Continuous Ultrasonic Reactors: Design, Mechanism and Application
Abstract
:1. Introduction
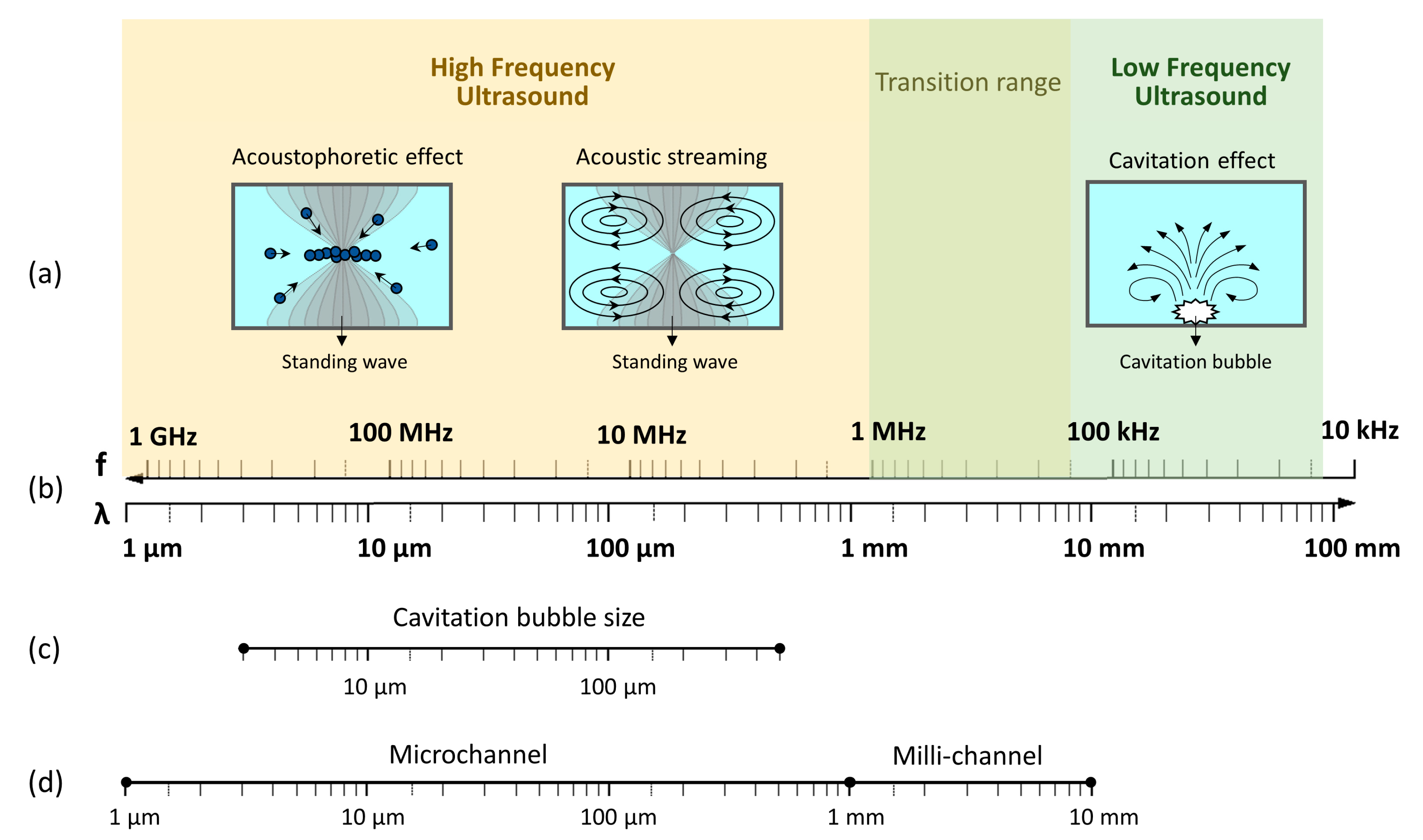
2. Physical Mechanisms of Ultrasound
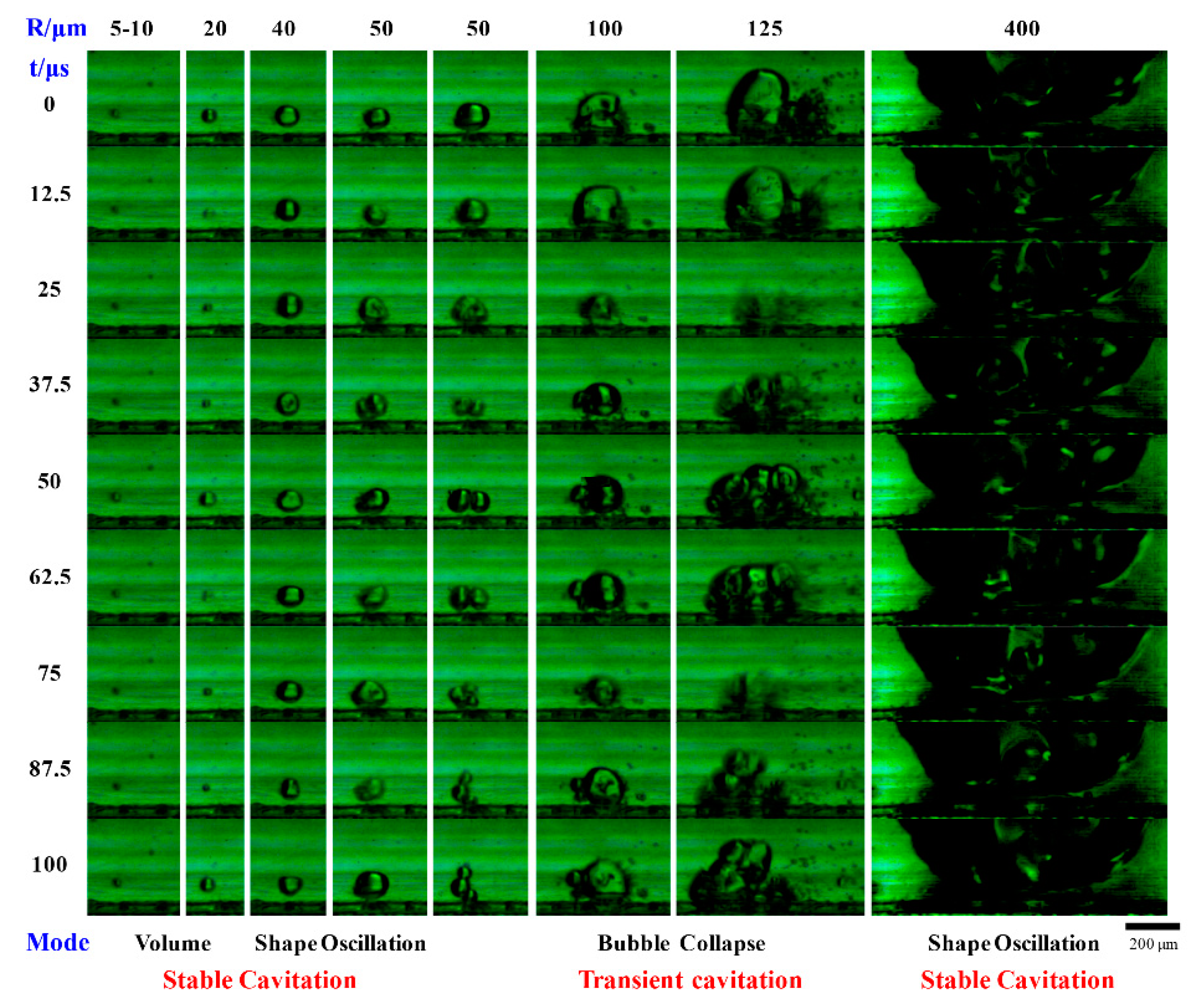
2.1. Cavitation Phenomena in Microchannels
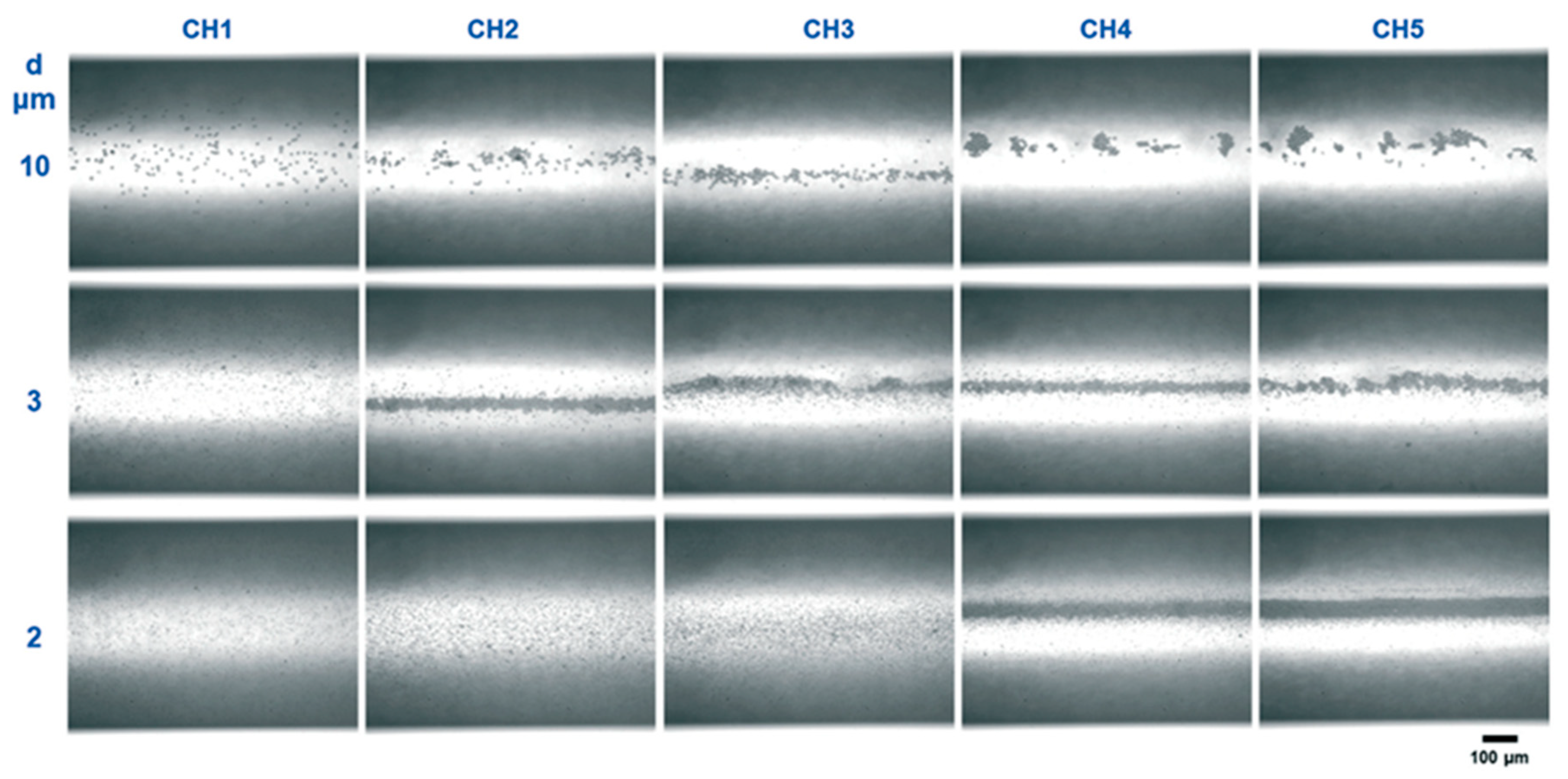
2.2. Standing Acoustic Waves in Microchannels: Acoustophoretic Force and Streaming
3. Reactor Fabrication
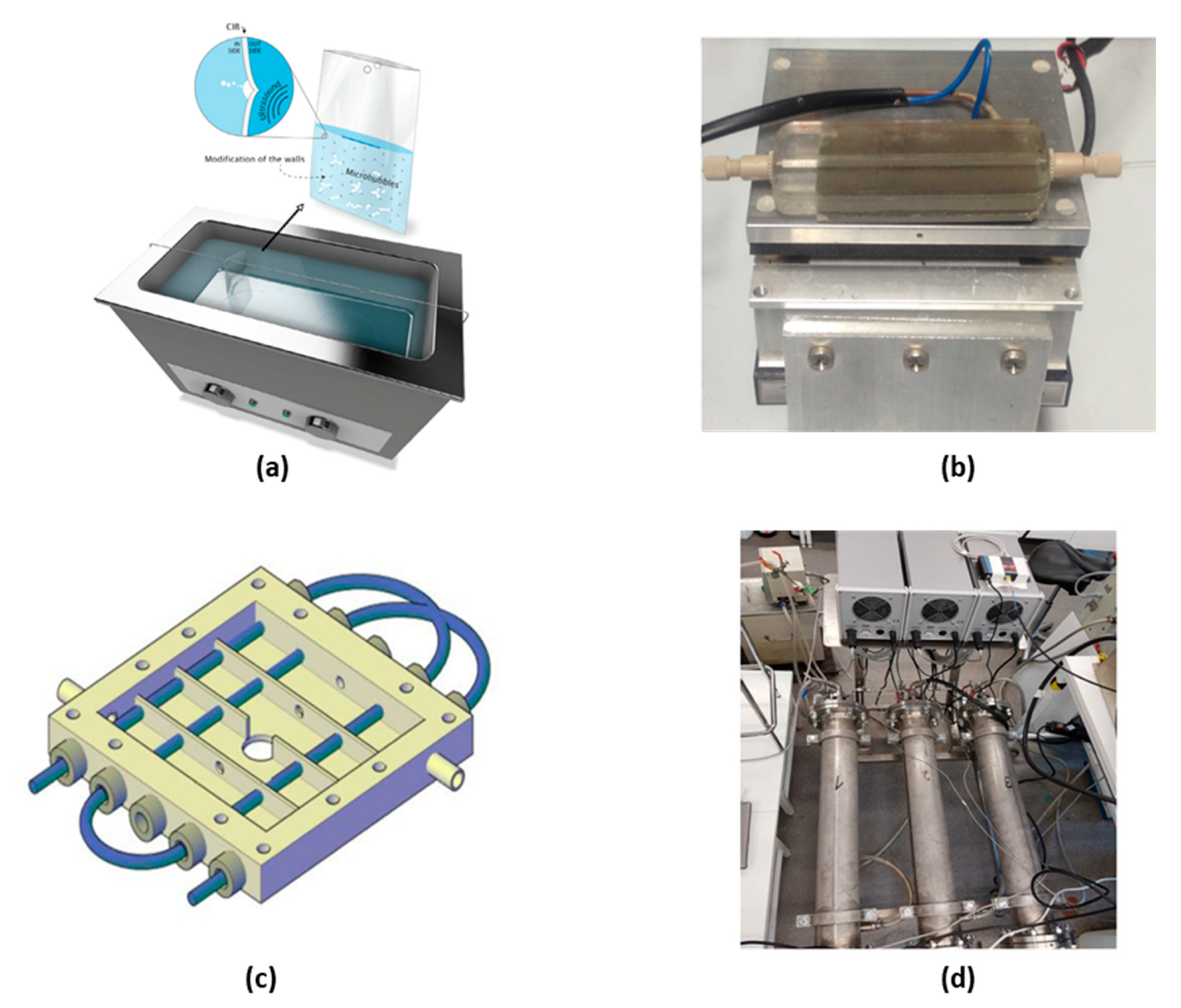
3.1. Reactor Design
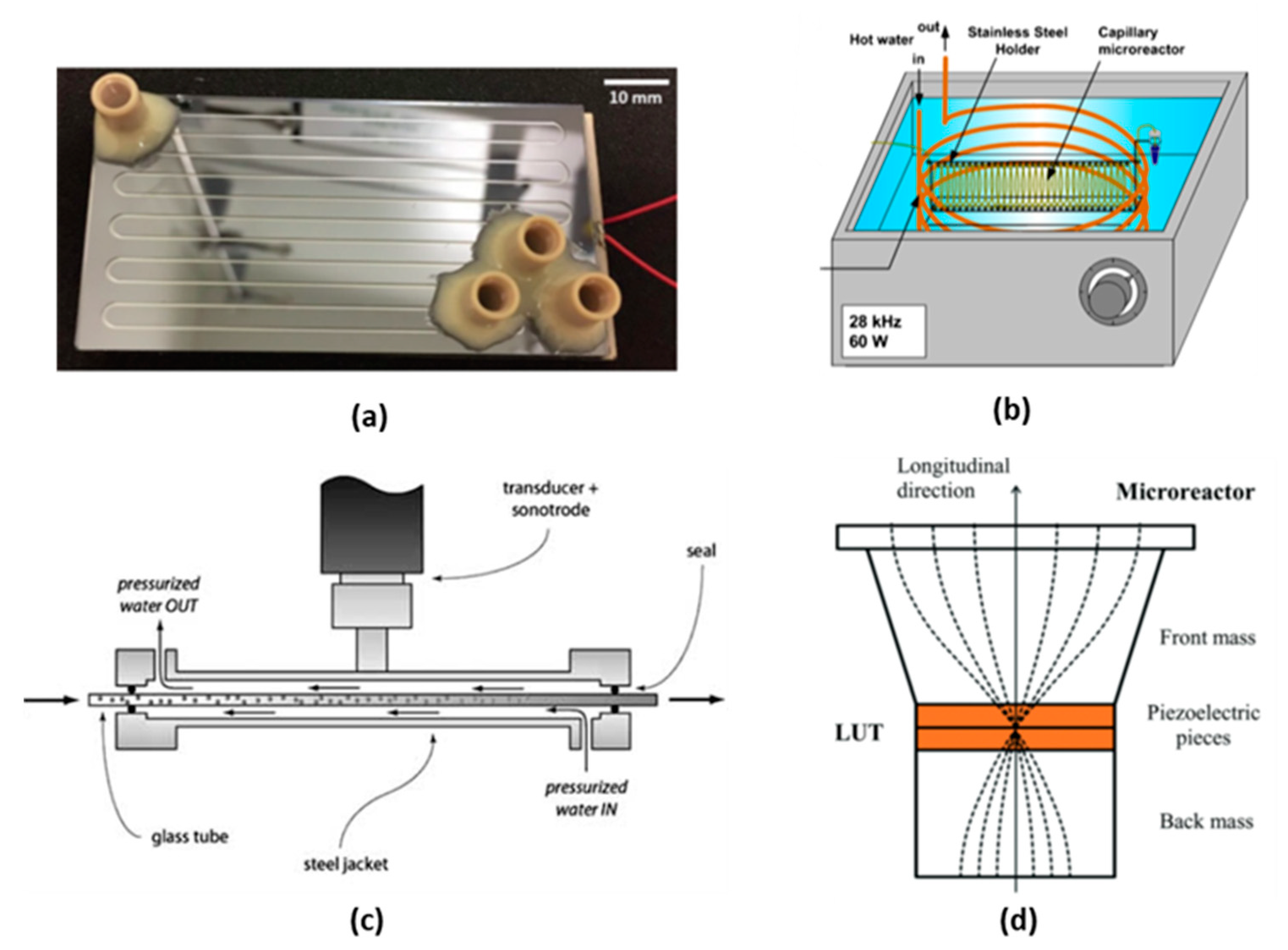
3.1.1. Piezoelectric Plate Based Reactor
3.1.2. Langevin-Type Transducer Based Reactor
3.2. Reactor Characterization
4. Applications
4.1. Gas–Liquid Systems
4.2. Liquid–Liquid Systems
4.3. Liquid–Solid Systems
5. Scale-Up of Ultrasound Reactors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in Microstructured Reactors. Angew. Chemie Int. Ed. 2004, 43, 406–446. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.F. Flow Chemistry—Microreaction Technology Comes of Age. AIChE 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Plou, P.; Macchi, A.; Roberge, D.M. From Batch to Continuous Chemical Synthesis A Toolbox Approach. Org. Process Res. Dev. 2014, 18, 1286–1294. [Google Scholar] [CrossRef]
- Sobieszuk, P.; Aubin, J.; Pohorecki, R. Hydrodynamics and Mass Transfer in Gas-Liquid Flows in Microreactors. Chem. Eng. Technol. 2012, 35, 1346–1358. [Google Scholar] [CrossRef]
- Yoshida, J.I.; Kim, H.; Nagaki, A. Green and Sustainable Chemical Synthesis Using Flow Microreactors. ChemSusChem 2011, 4, 331–340. [Google Scholar] [CrossRef]
- Elvira, K.S.; I Solvas, X.C.; Wootton, R.C.R.; Demello, A.J. The Past, Present and Potential for Microfluidic Reactor Technology in Chemical Synthesis. Nat. Chem. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. Novel Process Windows for Enabling, Accelerating, and Uplifting Flow Chemistry. ChemSusChem 2013, 6, 746–789. [Google Scholar] [CrossRef]
- Cole, K.P.; Groh, J.M.; Johnson, M.D.; Burcham, C.L.; Campbell, B.M.; Diseroad, W.D.; Heller, M.R.; Howell, J.R.; Kallman, N.J.; Koenig, T.M.; et al. Kilogram-Scale Prexasertib Monolactate Monohydrate Synthesis under Continuous-Flow CGMP Conditions. Science 2017, 356, 1144–1150. [Google Scholar] [CrossRef] [Green Version]
- Kockmann, N. Modular Equipment for Chemical Process Development and Small-Scale Production in Multipurpose Plants. ChemBioEng Rev. 2016, 3, 1–12. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Why Flow Means Green—Evaluating the Merits of Continuous Processing in the Context of Sustainability. Curr. Opin. Green Sustain. Chem. 2017, 7, 6–12. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous Manufacturing—the Green Chemistry Promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef] [Green Version]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-Demand Continuous-Flow Production of Pharmaceuticals in a Compact, Reconfigurable System. Science 2016, 352, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Kuhn, S. Strategies for Solids Handling in Microreactors. Chim. Oggi/Chemistry Today 2014, 32, 62–67. [Google Scholar]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S. Fouling in Microstructured Devices: A Review. Chem. Commun. 2015, 51, 8213–8228. [Google Scholar] [CrossRef]
- Hartman, R.L.; Naber, J.R.; Zaborenko, N.; Buchwald, S.L.; Jensen, K.F. Overcoming the Challenges of Solid Bridging and Constriction during Pd-Catalyzed C-N Bond Formation in Microreactors. Org. Process Res. Dev. 2010, 14, 1347–1357. [Google Scholar] [CrossRef]
- Wyss, H.M.; Blair, D.L.; Morris, J.F.; Stone, H.A.; Weitz, D.A. Mechanism for Clogging of Microchannels. Phys. Rev. E 2006, 74, 1–4. [Google Scholar] [CrossRef]
- Dressaire, E.; Sauret, A. Clogging of Microfluidic Systems. Soft Matter 2017, 13, 37–48. [Google Scholar] [CrossRef]
- Roberge, D.M.; Ducry, L.; Bieler, N.; Cretton, P.; Zimmermann, B. Microreactor Technology: A Revolution for the Fine Chemical and Pharmaceutical Industries? Chem. Eng. Technol. 2005, 28, 318–323. [Google Scholar] [CrossRef]
- Hartman, R.L. Managing Solids in Microreactors for the Upstream Continuous Processing of Fine Chemicals. Org. Process Res. Dev. 2012, 16, 870–887. [Google Scholar] [CrossRef]
- Henry, C.; Minier, J.P.; Lefèvre, G. Towards a Description of Particulate Fouling: From Single Particle Deposition to Clogging. Adv. Colloid Interface Sci. 2012, 185–186, 34–76. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A Review on Passive and Active Mixing Principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Kashid, M.N.; Renken, A.; Kiwi-Minsker, L. Microstructured Devices for Chemical Processing; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar] [CrossRef]
- Abolhasani, M.; Jensen, K.F. Oscillatory Multiphase Flow Strategy for Chemistry and Biology. Lab Chip 2016, 16, 2775–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Zhao, S.; Zhang, Y.; Yao, C.; Yuan, Q.; Chen, G. Mixing and Residence Time Distribution in Ultrasonic Microreactors. AIChE J. 2016, 63, 1404–1418. [Google Scholar] [CrossRef]
- Gobert, S.R.L.; Kuhn, S.; Braeken, L.; Thomassen, L.C.J. Characterization of Milli- and Microflow Reactors: Mixing Efficiency and Residence Time Distribution. Org. Process Res. Dev. 2017, 21, 531–542. [Google Scholar] [CrossRef]
