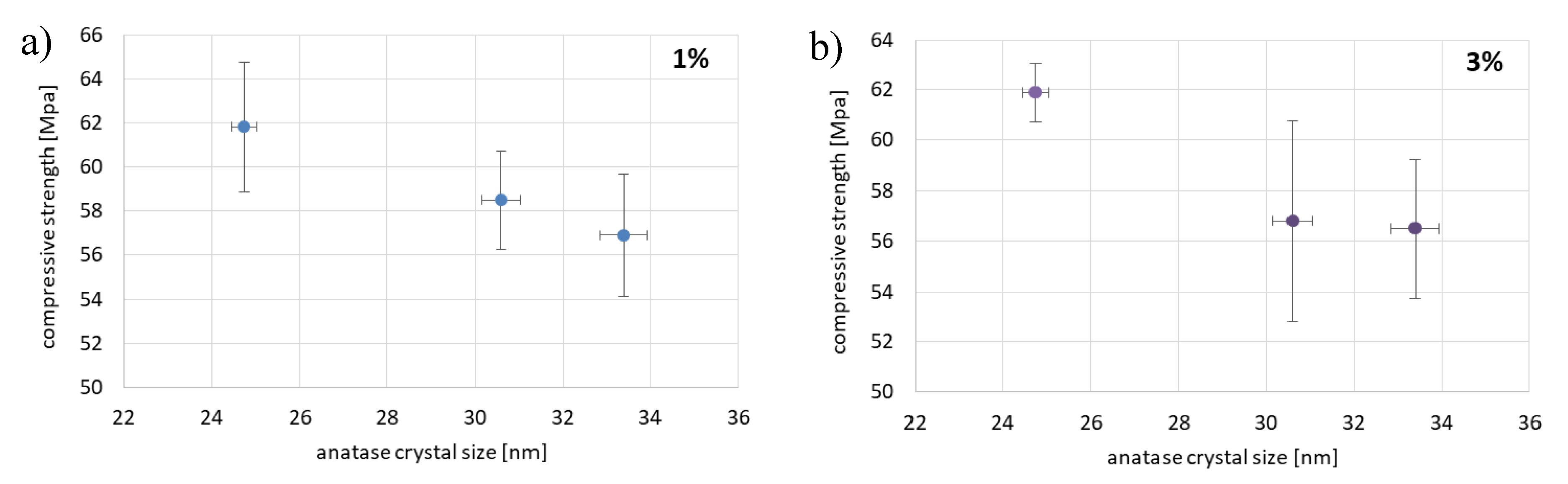
3.1. The Compressive and the Flexural Strength
3.2. The Initial and the Final Setting Time
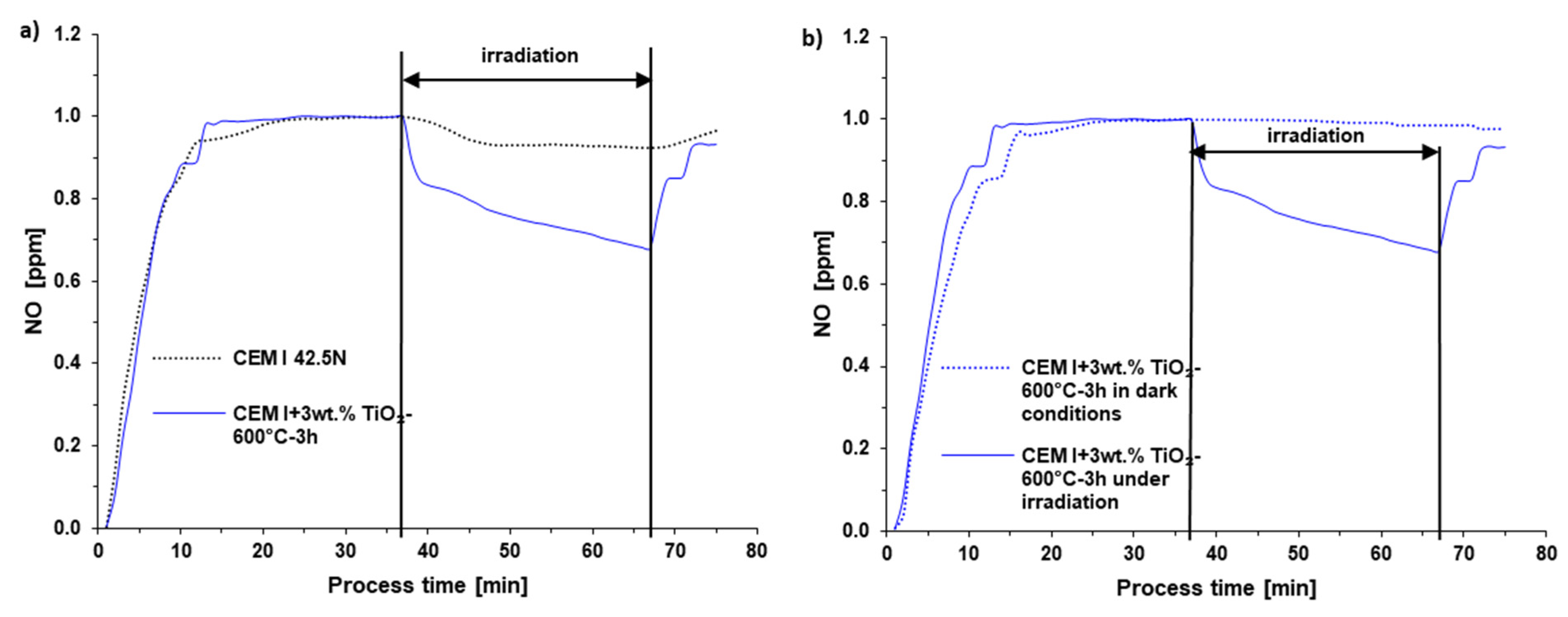
3.3. NOx Decomposition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamidi, F.; Aslani, F. TiO2-based photocatalytic cementitious composites: Materials, properties, influential parameters, and assessment techniques. Nanomaterials 2019, 9, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Viles, H.A. Efficiency and durability of a self-cleaning coating on concrete and stones under both natural and artificial ageing trials. Appl. Surf. Sci. 2018, 433, 312–320. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol-gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, Z.; Zhu, Y. Highly efficient visible photocatalytic disinfection and degradation performances of microtubular nanoporous g-C3N4 via hierarchical construction and defects engineering. J. Mater. Sci Technol. 2020, 49, 133–143. [Google Scholar] [CrossRef]
- Kaja, A.M.; Brouwers, H.J.H.; Yu, Q.L. NOx degradation by photocatalytic mortars: The underlying role of the CH and C-S-H carbonation. Cem. Concr. Res. 2019, 125, 105805–105816. [Google Scholar] [CrossRef]
- Baltes, L.; Patachia, S.; Tierean, M.; Ekincioglu, O.; Ozkul, H.M. Photoactive glazed polymer-cement composite. Appl. Surf. Sci. 2018, 438, 84–95. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Materiomics 2017, 3, 3–16. [Google Scholar] [CrossRef]
- Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Grande, M.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic BiOX mortars under visible light irradiation: Compatibility, NOx efficiency and nitrate selectivity. Catalysts 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Devahasdin, S.; Fan, Ch. Jr.; Li, K.; Chen, D.H. TiO2 photocatalytic oxidation of nitric oxide: Transient behavior and reaction kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Balbuena, J.; Cruz-Yusta, M.; Sánchez, L.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytic NOx abatement by calcium aluminate cements modified with TiO2: Improved NO2 conversion. Cem. Concr. Res. 2015, 70, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Shelimov, B.N.; Tolkachev, N.N.; Tkachenko, O.P.; Baeva, G.N.; Klementiev, K.V.; Stakheev, A.Y.; Kazansky, V.B. Enhacement effect of TiO2 dispersion over alumina on the photocatalytic removal of NOx admixtures from O2-N2 flow. J. Photochem. Photobiol. A Chem. 2008, 195, 81–88. [Google Scholar] [CrossRef]
