Electrodeposition of Aluminum Coatings from AlCl3-NaCl-KCl Molten Salts with TMACl and NaI Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrolytes Preparation
2.2. Electrolyte Volatility Tests
2.3. Electrochemical Tests
2.4. Electrodeposition
2.5. Characterization of the Al Coatings
3. Results and Discussion
3.1. Electrolyte Volatility
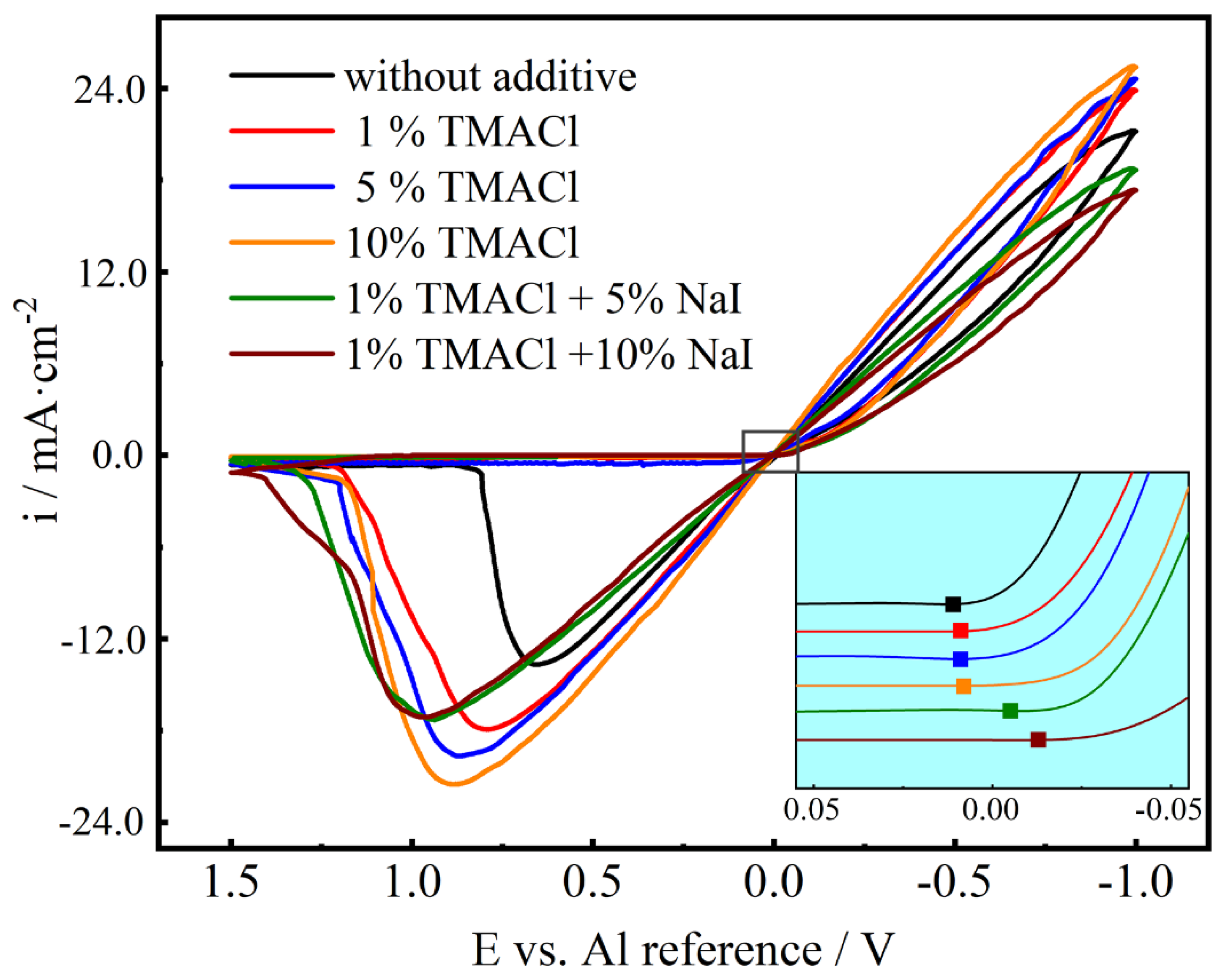
3.2. Voltammetric Behavior
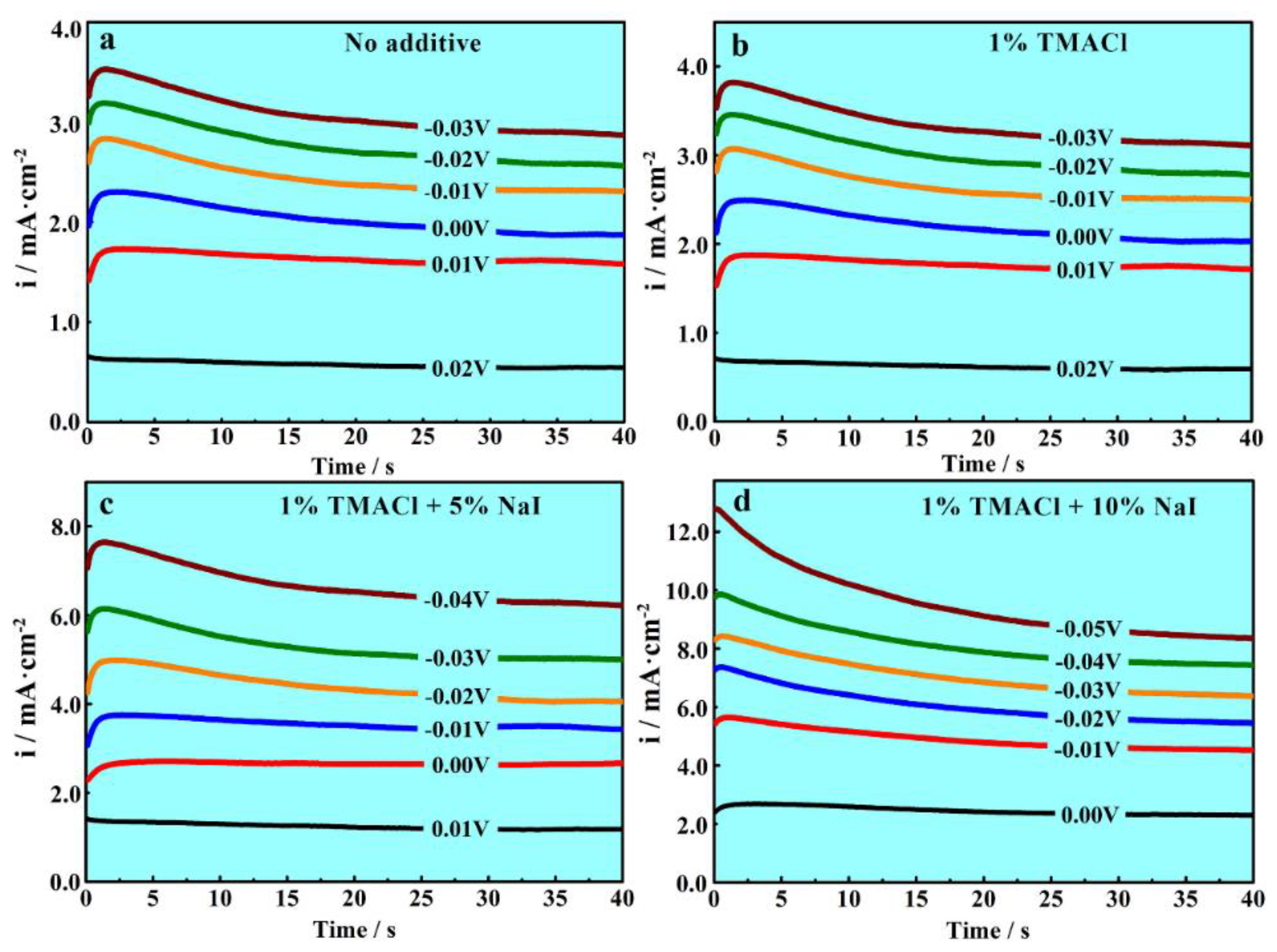
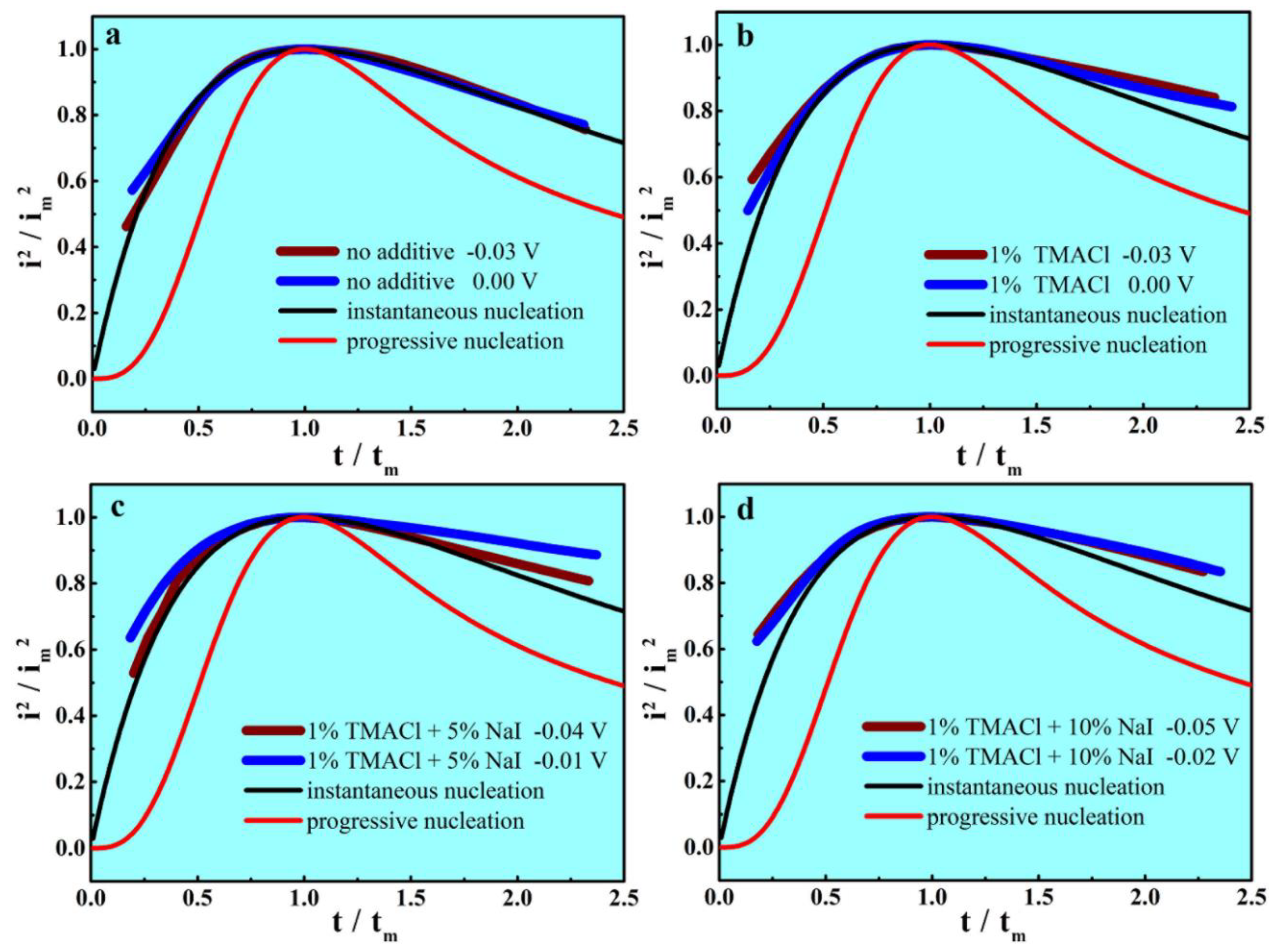