- Fernandez Rivas, D.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. 2016, 374. [Google Scholar] [CrossRef] [Green Version]
- Leighton, T.G. What Is Ultrasound? Prog. Biophys. Mol. Biol. 2007, 93, 3–83. [Google Scholar] [CrossRef]
- Gogate, P.R. Cavitational Reactors for Process Intensification of Chemical Processing Applications: A Critical Review. Chem. Eng. Process. 2008, 47, 515–527. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power Ultrasound in Organic Synthesis: Moving Cavitational Chemistry from Academia to Innovative and Large-Scale Applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef]
- Gogate, P.R.; Sutkar, V.S.; Pandit, A.B. Sonochemical Reactors: Important Design and Scale up Considerations with a Special Emphasis on Heterogeneous Systems. Chem. Eng. J. 2011, 166, 1066–1082. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Leighton, T.G. Bubble Population Phenomena in Acoustic Cavitation. Ultrason. Sonochem. 1995, 2, S123–S136. [Google Scholar] [CrossRef]
- Stankiewicz, A. Alternative Sources and Forms of Energy for Intensification of Chemical and Biochemical Processes. Chem. Eng. Res. Des. 2006, 84, 511–521. [Google Scholar] [CrossRef]
- Dong, Z.; Yao, C.; Zhang, X.; Xu, J.; Chen, G.; Zhao, Y.; Yuan, Q. A High-Power Ultrasonic Microreactor and Its Application in Gas-Liquid Mass Transfer Intensification. Lab Chip 2015, 15, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rivas, D.; Cintas, P.; Gardeniers, H.J.G.E. Merging Microfluidics and Sonochemistry: Towards Greener and More Efficient Micro-Sono-Reactors. Chem. Commun. 2012, 48, 10935–10947. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, G.; Zhao, S.; Yuan, Q. Sonochemical Microreactor—Synergistic Combination of Ultrasound and Microreactor. J. Chem. Ind. Eng. 2018, 69, 102–115. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Shi, J.; Juluri, B.K.; Huang, T.J. A Millisecond Micromixer via Single-Bubble-Based Acoustic Streaming. Lab Chip 2009, 9, 2738–2741. [Google Scholar] [CrossRef]
- Dong, Z.; Yao, C.; Zhang, Y. Hydrodynamics and Mass Transfer of Oscillating Gas-Liquid Flow in Ultrasonic Microreactors. AIChE J. 2016, 62, 1294–1307. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, C.; Dong, Z.; Liu, Y.; Chen, G.; Yuan, Q. Intensification of Liquid-Liquid Two-Phase Mass Transfer by Oscillating Bubbles in Ultrasonic Microreactor. Chem. Eng. Sci. 2018, 186, 122–134. [Google Scholar] [CrossRef]
- Na Kim, H.; Suslick, K.S. The Effects of Ultrasound on Crystals: Sonocrystallization and Sonofragmentation. Crystals 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Jordens, J.; Appermont, T.; Gielen, B.; Van Gerven, T.; Braeken, L. Sonofragmentation: Effect of Ultrasound Frequency and Power on Particle Breakage. Cryst. Growth Des. 2016, 16, 6167–6177. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, C.; Dong, Z.; Chen, G.; Yuan, Q. Role of Ultrasonic Oscillation in Chemical Processes in Microreactors: A Mesoscale Issue. Particuology 2019. [Google Scholar] [CrossRef]
- Kuhn, S.; Noël, T.; Gu, L.; Heider, P.L.; Jensen, K.F. A Teflon Microreactor with Integrated Piezoelectric Actuator to Handle Solid Forming Reactions. Lab Chip 2011, 11, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Kuhn, S.; Jensen, K.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Continuous-Flow Precipitation of Hydroxyapatite in Ultrasonic Microsystems. Chem. Eng. J. 2013, 215–216, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Delacour, C.; Lutz, C.; Kuhn, S. Pulsed Ultrasound for Temperature Control and Clogging Prevention in Micro-Reactors. Ultrason. Sonochem. 2019, 55, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, F.J.; Juliano, P.; Barbosa-Cánovas, G.; Knoerzer, K. Separation of Suspensions and Emulsions via Ultrasonic Standing Waves—A Review. Ultrason. Sonochem. 2014, 21, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of Acoustophoresis in Continuous Flow Microsystems. Lab Chip 2012, 12, 1210–1223. [Google Scholar] [CrossRef]
- Kuznetsova, L.A.; Coakley, W.T. Applications of Ultrasound Streaming and Radiation Force in Biosensors. Biosens. Bioelectron. 2007, 22, 1567–1577. [Google Scholar] [CrossRef]
- Dong, Z.; Fernandez Rivas, D.; Kuhn, S. Acoustophoretic Focusing Effects on Particle Synthesis and Clogging in Microreactors. Lab Chip 2019, 19, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of Acoustic Streaming in Microfluidic Devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef]
- Wyatt Shields, C., IV; Reyes, C.D.; López, G.P. Microfluidic Cell Sorting: A Review of the Advances in the Separation of Cells from Debulking to Rare Cell Isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [Green Version]