- Araña, J.; Garzón Sousa, D.; González Díaz, O.; Pulido Melián, E.; Doña Rodríguez, J.M. Effect of NO2 and NO3−/HNO3 adsorption on NO photocatalytic conversion. Appl. Catal. B Environ. 2019, 244, 660–670. [Google Scholar] [CrossRef]
- Makul, N. Modern sustainable cement and concrete composites: Review of current status, challenges and guidelines. Sustain. Mater. Technol. 2020, 25, 155–170. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Mahy, J.G.; Paez, C.A.; Hollevoet, J.; Courard, L.; Boonen, E.; Lambert, S.D. Durable photocatalytic thin coatings for road applications. Constr. Build. Mater. 2019, 215, 422–434. [Google Scholar] [CrossRef]
- García, L.D.; Pastor, J.M.; Peña, J. Self-cleaning and depolluting glass reinforced concrete panels: Fabrication, optimization and durability evaluation. Constr. Build. Mater. 2018, 162, 9–19. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M.; Cantú-Castro, L.V.F. Self-cleaning coatings based on fly ash and bismuth-photocatalysts: Bi2O3, Bi2O2CO3, BiOI, BiVO4, BiPO4. Constr. Build. Mater. 2019, 220, 206–213. [Google Scholar] [CrossRef]
- Feng, S.; Liu, F.; Fu, X.; Peng, X.; Zhu, J.; Zeng, Q. Photocatalytic performances and durability of TiO2/cement composites prepared by a smear method for organic wastewater degradation. Ceram. Int. 2019, 45, 23061–23069. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Stephan, D.; Huang, S.; Zhang, L.; Yang, P.; Cheng, X. SiO2/TiO2 composite powders deposited on cement-based materials: Rhodamine B removal and the bonding mechanism. Constr. Build. Mater. 2020, 241, 118–124. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z.; Hou, P.; Yang, P.; Cheng, X.; Huang, S. Rhodamine B removal of TiO2@SiO2 core-shell nanocomposites coated to buildings. Crystals 2020, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, C.; Valle, A.; Castellote, M.; Badamonde, A.; Faraldos, M. TiO2 and TiO2-SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B Environ. 