3.3. Chronoamperometric Investigations
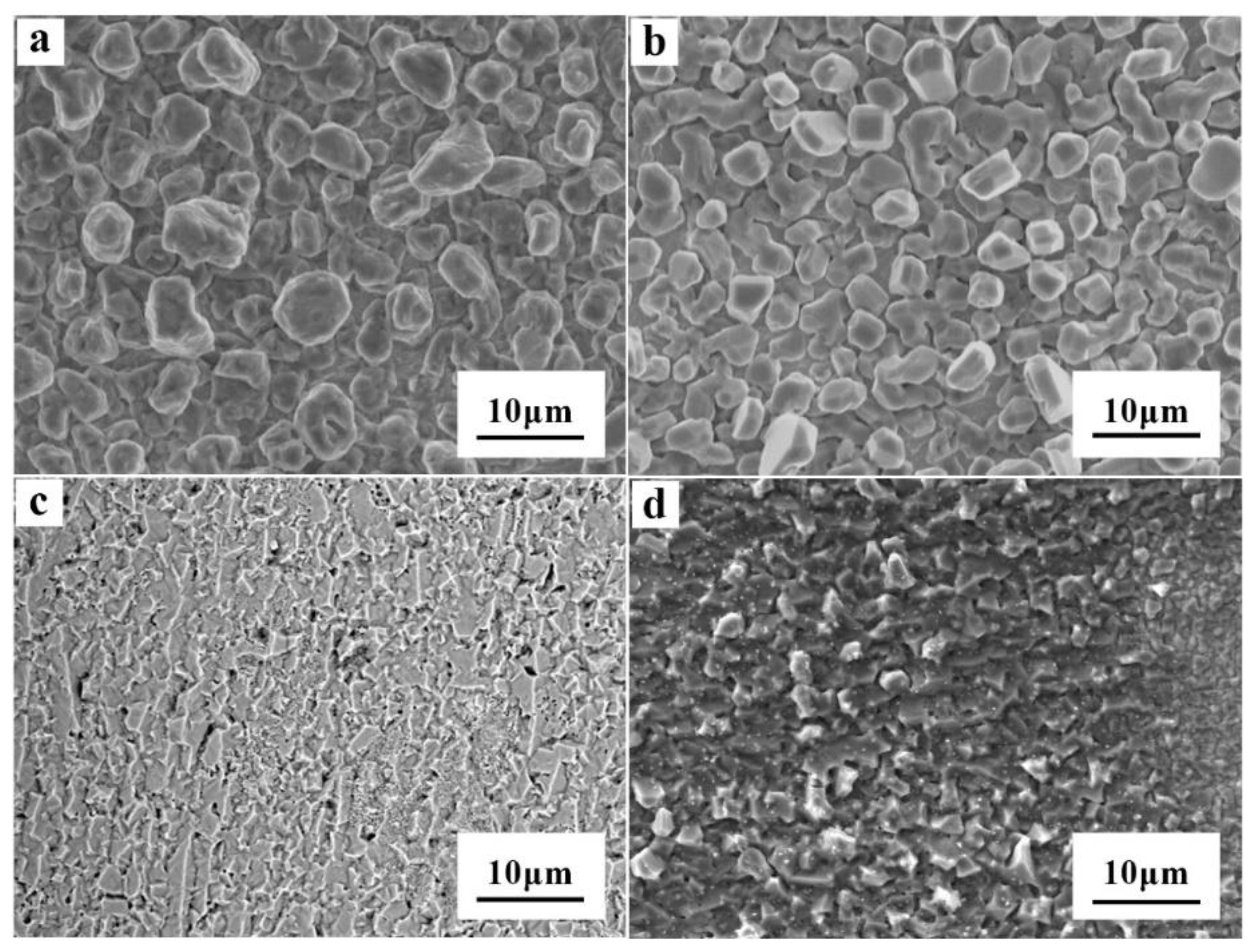
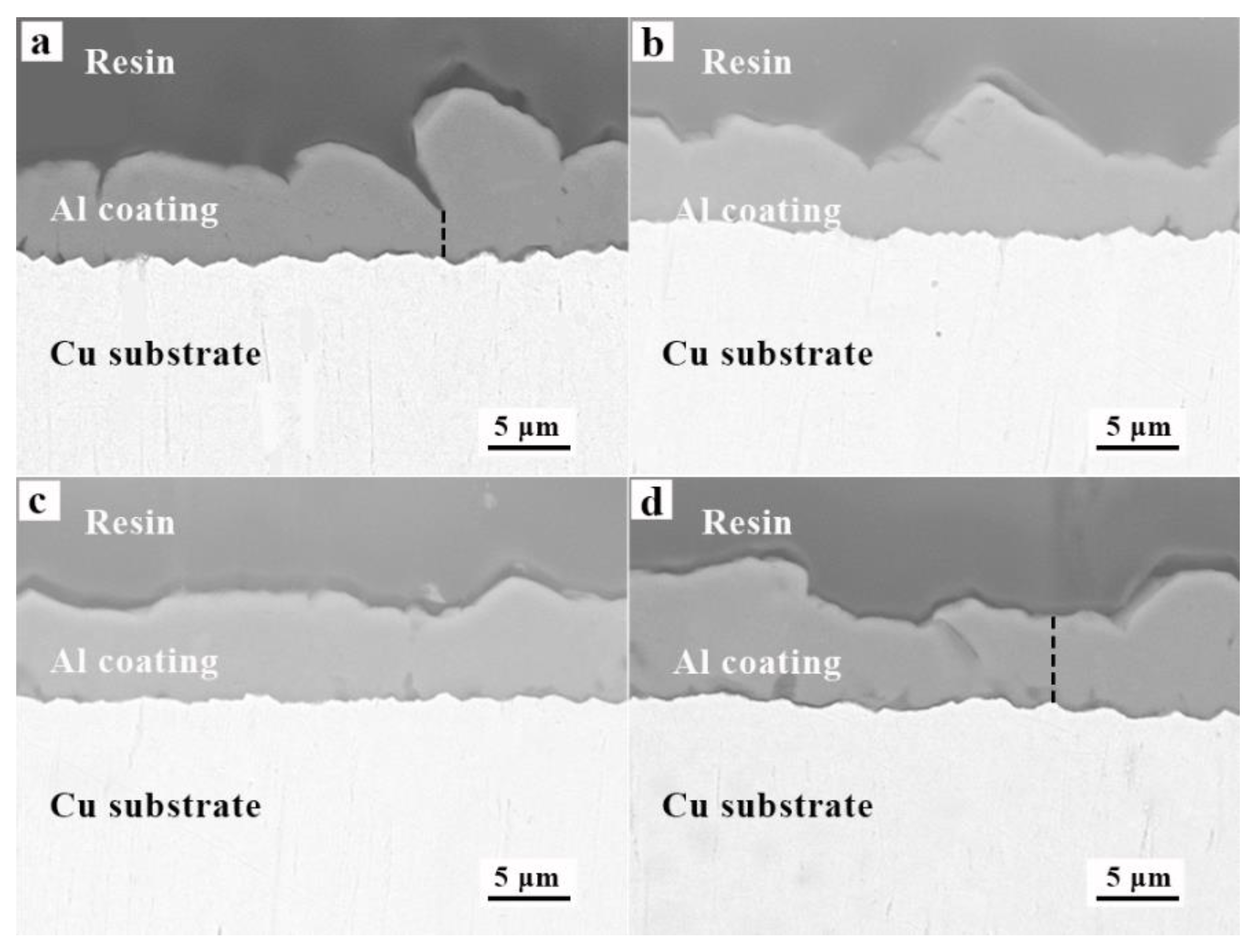
3.4. Morphology of Al Coatings
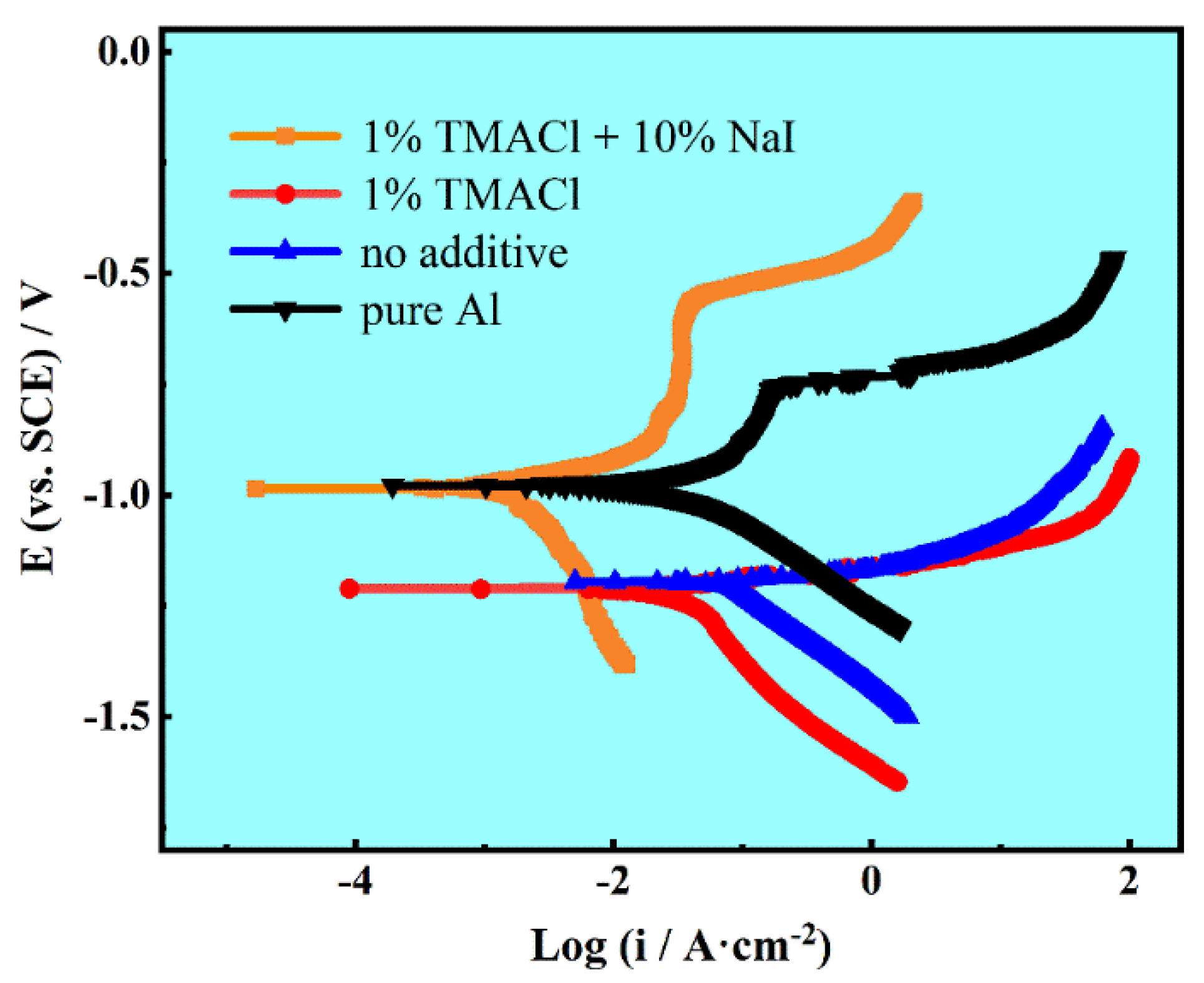
3.5. Corrosion Behaviors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shiva, P. Studies on the Hall-Heroult aluminum electrowinning process. J. Brazil. Chem. Soc. 2000, 11, 245–251. [Google Scholar]
- The Year Book of Nonferrous Metals Industry of China; Nonferrous Metals Industry Press: Beijing, China, 2018.