- Evander, M.; Nilsson, J. Acoustofluidics 20: Applications in Acoustic Trapping. Lab Chip 2012, 12, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Gelin, P.; Van Lindt, J.; Bratek-Skicki, A.; Stroobants, S.; Krzek, M.; Ziemecka, I.; Tompa, P.; De Malsche, W.; Maes, D. Focusing of Microcrystals and Liquid Condensates in Acoustofluidics. Crystals 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Sriphutkiat, Y.; Zhou, Y. Particle Accumulation in a Microchannel and Its Reduction by a Standing Surface Acoustic Wave (SSAW). Sensors 2017, 17, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooze, J.; Rebrov, E.V.; Schouten, J.C.; Keurentjes, J.T.F. Dissolved Gas and Ultrasonic Cavitation—A Review. Ultrason. Sonochem. 2013, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rivas, D.; Stricker, L.; Zijlstra, A.G.; Gardeniers, H.J.G.E.; Lohse, D.; Prosperetti, A. Ultrasound Artificially Nucleated Bubbles and Their Sonochemical Radical Production. Ultrason. Sonochem. 2013, 20, 510–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauer, M.; Belova-Magri, V.; Cairós, C.; Linka, G.; Mettin, R. High-Speed Imaging of Ultrasound Driven Cavitation Bubbles in Blind and through Holes. Ultrason. Sonochem. 2018, 48, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Grieser, F.; Choi, P.K.; Enomoto, N.; Harada, H.; Okitsu, K.; Yasui, K. Sonochemistry and the Acoustic Bubble; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Anderson, B., Ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Ashokkumar, M. The Characterization of Acoustic Cavitation Bubbles—An Overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Leong, T.; Ashokkumar, M.; Sandra, K. The Fundamentals of Power Ultrasound—A Review. Acoust. Aust. 2011, 39, 54–63. [Google Scholar]
- Birkin, P.R.; Offin, D.G.; Vian, C.J.B.; Leighton, T.G. Investigation of Noninertial Cavitation Produced by an Ultrasonic Horn. J. Acoust. Soc. Am. 2011, 130, 3297–3308. [Google Scholar] [CrossRef]
- Lauterborn, W.; Kurz, T. Physics of Bubble Oscillations. Rep. Prog. Phys. 2010, 73, 1–88. [Google Scholar] [CrossRef]
- Collis, J.; Manasseh, R.; Liovic, P.; Tho, P.; Ooi, A.; Petkovic-Duran, K.; Zhu, Y. Cavitation Microstreaming and Stress Fields Created by Microbubbles. Ultrasonics 2010, 50, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jalal, J.; Leong, T.S.H. Microstreaming and Its Role in Applications: A Mini-Review. Fluids 2018, 3, 93. [Google Scholar] [CrossRef] [Green Version]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Towata, A.; Kozuka, T. Bubble Motions Confined in a Microspace Observed with Stroboscopic Technique. Ultrason. Sonochem. 2007, 14, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yao, C.; Zhang, Q.; Chen, G.; Yuan, Q. Acoustic Cavitation and Ultrasound-Assisted Nitration Process in Ultrasonic Microreactors: The Effects of Channel Dimension, Solvent Properties and Temperature. Chem. Eng. J. 2019, 374, 68–78. [Google Scholar] [CrossRef]
- Iida, Y.; Yasui, K.; Tuziuti, T.; Sivakumar, M.; Endo, Y. Ultrasonic Cavitation in Microspace. Chem. Commun. 2004, 2280–2281. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rivas, D.; Prosperetti, A.; Zijlstra, A.G.; Lohse, D.; Gardeniers, H.J.G.E. Efficient Sonochemistry through Microbubbles Generated with Micromachined Surfaces. Angew. Chemie. Int. Ed. 2010, 49, 9699–9701. [Google Scholar] [CrossRef] [Green Version]
- Bezagu, M.; Arseniyadis, S.; Cossy, J.; Couture, O.; Tanter, M.; Monti, F.; Tabeling, P. A Fast and Switchable Microfluidic Mixer Based on Ultrasound-Induced Vaporization of Perfluorocarbon. Lab Chip 2015, 15, 2025–2029. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Krishna Juluri, B.; Jun Huang, T. A Fast Microfluidic Mixer Based on Acoustically Driven Sidewall-Trapped Microbubbles. Microfluid. Nanofluid. 2009, 7, 727–731. [Google Scholar] [CrossRef]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating Bubbles: A Versatile Tool for Lab on a Chip Applications. Lab Chip 2012, 12, 4216–4227. [Google Scholar] [CrossRef]
- Tovar, A.R.; Lee, A.P. Lateral Cavity Acoustic Transducer. Lab Chip 2009, 9, 41–43. [Google Scholar] [CrossRef]
- Tovar, A.R.; Patel, M.V.; Lee, A.P. Lateral Air Cavities for Microfluidic Pumping with the Use of Acoustic Energy. Microfluid. Nanofluid. 2011, 10, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Ozcelik, A.; Ahmed, D.; Xie, Y.; Nama, N.; Qu, Z.; Ahsan Nawaz, A.; Jun Huang, T. An Acoustofluidic Micromixer via Bubble Inception and Cavitation from Microchannel Sidewalls. Anal. Chem. 2014, 86, 5083–5088. [Google Scholar] [CrossRef] [PubMed]
- Ohl, S.W.; Ow, D.S.; Klaseboer, E.; Wong, V.V.; Dumke, R.; Ohl, C.D. Sonochemistry and Sonoluminescence in Microfluidics. Proc. Natl. Acad. Sci. USA 2011, 108, 5996–5998. [Google Scholar] [CrossRef] [Green Version]
- Ohl, S.W.; Ow, D.S.W.; Klaseboer, E.; Wong, V.V.; Camattari, A.; Ohl, C.D. Creation of Cavitation Activity in a Microfluidic Device through Acoustically Driven Capillary Waves. Lab Chip 2010, 10, 1848–1855. [Google Scholar] [CrossRef]
- Prest, J.E.; Treves Brown, B.J.; Fielden, P.R.; Wilkinson, S.J.; Hawkes, J.J. Scaling-up Ultrasound Standing Wave Enhanced Sedimentation Filters. Ultrasonics 2015, 56, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Petersson, F.; Jönsson, H.; Laurell, T. Acoustic Control of Suspended Particles in Micro Fluidic Chips. Lab Chip 2004, 4, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fornell, A.; Garofalo, F.; Nilsson, J.; Bruus, H.; Tenje, M. Intra-Droplet Acoustic Particle Focusing: Simulations and Experimental Observations. Microfluid. Nanofluid3. 2018, 22, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lenshof, A.; Evander, M.; Laurell, T.; Nilsson, J. Acoustofluidics 5: Building Microfluidic Acoustic Resonators. Lab Chip 2012, 12, 684–695. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip Integrated Strategies for Acoustic Separation and Manipulation of Cells and Particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef]
