Leaching of Carbon Reinforced Concrete—Part 1: Experimental Investigations
Abstract
:1. Introduction
- Wet–dry stress: In porous building materials, the drying phases cause a transport of water and dissolved substances to the surface by capillary transport during drying dissolved substances precipitate at the surface. This leads to an increased availability for leaching or wash-off in the following rain [11,13]. The microstructure plays a major role for the transport processes, especially under wet–dry stress. This might lead to changing release patterns over time, as moisture transport additionally fosters transformations of the matrix structure by causing pore changes through, e.g., micro-cracking or shrinkage [11,13,15,18].
- Substance-dependent leaching: Leaching rates depend on physical and chemical properties of the substances and the building material, especially the solubility of the particular substances, which can be pH dependent [9,17,19,21,22]. For intermittent moistened construction elements, the pH of the material, not of the leachate, is pivotal [13,19]. Wet–dry stress, temperature changes and air contact can alter the matrices over time; for example, by carbonation, which leads to decreasing pH values. This influences the long-term leaching [11,19,21,22].
2. Materials and Methods
2.1. Materials
2.1.1. Concrete Composition
2.1.2. Reinforcement
2.2. Test Specimens
2.3. Methods
2.3.1. pH Dependence Test (pHstat)
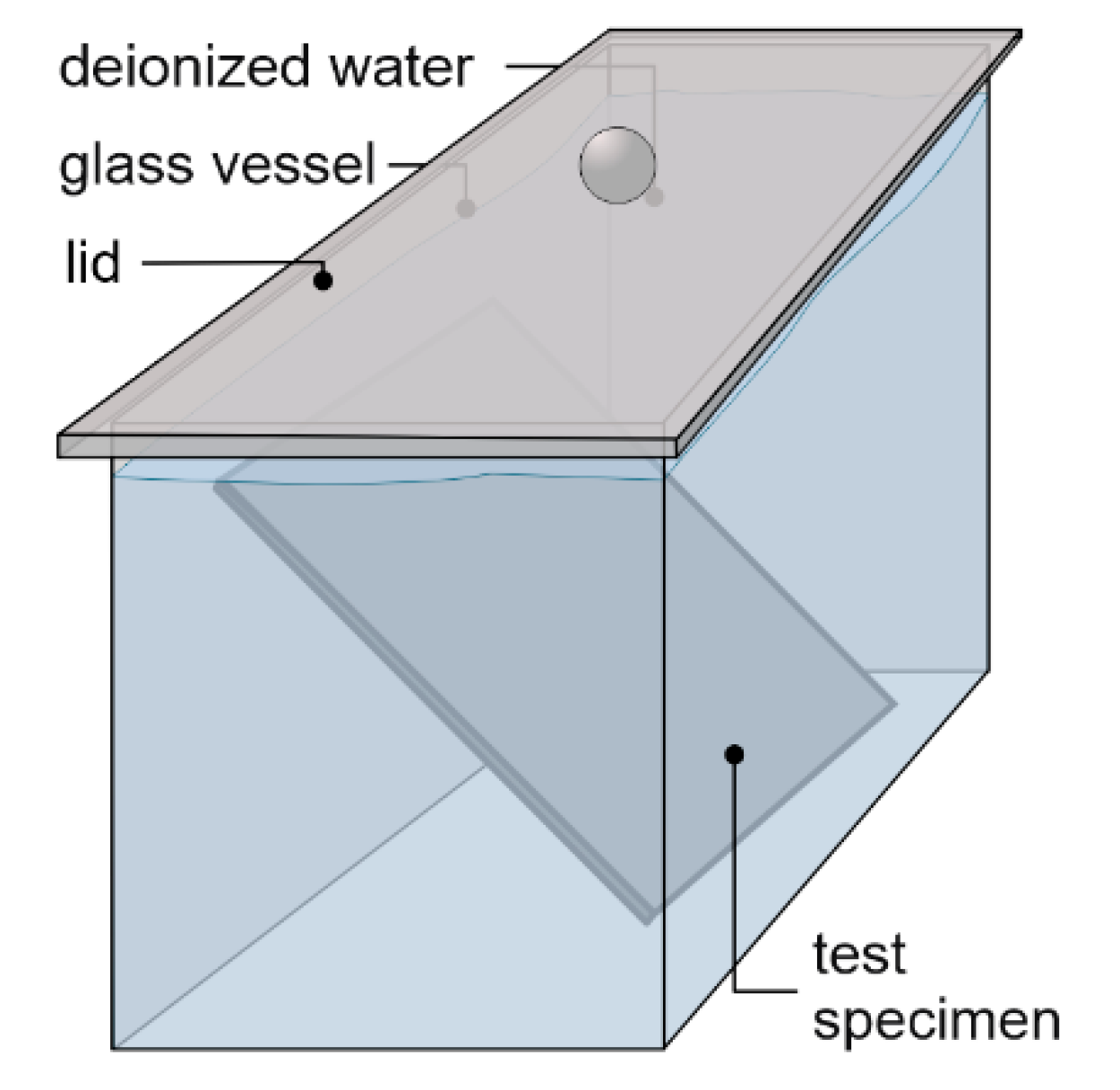
2.3.2. Dynamic Surface Leaching Test (DSLT)
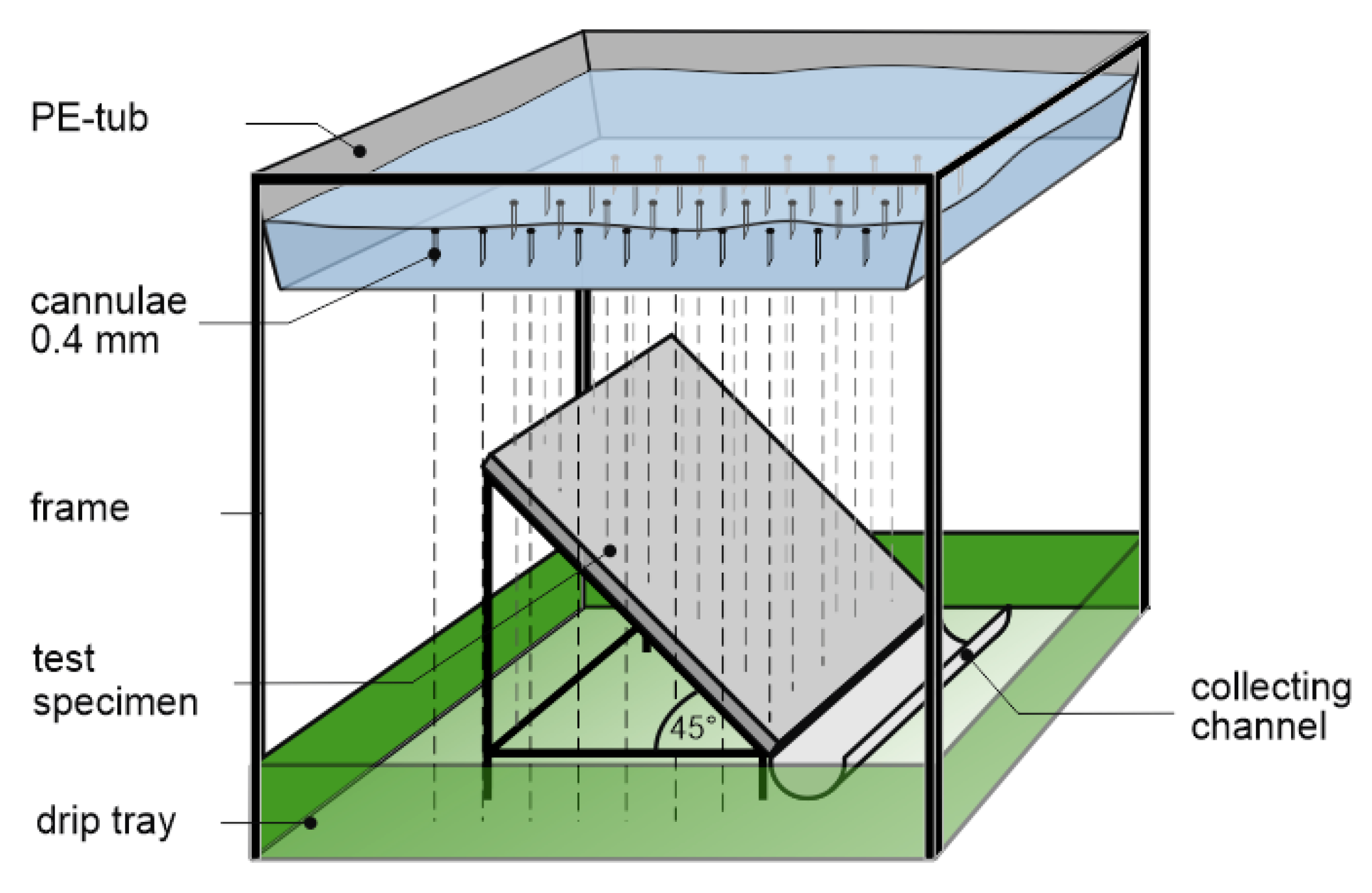
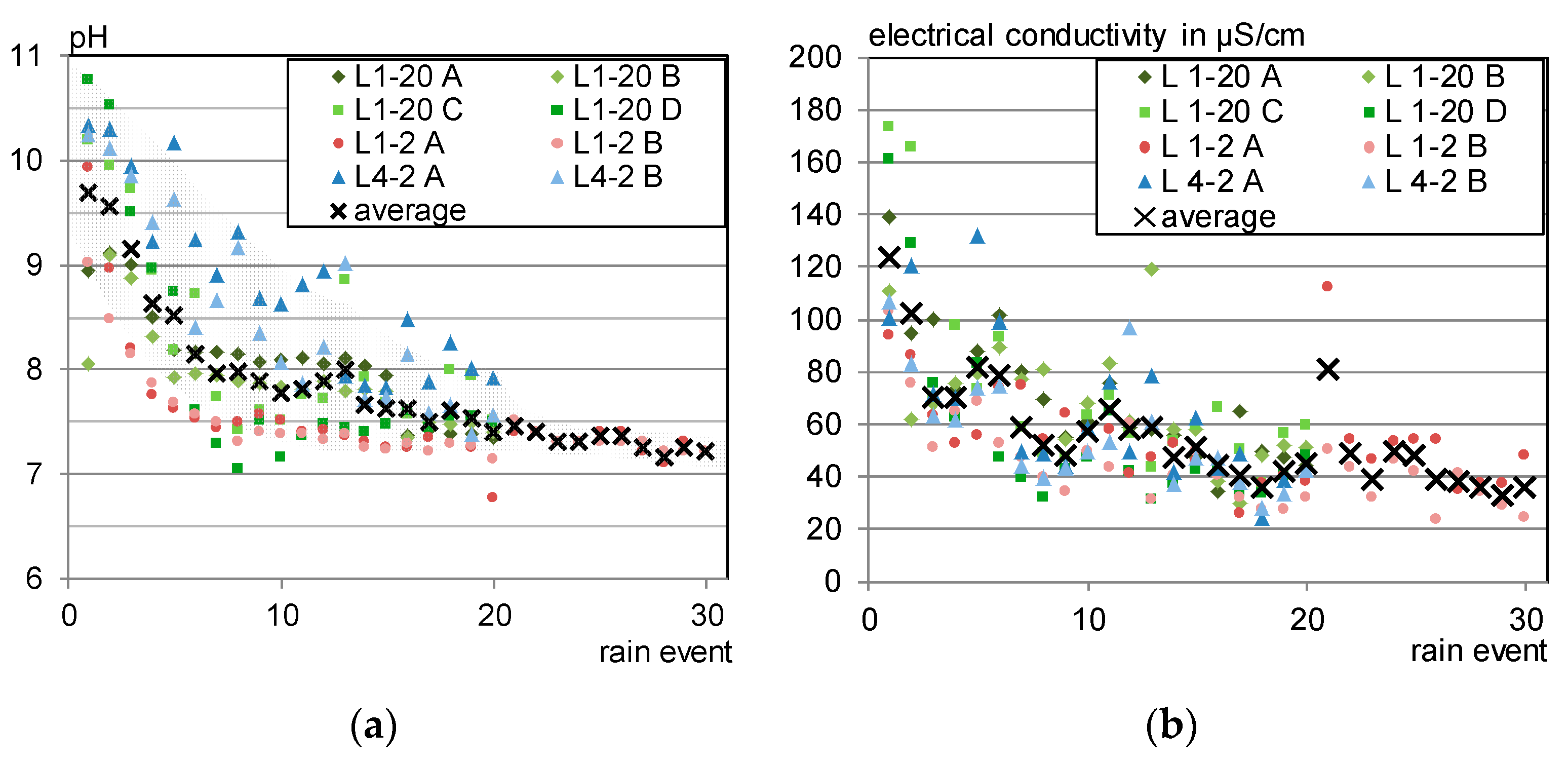