- Shi, W.M.; Wang, G.G.; Zhao, X.; Feng, X.H.; Wu, J. Price determination in the electrolytic aluminum industry: The role of electricity prices. Resour. Policy 2018, 59, 274–281. [Google Scholar] [CrossRef]
- Chen, X.P.; Li, W.X.; Qiu, S.L. Research on carbondioxide emissions of electrolytic aluminium industry. Light Met. 2012, 7, 33–36. [Google Scholar]
- Lin, B.Q.; Xu, L. Energy conservation of electrolytic aluminum industry in China. Renew. Sustain. Energy Rev. 2015, 43, 676–686. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Q.; Zhang, M.X.; Niu, W.J.; Tang, C.B.; Wang, K.S.; Rui, X.; Zhai, L.; Wang, L. The influence of cold and detonation thermal spraying processes on the microstructure and properties of Al-based composite coatings on Mg alloy. Surf. Coat. Technol. 2018, 352, 627–633. [Google Scholar] [CrossRef]
- Belova, I.V.; Heuskin, D.; Sondermann, E.; Ignatzi, B.; Kargl, F.; Murch, G.E.; Meyer, A. Combined interdiffusion and self-diffusion analysis in Al-Cu liquid diffusion couple. Scr. Mater. 2018, 143, 40–43. [Google Scholar] [CrossRef]
- Cho, L.; Seo, E.J.; Sulistiyo, D.H.; Jo, K.R.; Kim, S.W.; Oh, J.K.; Cho, Y.R.; De Cooman, B.C. Influence of vanadium on the hydrogen embrittlement of aluminized ultra-high strength press hardening steel. Mater. Sci. Eng. A 2018, 735, 448–455. [Google Scholar] [CrossRef]
- Luo, X.X.; Yao, Z.J.; Zhang, P.Z.; Gu, D.D. Al2O3 nanoparticles reinforced Fe-Al laser cladding coatings with enhanced mechanical properties. J. Alloy. Compd. 2018, 755, 41–45. [Google Scholar] [CrossRef]
- Christoglou, C.; Voudouris, N.; Angelopoulos, G.N.; Pant, M.; Dahl, W. Deposition of aluminum on magnesium by a CVD process. Surf. Coat. Technol. 2004, 184, 149–155. [Google Scholar] [CrossRef]
- Wojciech, S.; Dagmara, P.; Ginter, N. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar]
- Howie, R.C.; Macmillan, D.W. The electrodeposition of aluminium from molten aluminium chloride/sodium chloride. J. Appl. Electrochem. 1972, 2, 217–222. [Google Scholar] [CrossRef]
- Nayak, B.; Misra, M. The electrodeposition of aluminium on brass from a molten aluminium chloride-sodium chloride bath. J. Appl. Electrochem. 1977, 7, 45–50. [Google Scholar] [CrossRef]
- Moffat, T.P. Electrodeposition of Al-Cr Metallic Glass. J. Electrochem. Soc. 1994, 141, L115–L117. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.G.; Gobal, F.; Danaee, I. Effect of potential on the early stage of nucleation and growth during aluminum electrocrystallization from molten salt (AlCl3-NaCl-KCl). J. Electroanal. Chem. 2006, 588, 190–196. [Google Scholar] [CrossRef]