- Perfetti, C.; Saverio Iorio, C. Three-Dimensional Matrixlike Focusing of Microparticles in Flow through Minichannel Using Acoustic Standing Waves: An Experimental and Modeling Study. Acoust. Sci. Technol. 2016, 37, 221–230. [Google Scholar] [CrossRef]
- Petersson, F.; Nilsson, A.; Holm, C.; Jönsson, H.; Laurell, T. Continuous Separation of Lipid Particles from Erythrocytes by Means of Laminar Flow and Acoustic Standing Wave Forces. Lab Chip 2005, 5, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Lenshof, A.; Tajudin, A.A.; Järås, K.; Swärd-Nilsson, A.M.; Åberg, L.; Marko-Varga, G.; Malm, J.; Lilja, H.; Laurell, T. Acoustic Whole Blood Plasmapheresis Chip for PSA Microarray Diagnostics. Anal. Chem. 2009, 81, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.W., IV; Cruz, D.F.; Ohiri, K.A.; Yellen, B.B.; Lopez, G.P. Fabrication and Operation of Acoustofluidic Devices Supporting Bulk Acoustic Standing Waves for Sheathless Focusing of Particles. J. Vis. Exp. 2016, 109, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giddings, J.C. A System Based on Split-Flow Lateral-Transport Thin (SPLITT) Separation Cells for Rapid and Continuous Particle Fractionation. Sep. Sci. Technol. 1985, 20, 749–768. [Google Scholar] [CrossRef]
- Dykes, J.; Lenshof, A.; Åstrand-Grundström, I.-B.; Laurell, T.; Scheding, S. Efficient Removal of Platelets from Peripheral Blood Progenitor Cell Products Using a Novel Micro-Chip Based Acoustophoretic Platform. PLoS ONE 2011, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ayan, B.; Ozcelik, A.; Bachman, H.; Tang, S.Y.; Xie, Y.; Wu, M.; Li, P.; Huang, T.J. Acoustofluidic Coating of Particles and Cells. Lab Chip 2016, 16, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Goddard, G.; Kaduchak, G. Ultrasonic Particle Concentration in a Line-Driven Cylindrical Tube. Acoust. Soc. Am. 2005, 117, 3440–3447. [Google Scholar] [CrossRef]
- Bengtsson, M.; Laurell, T. Ultrasonic Agitation in Microchannels. Anal. Bioanal. Chem. 2004, 378, 1716–1721. [Google Scholar] [CrossRef]
- Johansson, L.; Johansson, S.; Nikolajeff, F.; Thorslund, S. Effective Mixing of Laminar Flows at a Density Interface by an Integrated Ultrasonic Transducer. Lab Chip 2009, 9, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Udepurkar, A.P.; Kuhn, S. Synergistic Effects of the Alternating Application of Low and High Frequency Ultrasound for Particle Synthesis in Microreactors. Ultrason. Sonochem. 2020, 60, 104800. [Google Scholar] [CrossRef]
- Gröschl, M. Ultrasonic Separation of Suspended Particles—Part I: Fundamentals. Acustica 1998, 84, 432–447. [Google Scholar]
- Hill, M.; Shen, Y.; Hawkes, J.J. Modelling of Layered Resonators for Ultrasonic Separation. Ultrasonics 2002, 40, 385–392. [Google Scholar] [CrossRef]
- Hill, M.; Townsend, R.J.; Harris, N.R. Modelling for the Robust Design of Layered Resonators for Ultrasonic Particle Manipulation. Ultrasonics 2008, 48, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljbour, S.; Yamada, H.; Tagawa, T. Ultrasound-Assisted Phase Transfer Catalysis in a Capillary Microreactor. Chem. Eng. Process. 2009, 48, 1167–1172. [Google Scholar] [CrossRef]
- Freitas, S.; Hielscher, G.; Merkle, H.P.; Gander, B. Continuous Contact- and Contamination-Free Ultrasonic Emulsification—A Useful Tool for Pharmaceutical Development and Production. Ultrason. Sonochem. 2006, 13, 76–85. [Google Scholar] [CrossRef]
- Mathieson, A.; Cardoni, A.; Cerisola, N.; Lucas, M. The Influence of Piezoceramic Stack Location on Nonlinear Behavior of Langevin Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1126–1133. [Google Scholar] [CrossRef]
- Harvey, G.; Gachagan, A.; Mutasa, T. Review of High-Power Ultrasound-Industrial Applications and Measurement Methods. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 481–495. [Google Scholar] [CrossRef] [Green Version]
- Sancheti, S.V.; Gogate, P.R. A Review of Engineering Aspects of Intensification of Chemical Synthesis Using Ultrasound. Ultrason. Sonochem. 2017, 36, 527–543. [Google Scholar] [CrossRef]
- Lin, S. The Mechanism and Design of Ultrasound Transducer; Science Press: Beijing, China, 2004. [Google Scholar]
- Navarro-Brull, F.J.; Teixeira, A.R.; Giri, G.; Gómez, R. Enabling Low Power Acoustics for Capillary Sonoreactors. Ultrason. Sonochem. 2019, 56, 105–113. [Google Scholar] [CrossRef]
- Navarro-Brull, F.J.; Poveda, P.; Ruiz-Femenia, R.; Bonete, P.; Ramis, J.; Gómez, R. Guidelines for the Design of Efficient Sono-Microreactors. Green Process. Synth. 2014, 3, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Valitov, G.; Jamshidi, R.; Rossi, D.; Gavriilidis, A.; Mazzei, L. Effect of Acoustic Streaming on Continuous Flow Sonocrystallization in Millifluidic Channels. Chem. Eng. J. 2020, 379, 1–13. [Google Scholar] [CrossRef]