2015, 178, 155–164. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.J.; Santana Rodríguez, R.; Darias, R.; González Díaz, O.; Pérez Luzardo, J.M.; Doña Rodríguez, J.M.; Pulido Melián, E. Effect of TiO2 addition on mortars: Characterization and photoactivity. Appl. Sci. 2019, 9, 2598. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Yang, J.; Yang, E.H. Self-cleaning engineered cementitious composites. Cem. Concr. Compos. 2015, 64, 74–83. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insight into self-cleaning and depolluting chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Tyukavkina, V.V.; Gerasimova, L.G.; Semushin, V.V. Properties of compositions based on cement and modified nanodispersed titanium dioxide. Inorg. Mater. 2019, 10, 122–126. [Google Scholar] [CrossRef]
- Zając, K.; Janus, M.; Kuźmiński, K.; Morawski, A.W. Preparation of gypsum building materials with photocatalytic properties. A strong emphasis on waste gypsum from flue gas desulfurization. Przem. Chem. 2016, 95, 2222–2226. [Google Scholar]
- Janus, M.; Zając, K.; Ehm, C.; Stephan, D. Fast Method for Testing the Photocatalytic Performance of Modified Gypsum. Catalysts 2019, 9, 693. [Google Scholar] [CrossRef] [Green Version]
- Janus, M.; Mądraszewski, Sz.; Zając, K.; Kusiak-Nejman, E.; Morawski, A.; Stepan, D. Photocatalytic activity and mechanical properties of cements modified with TiO2/N. Materials 2020, 12, 3756. [Google Scholar] [CrossRef] [Green Version]
- Gies, A.; Knöfel, D. Influence of sulfur on the composition of belite-rich clinkers and the technological properties of the resulting cements. Cem. Concr. Res. 1987, 17, 317–328. [Google Scholar] [CrossRef]
- Yamashita, M.; Harada, T.; Sakai, E.; Tsuchiya, K. Influence of sulfur trioxide in clinker on the hydration heat and physical properties of Portland cement. Constr. Build. Mater. 2020, 250, 118844. [Google Scholar] [CrossRef]
- Xu, M.; Bao, Y.; Wu, T.; Xia, T.; Clark, H.L.; Shi, H.; Li, V.C. Influence of TiO2 incorporation methods on NOx abatement in Engineered Cementitious Composites. Const. Build. Mater. 2019, 221, 375–383. [Google Scholar] [CrossRef]