- Zhang, J.F.; Yan, C.W.; Wang, F.H. Electrodeposition of Al-Mn alloy on AZ31B magnesium alloy in molten salts. Appl. Surf. Sci. 2009, 255, 4926–4932. [Google Scholar] [CrossRef]
- Li, Y.L.; Zhao, P.; Dai, Y.H.; Yao, M.Q.; Gan, H.B.; Hu, W.C. Electrochemical deposition of Al-Mg alloys on tungsten wires from AlCl3-NaCl-KCl melts. Fusion Eng. Des. 2016, 103, 8–12. [Google Scholar] [CrossRef]
- Mikito, U.; Ryuichi, I.; Toshiaki, O. Composition and structure of Al-Sn alloys formed by constant potential electrolysis in an AlCl3-NaCl-KCl-SnCl2 molten salt. Electrochim. Acta 2013, 100, 281–284. [Google Scholar]
- Vukićević, N.M.; Cvetković, V.S.; Jovanović, L.S.; Stevanović, S.I.; Jovićević, J.N. Alloy Formation by Electrodeposition of Niobium and Aluminium on Gold from Chloroaluminate Melts. Int. J. Electrochem. Sci. 2017, 12, 1075–1093. [Google Scholar]
- Kazuki, S.; Hisayoshi, M.; Mikito, U. Electrodeposition of Al-Ta alloys in NaCl-KCl-AlCl3 molten salt containing TaCl5. Appl. Surf. Sci. 2016, 388, 794–798. [Google Scholar]
- Cao, R.X.; Tao, Z.C.; Wang, H.B.; Guo, Q.G. Electrodeposition of an aluminum coating on a graphite surface from a molten AlCl3-NaCl-KCl mixture. Abstract/Carbon 2015, 93, 1085–1088. [Google Scholar] [CrossRef]
- Guinea, E.; Asier Salicio-Paz Iriarte, A.; Grande, H.J.; Medina, E.; García-Lecina, E. Robust Aluminum Electrodeposition from Ionic Liquid Electrolytes Containing Light Aromatic Naphta as Additive. ChemistryOpen 2019, 8, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Jin, Z.; Xu, F.; Yu, Y.; Wei, G.; Ge, H.; Zhang, Z.; Cao, C. Effect of potential on aluminium early-stage electrodeposition onto NdFeB magnet. Surf. Eng. 2017, 33, 375–382. [Google Scholar] [CrossRef]
- Fellner, P.; Chrenková-Paučírová, M.; Matiašovský, K. Electrolytic aluminum plating in molten salt mixtures based on AlCl3 I: Influence of the addition of tetramethylammonium chloride. Surf. Technol. 1981, 14, 101–108. [Google Scholar] [CrossRef]
- Liu, L.; Lu, X.M.; Cai, Y.J.; Zheng, Y.; Zhang, S.J. Influence of Additives on the Speciation, Morphology, and Nanocrystallinity of Aluminium Electrodeposition. Aust. J. Chem. 2012, 65, 1523–1528. [Google Scholar] [CrossRef]
- Masao, M.; Yuki, K.; Tetsuji, H. Hull cell tests for evaluating the effects of polyethylene amines as brighteners in the electrodeposition of aluminum from dimethylsulfone-AlCl3 baths. Electrochim. Acta 2014, 120, 423–428. [Google Scholar]