- John, J.J.; Kuhn, S.; Braeken, L.; Van Gerven, T. Ultrasound Assisted Liquid–Liquid Extraction with a Novel Interval-Contact Reactor. Chem. Eng. Process. Process Intensif. 2017, 113, 35–41. [Google Scholar] [CrossRef]
- Tseng, Q.; Lomonosov, A.M.; Furlong, E.E.M.; Merten, C.A. Fragmentation of DNA in a Sub-Microliter Microfluidic Sonication Device. Lab Chip 2012, 12, 4677–4682. [Google Scholar] [CrossRef] [PubMed]
- John, J.J.; Kuhn, S.; Braeken, L.; Van Gerven, T. Ultrasound Assisted Liquid-Liquid Extraction in Microchannels-A Direct Contact Method. Chem. Eng. Process. Process Intensif. 2016, 102, 37–46. [Google Scholar] [CrossRef]
- Hübner, S.; Kressirer, S.; Kralisch, D.; Bludszuweit-Philipp, C.; Lukow, K.; Jänich, I.; Schilling, A.; Hieronymus, H.; Liebner, C.; Jähnisch, K. Ultrasound and Microstructures—A Promising Combination? ChemSusChem 2012, 5, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Roberge, D.; Raimone, F.; Quittmann, W.; Gottsponer, M.; Eyholzer, M. Method for Preventing Plugging of a Continuous-Reaction Channel-System and Micro-Reactor for Carrying out the Method. US20150158007A1, 11 June 2015. [Google Scholar]
- Iranmanesh, I.; Ohlin, M.; Ramachandraiah, H.; Ye, S.; Russom, A.; Wiklund, M. Acoustic Micro-Vortexing of Fluids, Particles and Cells in Disposable Microfluidic Chips. Biomed. Microdevices 2016, 18, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Horie, T.; Sumino, M.; Tanaka, T.; Matsushita, Y.; Ichimura, T.; Yoshida, J.-I. Photodimerization of Maleic Anhydride in a Microreactor without Clogging. Org. Process Res. Dev. 2010, 14, 405–410. [Google Scholar] [CrossRef]
- Noël, T.; Naber, J.R.; Hartman, R.L.; Mcmullen, J.P.; Jensen, K.F.; Buchwald, S.L. Palladium-Catalyzed Amination Reactions in Flow: Overcoming the Challenges of Clogging via Acoustic Irradiation. Chem. Sci. 2011, 2, 287–290. [Google Scholar] [CrossRef]
- Aljbour, S.; Tagawa, T.; Yamada, H. Ultrasound-Assisted Capillary Microreactor for Aqueous-Organic Multiphase Reactions. J. Ind. Eng. Chem. 2009, 15, 829–834. [Google Scholar] [CrossRef]
- John, J.J.; Kuhn, S.; Braeken, L.; Van Gerven, T. Temperature Controlled Interval Contact Design for Ultrasound Assisted Liquid-Liquid Extraction. Chem. Eng. Res. Des. 2017, 125, 146–155. [Google Scholar] [CrossRef]
- Choi, P.; Kaneko, Y.; Meguro, T. Enhancement of Sonoluminescence and Bubble Dynamics Using Pulsed Ultrasound at 103 KHz. Jpn. J. Appl. Phys. 2008, 47, 4111–4114. [Google Scholar] [CrossRef]
- Ciaravino, V.; Flynn, H.G.; Miller, M.W. Pulsed Enhancement of Acoustic Cavitation: A Postulated Model. Ultrasound Med. Biol. 1981, 7, 159–166. [Google Scholar] [CrossRef]
- Gielen, B.; Kusters, P.; Jordens, J.; Thomassen, L.C.J.; Van Gerven, T.; Braeken, L. Energy Efficient Crystallization of Paracetamol Using Pulsed Ultrasound. Chem. Eng. Process. Process Intensif. 2017, 114, 55–66. [Google Scholar] [CrossRef]
- Sutkar, V.S.; Gogate, P.R. Design Aspects of Sonochemical Reactors: Techniques for Understanding Cavitational Activity Distribution and Effect of Operating Parameters. Chem. Eng. J. 2009, 155, 26–36. [Google Scholar] [CrossRef]
- Rossi, D.; Jamshidi, R.; Saffari, N.; Kuhn, S.; Gavriilidis, A.; Mazzei, L. Continuous-Flow Sonocrystallization in Droplet-Based Microfluidics. Cryst. Growth Des. 2015, 15, 5519–5529. [Google Scholar] [CrossRef]
- Verhaagen, B.; Liu, Y.; Pérez, A.G.; Castro-Hernandez, E.; Fernandez Rivas, D. Scaled-Up Sonochemical Microreactor with Increased Efficiency and Reproducibility. ChemistrySelect 2016, 2, 136–139. [Google Scholar] [CrossRef] [Green Version]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A Standard Method to Calibrate Sonochemical Efficiency of an Individual Reaction System. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Asakura, Y. Experimental Methods in Sonochemistry. In Sonochemistry and the Acoustic Bubble; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 119–150. [Google Scholar] [CrossRef]
- Jordens, J.; De Coker, N.; Gielen, B.; Van Gerven, T.; Braeken, L. Ultrasound Precipitation of Manganese Carbonate: The Effect of Power and Frequency on Particle Properties. Ultrason. Sonochem. 2015, 26, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Jordens, J.; Honings, A.; Degrève, J.; Braeken, L.; Van Gerven, T. Investigation of Design Parameters in Ultrasound Reactors with Confined Channels. Ultrason. Sonochem. 2013, 20, 1345–1352. [Google Scholar] [CrossRef] [Green Version]
- Pohl, B.; Jamshidi, R.; Brenner, G.; Peuker, U.A. Experimental Study of Continuous Ultrasonic Reactors for Mixing and Precipitation of Nanoparticles. Chem. Eng. Sci. 2012, 69, 365–372. [Google Scholar] [CrossRef]
- Sáez, V.; Frías-Ferrer, A.; Iniesta, J.; González-García, J.; Aldaz, A.; Riera, E. Chacterization of a 20 KHz Sonoreactor. Part I: Analysis of Mechanical Effects by Classical and Numerical Methods. Ultrason. Sonochem. 2005, 12, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshidi, R.; Rossi, D.; Saffari, N.; Gavriilidis, A.; Mazzei, L. Investigation of the Effect of Ultrasound Parameters on Continuous Sonocrystallization in a Millifluidic Device. Cryst. Growth Des. 2016, 16, 4607–4619. [Google Scholar] [CrossRef]