| Materials | Mass of Used Materials [g] | |
|---|---|---|
| 1% | 3% | |
| CEM I 42.5 N | 444.5 | 436.5 |
| TiO2 1 | 4.5 | 13.5 |
| Standard Sand | 1350 | 1350 |
| water | 180 | 180 |
| Samples | The Initial Setting Time [min] | The Final Setting Time [min] |
|---|---|---|
| CEM I 42.5 N | 218 | 305 |
| CEM + 1 wt.%TiO2-300 °C-1 h | 244 | 354 |
| CEM + 1 wt.%TiO2-300 °C-3 h | 259 | 375 |
| CEM + 1 wt.%TiO2-300 °C-5 h | 244 | 332 |
| CEM + 3 wt.%TiO2-300 °C-1 h | 217 | 296 |
| CEM + 3 wt.%TiO2-300 °C-3 h | 220 | 292 |
| CEM + 3 wt.%TiO2-300 °C-5 h | 203 | 289 |
| CEM + 1 wt.%TiO2-600 °C-1 h | 246 | 379 |
| CEM + 1 wt.%TiO2-600 °C-3 h | 273 | 375 |
| CEM + 1 wt.%TiO2-600 °C-5 h | 263 | 384 |
| CEM + 3 wt.%TiO2-600 °C-1 h | 232 | 315 |
| CEM + 3 wt.%TiO2-600 °C-3 h | 245 | 344 |
| CEM + 3 wt.%TiO2-600 °C-5 h | 255 | 366 |
| Samples | Mass% of Sulfur |
|---|---|
| TiO2 | 2.48 |
| TiO2-300 °C-1 h | 2.33 |
| TiO2-300 °C-3 h | 2.30 |
| TiO2-300 °C-5 h | 2.32 |
| TiO2-600 °C-1 h | 1.09 |
| TiO2-600 °C-3 h | 0.76 |
| TiO2-600 °C-5 h | 0.67 |
| Samples | NO (II) Removal [%] |
|---|---|
| CEM I 42.5N | 6.3 |
| CEM + 1 wt.%TiO2-300 °C-1 h | 6.2 |
| CEM + 1 wt.%TiO2-300 °C-3 h | 9.1 |
| CEM + 1 wt.%TiO2-300 °C-5 h | 10.4 |
| CEM + 3 wt.%TiO2-300 °C-1 h | 12.8 |
| CEM + 3 wt.%TiO2-300 °C-3 h | 10.9 |
| CEM + 3 wt.%TiO2-300 °C-5 h | 17.4 |
| CEM + 1 wt.%TiO2-600 °C-1 h | 10.3 |
| CEM + 1 wt.%TiO2-600 °C-3 h | 13.7 |
| CEM + 1 wt.%TiO2-600 °C-5 h | 15.6 |
| CEM + 3 wt.%TiO2-600 °C-1 h | 15.8 |
| CEM + 3 wt.%TiO2-600 °C-3 h | 25.3 |
| CEM + 3 wt.%TiO2-600 °C-5 h | 16.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janus, M.; Mądraszewski, S.; Zając, K.; Kusiak-Nejman, E. A New Preparation Method of Cement with Photocatalytic Activity. Materials 2020, 13, 5540. https://doi.org/10.3390/ma13235540
Janus M, Mądraszewski S, Zając K, Kusiak-Nejman E. A New Preparation Method of Cement with Photocatalytic Activity. Materials. 2020; 13(23):5540. https://doi.org/10.3390/ma13235540
Chicago/Turabian StyleJanus, Magdalena, Szymon Mądraszewski, Kamila Zając, and Ewelina Kusiak-Nejman. 2020. "A New Preparation Method of Cement with Photocatalytic Activity" Materials 13, no. 23: 5540. https://doi.org/10.3390/ma13235540