- Zhang, Q.Q.; Wang, Q.; Zhang, S.J.; Lu, X.M. Effect of nicotinamide on electrodeposition of Al from aluminium chloride (AlCl3)-1-butyl-3-methylimidazolium chloride ([Bmim]Cl) ionic liquids. J. Solid State Electrochem. 2014, 18, 257–267. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.G.; Gobal, F.; Danaee, I. Electrodeposition of aluminum from molten AlCl3-NaCl-KCl mixture. J. Appl. Electrochem. 2006, 36, 1169–1173. [Google Scholar] [CrossRef]
- Delimarsky, Y.K.; Tumanova, N.K. A study of the effect of surfactants on electrode processes in molten salts. Electrochim. Acta 1979, 24, 19–24. [Google Scholar] [CrossRef]
- Paučírová, M.; Matiašovský, K. Electrolytic aluminium-plating in fused salts based on chlorides. Electrodepos. Surf. Treat. 1975, 3, 121–128. [Google Scholar] [CrossRef]
- Yao, T.Y.; Yang, H.Y.; Wang, K.; Jiang, H.Y.; Chen, X.B.; Liu, H.Z.; Wang, Q.D.; Ding, W.J. Effects of additive NaI on electrodeposition of Al coatings in AlCl3-NaCl-KCl molten salts. Front. Chem. Ence Eng. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Fang, W.J.; Xing, Y.; Guo, Y.S.; Liu, R. Effects of dimethyl or diethyl carbonate as an additive on volatility and flash point of an aviation fuel. J. Hazard. Mater. 2009, 161, 1193–1201. [Google Scholar] [CrossRef]
- Robinson, J.; Osteryoung, R.A. The Electrochemical Behavior of Aluminum in the Low Temperature Molten Salt System n Butyl Pyridinium Chloride: Aluminum Chloride and Mixtures of This Molten Salt with Benzene. J. Electrochem. Soc. 1980, 127, 122–128. [Google Scholar] [CrossRef]
- Yang, H.Y.; Guo, X.W.; Chen, X.B.; Wang, S.H.; Wu, G.H.; Ding, W.J.; Birbilis, N. On the electrodeposition of nickel–zinc alloys from a eutectic-based ionic liquid. Electrochim. Acta 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Li, M.; Gao, B.L.; Chen, W.T.; Liu, C.Y.; Wang, Z.W.; Shi, Z.N.; Hu, X.W. Electrodeposition behavior of aluminum from urea-acetamide-lithium halide low-temperature molten salts. Electrochim. Acta 2013, 185, 148–155. [Google Scholar] [CrossRef]
- Li, M.; Gao, B.L.; Shi, Z.N.; Hua, X.W.; Wang, S.X.; Li, L.X.; Wang, Z.W.; Yu, J.Y. Electrochemical study of nickel from urea-acetamide-LiBr low-temperature molten salt. Electrochim. Acta 2015, 169, 82–89. [Google Scholar] [CrossRef]