- Higbie, R. The Rate of Absorption of a Pure Gas into a Still Liquid during Short Periods of Exposure. Trans. Am. Inst. Chem. Eng. 1935, 31, 365–389. [Google Scholar]
- Xie, Y.; Chindam, C.; Nama, N.; Yang, S.; Lu, M.; Zhao, Y.; Mai, J.D.; Costanzo, F.; Jun Huang, T. Exploring Bubble Oscillation and Mass Transfer Enhancement in Acoustic-Assisted Liquid-Liquid Extraction with a Microfluidic Device. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandiono, T.; Ohl, S.-W.; Ow, S.-W.D.; Ohl, C.-D. Microfluidic Devices and Methods for Providing an Emulsion of a Plurality of Fluids. WO2013/184075 A1, 2013. [Google Scholar]
- Ohl, S.-W.; Tandiono, T.; Klaseboer, E.; Ow, D.; Choo, A.; Li, F.; Ohl, C.-D. Surfactant-Free Emulsification in Microfluidics Using Strongly Oscillating Bubbles. J. Acoust. Soc. Am. 2014, 136, 2289. [Google Scholar] [CrossRef]
- Navarro-Brull, F.J.; Teixeira, A.R.; Zhang, J.; Gómez, R.; Jensen, K.F. Reduction of Dispersion in Ultrasonically-Enhanced Micropacked Beds. Ind. Eng. Chem. Res. 2018, 57, 122–128. [Google Scholar] [CrossRef]
- Zhao, S.; Dong, Z.; Chaoqun, Y.; Wen, Z.; Chen, G.; Yuan, Q. Liquid-Liquid Two-Phase Flow in Ultrasonic Microreactors: Cavitation, Emulsification and Mass Transfer Enhancement. AIChE J. 2018, 64, 1412–1423. [Google Scholar] [CrossRef]
- Stepišnik Perdih, T.; Zupanc, M.; Dular, M. Revision of the Mechanisms behind Oil-Water (O/W) Emulsion Preparation by Ultrasound and Cavitation. Ultrason. Sonochem. 2019, 51, 298–304. [Google Scholar] [CrossRef]
- Jordens, J.; Gielen, B.; Xiouras, C.; Hussain, M.N.; Stefanidis, G.D.; Thomassen, L.C.J.; Braeken, L.; Van Gerven, T. Sonocrystallisation: Observations, Theories and Guidelines. Chem. Eng. Process. Process Intensif. 2019, 139, 130–154. [Google Scholar] [CrossRef]
- De Castro, M.D.; Priego-Capote, F. Ultrasound-Assisted Crystallization (Sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef]
- Chen, Y.; Sabio, J.C.; Hartman, R.L. When Solids Stop Flow Chemistry in Commercial Tubing. J. Flow Chem. 2015, 5, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Shang, M.; Noël, T.; Su, Y.; Hessel, V. High Pressure Direct Synthesis of Adipic Acid from Cyclohexene and Hydrogen Peroxide via Capillary Microreactors. Ind. Eng. Chem. Res. 2016, 55, 2669–2676. [Google Scholar] [CrossRef]
- Yang, W.; Yang, H.; Ding, W.; Zhang, B.; Zhang, L.; Wang, L.; Yu, M.; Zhang, Q. High Quantum Yield ZnO Quantum Dots Synthesizing via an Ultrasonication Microreactor Method. Ultrason. Sonochem. 2016, 33, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, V.; Zaborenko, N.; Gu, L.; Jensen, K.F. Microfluidic Assisted Synthesis of Hybrid Au-Pd Dumbbell-like Nanostructures: Sequential Addition of Reagents and Ultrasonic Radiation. Cryst. Growth Des. 2017, 17, 2700–2710. [Google Scholar] [CrossRef]
- Hussain, M.N.; Jordens, J.; John, J.J.; Braeken, L.; Van Gerven, T. Enhancing Pharmaceutical Crystallization in a Flow Crystallizer with Ultrasound: Anti-Solvent Crystallization. Ultrason. Sonochem. 2019, 59, 104743. [Google Scholar] [CrossRef]
- Van Zwieten, R.; Verhaagen, B.; Schroën, K.; Fernández Rivas, D. Emulsification in Novel Ultrasonic Cavitation Intensifying Bag Reactors. Ultrason. Sonochem. 2017, 36, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Katayama, E.; Togashi, S.; Endo, Y. Production of AgCl Nanoparticles Using Microreactors. J. Chem. Eng. Japan 2010, 43, 1023–1028. [Google Scholar] [CrossRef]
- Sedelmeier, J.; Ley, S.V.; Baxendale, I.R.; Baumann, M. KMnO4-Mediated Oxidation as a Continuous Flow Process. Org. Lett. 2010, 12, 3618–3621. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, M.; Teng, P.; Zhao, D.; Lu, X.; Li, J.X. Ultrasound-Promoted Intramolecular Direct Arylation in a Capillary Flow Microreactor. Ultrason. Sonochem. 2012, 19, 250–256. [Google Scholar] [CrossRef]
- Gallaher, A.; Hannon, D.; Hardie, D. Improvements in and Relating to Sonochemistry. US20080217160, 28 July 2005. [Google Scholar]
- Koiranen, T.; Ekberg, B.; Häkkinen, A.; Varis, J.; Louhi-Kultanen, M. An Ultrasound Crystallization Device and an Ultrasound Crystallization System. US20190374872A1, 12 December 2019. [Google Scholar]
- Ezeanowi, N.; Koiranen, T. Effect of Process Parameters on a Novel Modular Continuous Crystallizer. In Proceedings of the 2nd International Process Intensification Conference (IPIC2), Leuven, Belgium, 27–29 May 2019. Abstract no. 203. [Google Scholar]
- Karvonen, V.; Häkkinen, A.; Louhi-Kultanen, M.; Koiranen, T. Method and Apparatus for Continuous Crystallization and Use Thereof. WO 2016/107968 AI, 2016. [Google Scholar]
- Jensen, K.F.; Reizman, B.J.; Newman, S.G. Tools for Chemical Synthesis in Microsystems. Lab Chip 2014, 14, 3206–3212. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, K.; Teixeira, A.R.; Jensen, K.F.; Luo, G. Design and Scaling Up of Microchemical Systems: A Review. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 13.1–13.21. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.G. Using Continuous Processes to Increase Production. Org. Process Res. Dev. 2012, 16, 852–869. [Google Scholar] [CrossRef]