- Tsuda, T.; Nohira, T.; Ito, Y. Nucleation and surface morphology of aluminum-lanthanum alloy electrodeposited in a LaCl3-saturatedAlCl3-EtMeImCl room temperature molten salt. Electrochim. Acta 2002, 47, 2817–2822. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Zhang, Q.Q.; Lu, X.M.; Zhang, S.J. Aluminum Deposition from Lewis Acidic 1-Butyl-3-Methylimidazolium Chloroaluminate Ionic Liquid ([Bmim]Cl/AlCl3) Modified with Methyl Nicotinate. ChemElectroChem 2015, 2, 1794–1798. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.Q.; Chen, B.; Lu, X.M.; Zhang, S.J. Electrodeposition of Bright Al Coatings from 1-Butyl-3-Methylimidazolium Chloroaluminate Ionic Liquids with Specific Additives. J. Electrochem. Soc. 2015, 162, D320–D324. [Google Scholar] [CrossRef]
- Osorio, W.R.; Peixoto, L.C.; Moutinho, D.J.; Gomes, L.G.; Ferreira, I.L.; Garcia, A. Corrosion resistance of directionally solidified Al–6Cu–1Si and Al–8Cu–3Si alloys castings. Mater. Des. 2011, 32, 3832–3837. [Google Scholar] [CrossRef]

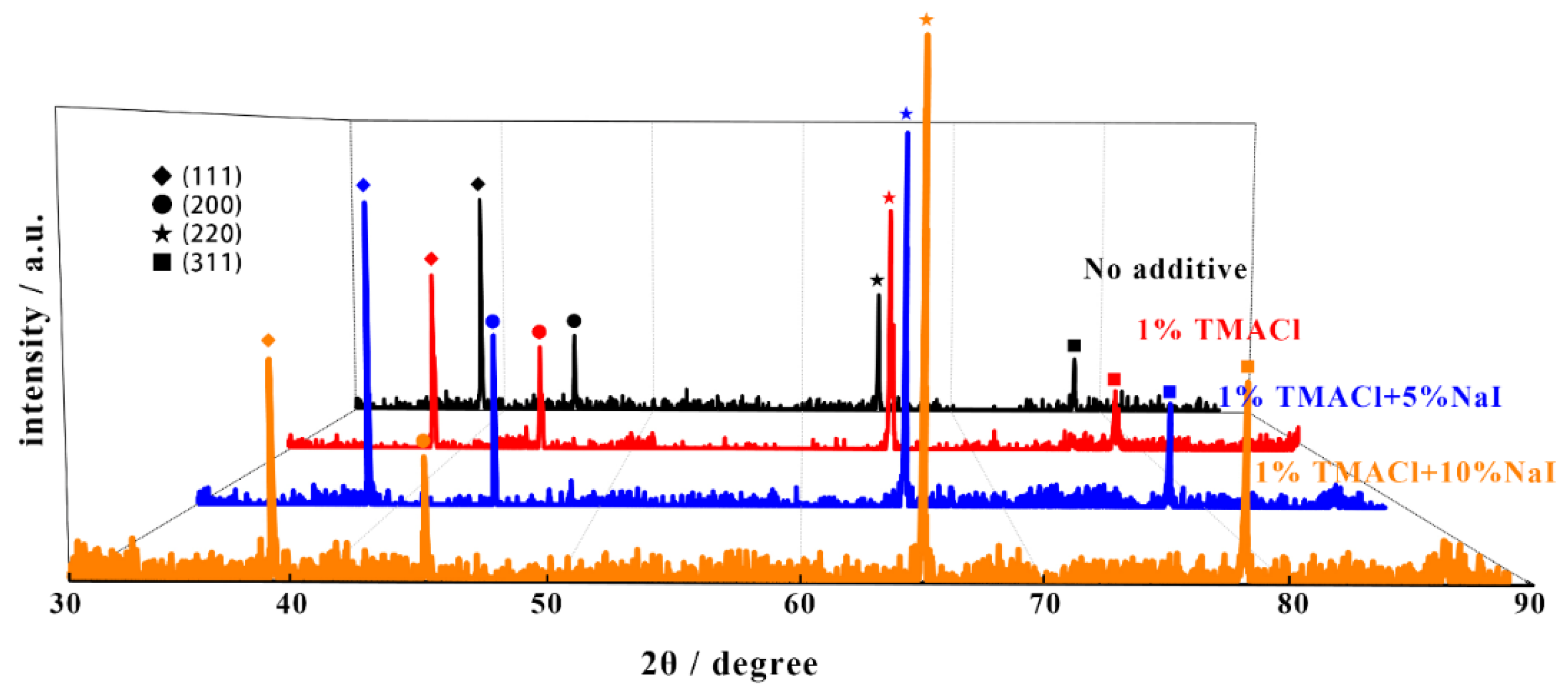
| Additive | TC(111) | TC(200) | TC(220) | TC(311) | Grain Size (nm) |
|---|---|---|---|---|---|
| No additives | 17.89% | 15.11% | 45.73% | 21.27% | 417 |
| 1% TMACl | 9.76% | 13.49% | 60.73% | 16.02% | 401 |
| 1% TMACl + 5% NaI | 10.51% | 14.04% | 57.49% | 17.46% | 395 |
| 1% TMACl + 10% NaI | 5.72% | 7.77% | 62.24% | 24.27% | 363 |
| Specimens | Ecorr (vs. SCE)/V | icorr/μA·cm−2 |
|---|---|---|
| Al coatings electrodeposited with 1% TMACl + 10% NaI. | −0.985 | 3.625 |
| Al coatings electrodeposited with 1% TMACl | −1.211 | 38.768 |
| Al coatings electrodeposited with No additive | −1.196 | 40.161 |
| Pure Al | −0.978 | 35.140 |
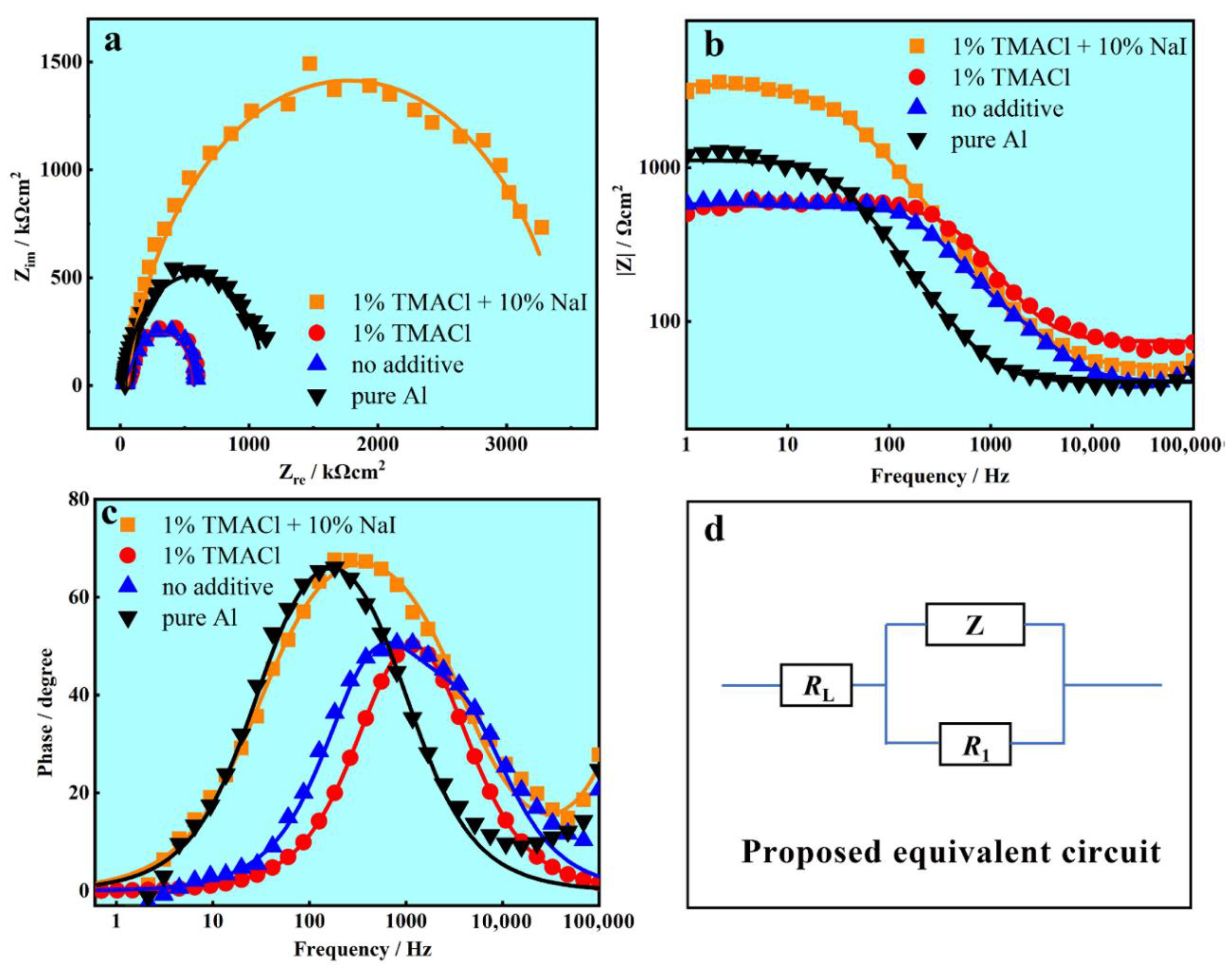
| Specimens | RL (Ω cm2) | Z (μF cm2) | R1 (Ω cm2) |
|---|---|---|---|
| Al coatings electrodeposited with 1% TMACl + 10% NaI. | 55.48 | 10.97 | 3258 |
| Al coatings electrodeposited with 1% TMACl | 74.03 | 76.42 | 488.5 |
| Al coatings electrodeposited with No additive | 44.47 | 10.66 | 52.32 |
| Pure Al | 41.13 | 46.12 | 1059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.; Yang, H.; Wang, K.; Wu, W.; Jiang, H.; Liu, H.; Wang, Q.; Ding, W. Electrodeposition of Aluminum Coatings from AlCl3-NaCl-KCl Molten Salts with TMACl and NaI Additives. Materials 2020, 13, 5506. https://doi.org/10.3390/ma13235506
Yao T, Yang H, Wang K, Wu W, Jiang H, Liu H, Wang Q, Ding W. Electrodeposition of Aluminum Coatings from AlCl3-NaCl-KCl Molten Salts with TMACl and NaI Additives. Materials. 2020; 13(23):5506. https://doi.org/10.3390/ma13235506
Chicago/Turabian StyleYao, Tianyu, Haiyan Yang, Kui Wang, Weiping Wu, Haiyan Jiang, Hezhou Liu, Qudong Wang, and Wenjiang Ding. 2020. "Electrodeposition of Aluminum Coatings from AlCl3-NaCl-KCl Molten Salts with TMACl and NaI Additives" Materials 13, no. 23: 5506. https://doi.org/10.3390/ma13235506