- Fernandez Rivas, D.; Verhaagen, B.; Galdamez Perez, A.; Castro-Hernandez, E.; Van Zwieten, R.; Schroen, K. A Novel Ultrasonic Cavitation Enhancer. J. Phys. Conf. Ser. 2015, 656, 012112. [Google Scholar] [CrossRef] [Green Version]
- Nickel, K.; Neis, U. Ultrasonic Disintegration of Biosolids for Improved Biodegradation. Ultrason. Sonochem. 2007, 14, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Gondrexon, N.; Renaudin, V.; Petrier, C.; Boldo, P.; Bernis, A.; Gonthier, Y. Degradation of Pentachlorophenol Aqueous Solutions Using a Continuous Flow Ultrasonic Reactor: Experimental Performance and Modelling. Ultrason. Sonochem. 1999, 5, 125–131. [Google Scholar] [CrossRef]
- Cintas, P.; Mantegna, S.; Gaudino, E.C.; Cravotto, G. A New Pilot Flow Reactor for High-Intensity Ultrasound Irradiation. Application to the Synthesis of Biodiesel. Ultrason. Sonochem. 2010, 17, 985–989. [Google Scholar] [CrossRef]


| Method | Type of Method | Objectives | Materials | Reference |
|---|---|---|---|---|
| Sonochemiluminescence of luminol | Experimental, Chemical, Qualitative | Observation of cavitation activity distribution | Aqueous solution of luminol and sodium hydroxide. | [76,120,121] |
| Dosimetries: salicylic acid, Fricke, Weissler, terephthalic acid | Experimental, Chemical, Qualitative | General cavitation activity measurement, cavitation yield | Analysis method: spectrophotometry, HPLC analysis. | [119,121,122] |
| Hydrophone measurement | Experimental, Physical, Quantitative | Acoustic pressure mapping. Observation of standing waves. | Hydrophone probe, oscilloscope. | [76,119,121] |
| Temperature mapping | Experimental, Physical, Qualitative | Temperature mapping to observe hot spots. | Thermal camera. | [106] |
| Calorimetric measurement | Experimental, Physical, Quantitative | Temperature rise measurements. Estimation of power density. | Temperature probe. | [30,122,123] |
| Impedance measurement | Experimental, Physical, Quantitative | Resonance conditions: resonance and anti-resonance frequency. | Impedance analyzer | [34,45,124] |
| Pressure acoustic mapping | Numerical, Quantitative | Helmholtz equation | Numerical simulation software | [119,120,125] |
| Simulation of primary and secondary effect | Numerical, Quantitative | Temperature, bubble yield | Numerical simulation software | [119,125] |
| Processes | Ultrasound Effect and Application | Reactor Description | Reactor Scale | Reference |
| Liquid (single phase) | Cavitation to improve mixing of dye and water | Langevin-type transducer reactor, direct coupling 20 kHz, 10–30 W Silicon microreactor: channel size 1 × 1 mm2, 0.5 × 0.5 mm2 and 0.5 × 0.25 mm2 | Laboratory scale | [24] |
| Cavitation to improve mixing of glycerol and water | Piezoelectric plate reactor 38.9 kHz, 160 Vpp PDMS microreactor: channel size 0.24 × 0.15 mm2 | Laboratory scale | [75] | |
| Ultrasound assisted nitration of toluene | Langevin-type transducer reactor, hybrid contact 21 kHz, 50 W Stainless steel capillary: inner diameter 0.6–1 mm | Laboratory scale | [67] | |
| Gas/liquid | Cavitation and surface wave oscillation to improve gas-liquid mass transfer for carbon dioxide absorption | Langevin-type transducer reactor, direct coupling 20 kHz, 10–50 W Silicon microreactor: channel size 1 × 1 mm2, 0.5 × 0.5 mm2 and 0.5 × 0.25 mm2 | Laboratory scale | [38] |
| Gas/liquid/solid | Sonication to partially fluidize a micro-packed-bed reactor to reduce gas-channeling | Langevin-type transducer reactor, direct coupling 38 kHz, 20 W Micropacked-bed reactor: inner diameter 3.175 mm, diameter of packed beads 0.2 mm | Laboratory scale | [133] |
| Liquid/liquid | Surface wave oscillation with the introduction of a gas phase to improve liquid-liquid extraction | Langevin-type transducer reactor, direct coupling 20 kHz, 5–30 W Silicon microreactor: channel size 1 × 1 mm2 | Laboratory scale | [39] |
| piezoelectric plate reactor 1–100 kHz, 10–20 Vpp PDMS microreactor: channel size 0.2 × 0.05 mm2 | Laboratory scale | [130] | ||
| Ultrasound assisted reactive extraction of p-nitrophenylacetate | Langevin-type transducer reactor, direct contact 20.3 kHz, 20–29 W PFA Capillary: inner diameter 0.8 mm | Laboratory scale | [108] | |
| Langevin-type transducer reactor, hybrid contact 20–65 kHz, 20 W PFA Capillary: inner diameter 0.8–2 mm | Scale up strategy: scale out | [115] | ||
| Cavitation to emulsify and improve mixing for the extraction of rhodamine B from water to 1-octanol | Langevin-type transducer reactor, direct coupling 20 kHz, 10–30 W Silicon microreactor: channel size 1 × 1 mm2 and 0.5 × 0.5 mm2 | Laboratory scale | [134] | |
| Ultrasound for oil-water emulsion and PLGA nanoparticle synthesis | Langevin-type transducer reactor, indirect coupling 24 kHz, 17–32 W Glass tube: inner diameter 2 mm | Laboratory scale | [98] | |
| Cavitation to enhance emulsification of hexadecane in SDS aqueous emulsion | Ultrasonic bath reactor 37 and 80 kHz, around 180 W Cavitation intensification bag: plastic bag with pits | Laboratory and large scale Scale up strategy: numbering up | [143] | |
| Processes | Ultrasound Effect and Application | Reactor Description | Reactor Scale | Reference |
| Liquid/solid Material synthesis | Cavitation leading to milder reaction conditions applied to Dumbbell shaped Au-Pd nanoparticle synthesis | Piezoelectric plate reactor 40 kHz, 30 W Silicon microreactor: square channel 0.4 × 0.4 mm2 | Laboratory scale | [141] |
| Cavitation to prevent of clogging for AgCl nanoparticle synthesis | Ultrasonic bath reactor 40 kHz, power not mentioned PTFE Tube: inner diameter 1 and 2 mm | Laboratory scale | [144] | |
| Cavitation to change structure of ZnO quantum dots due to high energy hotspots | Ultrasonic bath reactor 53 kHz, 72–180 W PTFE Tube: inner diameter 0.8 mm | Laboratory scale | [140] | |
| Cavitation to promote uniform particle shape and size, improved crystal quality applied to precipitation of hydroxyapatite. | Reactor type 1: ultrasonic bath reactor 40 kHz, 4–8 W Teflon Tube: inner diameter 1.02 mm Reactor type 2: piezoelectric plate reactor 50 kHz, 30 W Teflon microreactor: channel width 0.6 mm | Laboratory scale | [44] | |
| Cavitation for clogging prevention, particle size control applied to barium sulfate precipitation | Langevin-type transducer reactor, direct coupling 21–46 kHz, 11–23 W Silicon microreactor: square channel 0.6 × 0.6 mm2 | Laboratory scale | [45] | |
| Acoustophoresis for clogging prevention, particle size control applied to particle synthesis | Piezoelectric plate reactor 1.21 MHz, 0.3–3.3 W Silicon microreactor: square channel 0.6 × 0.6 mm2 | Laboratory scale | [49] | |
| Combining cavitation and acoustophoresis for particle synthesis | Piezoelectric plate reactor 61.7 kHz (8 W) and 1.21 MHz (1.6 W), pulse and switch mode Silicon microreactor: square channel 0.6 × 0.6 mm2 | Laboratory scale | [93] | |
| Liquid/solid Organic synthesis | Cavitation for clogging prevention applied to C–N cross coupling reaction | Ultrasonic bath reactor 41.5 kHz, power not mentioned PFA tube: inner diameter 1.01 mm | Laboratory scale | [113] |
| Ultrasonic bath reactor 41.5 kHz, power not mentioned PFA tube: inner diameter 0.5 and 1 mm | Laboratory scale | [15] | ||
| Piezoelectric plate reactor 50 kHz, 30 W Teflon microreactor: channel width 0.6 mm | Laboratory scale | [43] | ||
| Cavitation for clogging prevention applied to KMnO4 oxidation | Ultrasonic bath reactor 44 kHz, pulsed (5 s every minute), power not mentioned PFA tube: inner diameter 0.5 mm | Laboratory scale | [145] | |
| Cavitation for clogging prevention applied to photodimerization of maleic anhydride | Ultrasonic bath reactor 39 kHz, 100 W FEP tube: inner diameter 0.5–1.6 mm | Laboratory scale | [112] | |
| Cavitation for clogging prevention applied to arylation of aryl bromides | Ultrasonic bath reactor 40 kHz, 150 W Capillary coil: inner diameter 0.53 mm | Laboratory scale | [146] | |
| Liquid/solid Sonocrystallization | Enhanced nucleation with ultrasound for adipic acid crystallization | Langevin-type transducer reactor, indirect coupling 20 kHz, 750 W, Amplitude 21% PFA Capillary: inner diameter 1 mm | Laboratory scale | [120] |
| Enhanced anti-solvent mixing, reduced induction times and anti-solvent crystallization at a lower supersaturation with ultrasound for acetyl salicylic acid crystallization | Langevin-type transducer reactor, hybrid contact 42 kHz, 7–24 W PFA Capillary: inner diameter 2 mm | Laboratory scale | [142] | |
| Increased nucleation rate and smaller crystals size with pulsed ultrasound for adipic acid crystallization | Piezoelectric plate reactor 42–1090 kHz, pulsed, 400 mVpp, duty cycle 1%–7% Glass milli-reactor: channel 2 × 5 mm2 | Scale up strategy: micro to milliscale | [128] | |
| Backmixing lead to lower yield, smaller crystal size with ultrasound | Langevin-type transducer reactor, indirect coupling 20 kHz, 750 W, amplitude 21% FEP Capillary: diameter 1.55 and 3.2 mm | Laboratory scale | [105] | |
| Cavitation for clogging prevention applied to crystallization processes (Patent) | Piezoelectric plate reactor Piezoelectric ring attached to tubing with adaptable diameter | Scale up strategy: micro to milliscale and parallel numbering-up | [147] | |
| Langevin-type transducer reactor, direct coupling Reactor wrapped as a helix around a sonotrode | Scale up strategy: micro to milliscale and parallel numbering up | [148,149] | ||
| Langevin-type transducer reactor, indirect coupling Reactor wrapped as a helix and immersed in a jacketed beaker for temperature control. Ultrasonic transducer attached to the bottom of the beaker | Scale up strategy: micro to milliscale | [150] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Delacour, C.; Mc Carogher, K.; Udepurkar, A.P.; Kuhn, S. Continuous Ultrasonic Reactors: Design, Mechanism and Application. Materials 2020, 13, 344. https://doi.org/10.3390/ma13020344
Dong Z, Delacour C, Mc Carogher K, Udepurkar AP, Kuhn S. Continuous Ultrasonic Reactors: Design, Mechanism and Application. Materials. 2020; 13(2):344. https://doi.org/10.3390/ma13020344
Chicago/Turabian StyleDong, Zhengya, Claire Delacour, Keiran Mc Carogher, Aniket Pradip Udepurkar, and Simon Kuhn. 2020. "Continuous Ultrasonic Reactors: Design, Mechanism and Application" Materials 13, no. 2: 344. https://doi.org/10.3390/ma13020344