2.3.3. Laboratory Irrigation
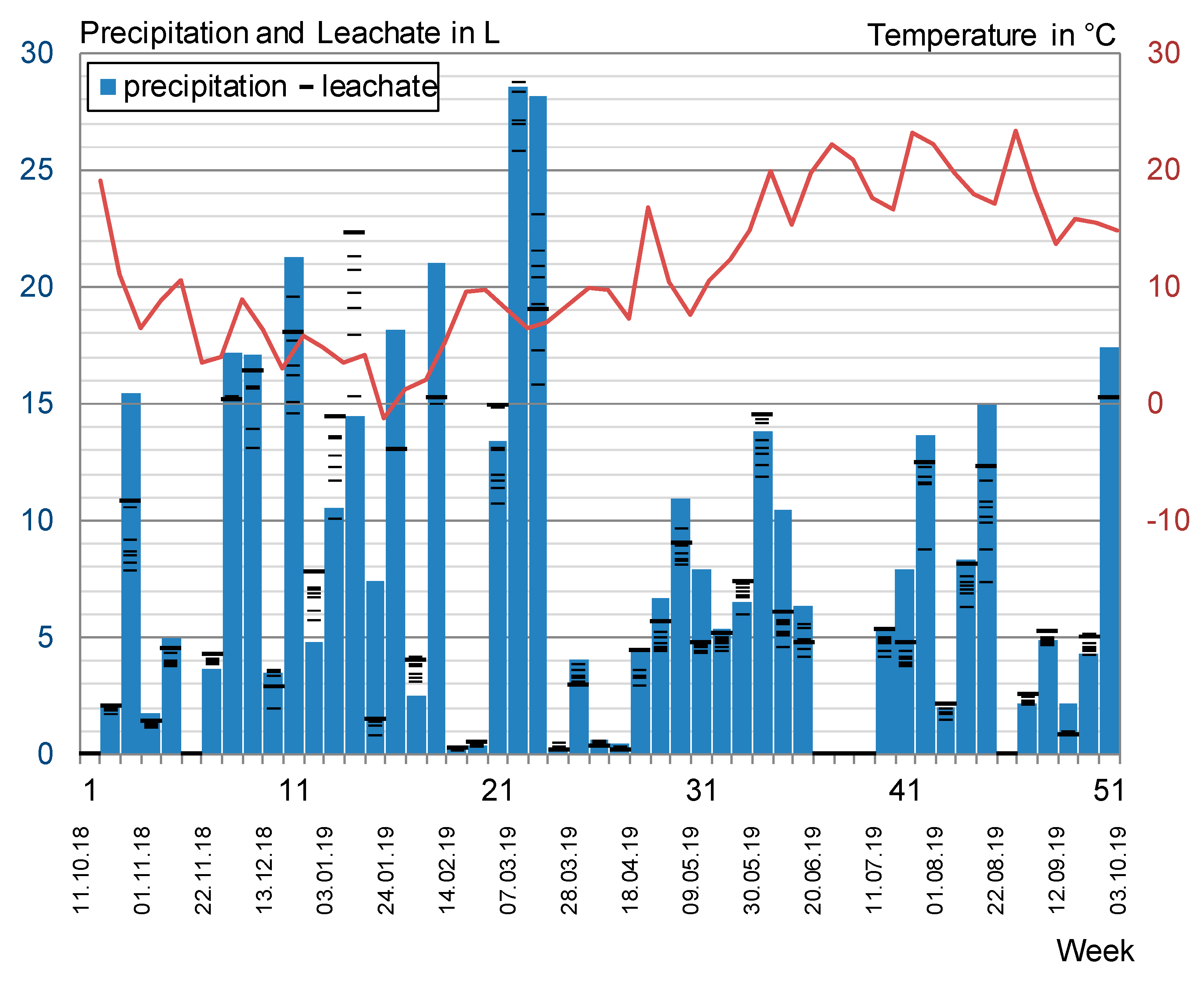
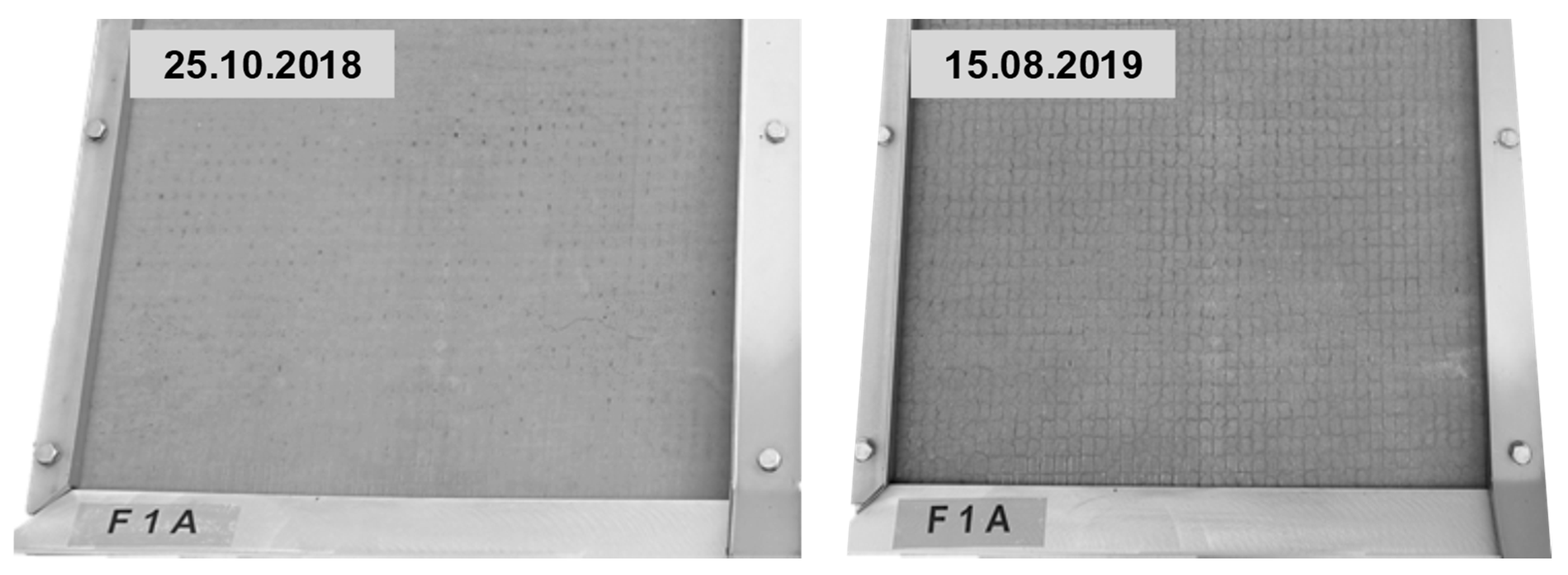
2.3.4. Outdoor Testing
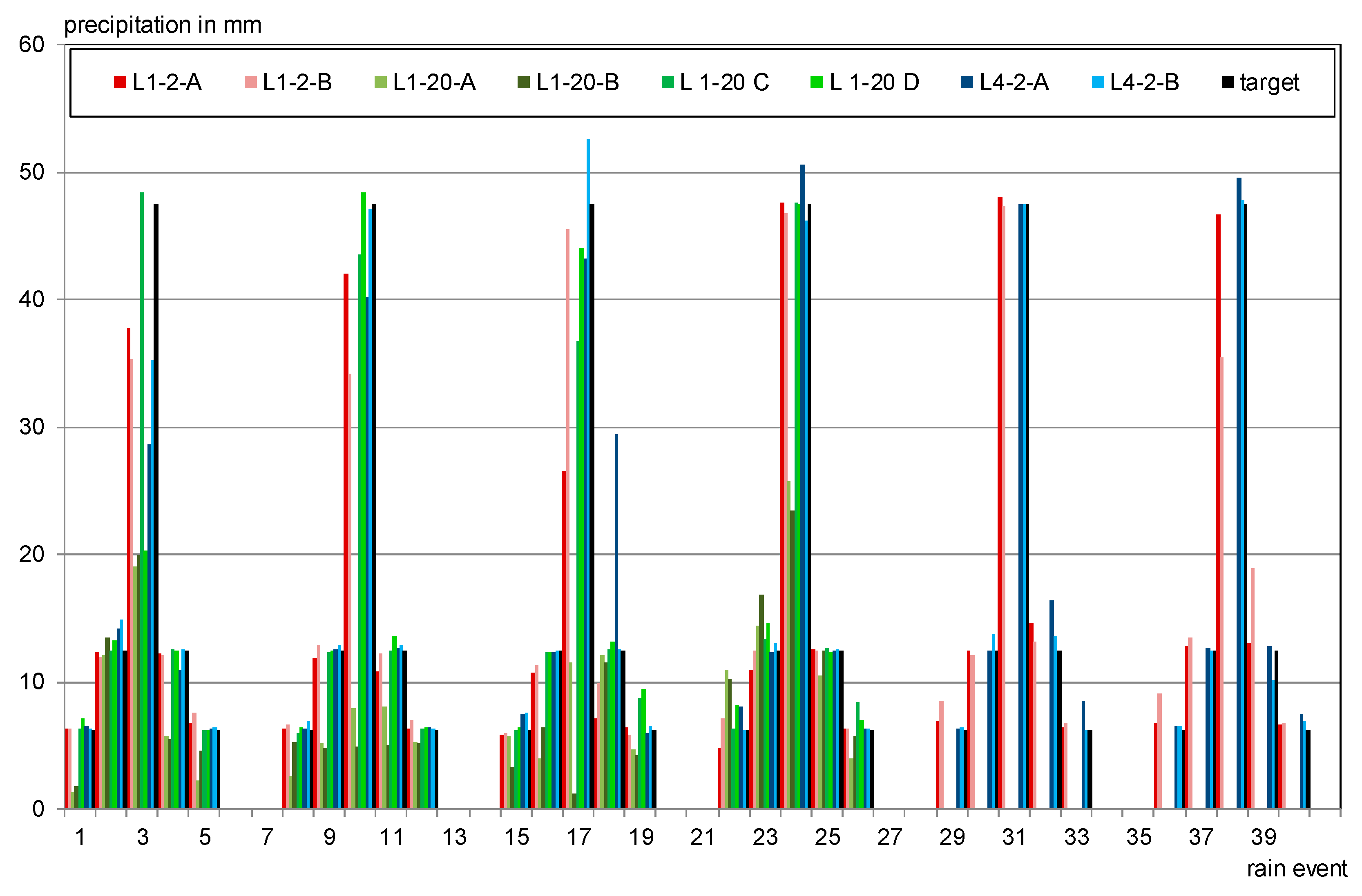
2.4. Testing Program
3. Results
3.1. Preliminary Note
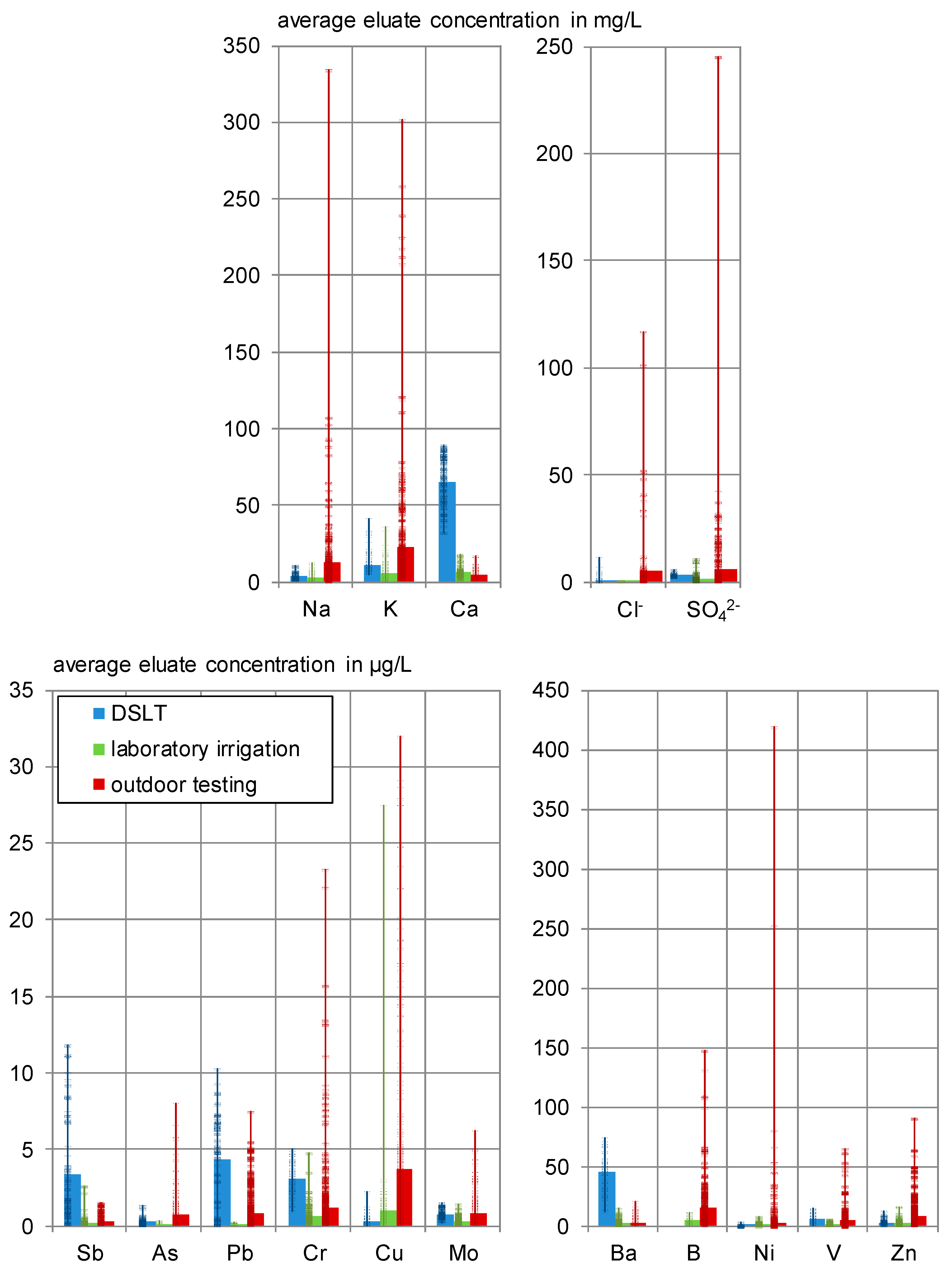
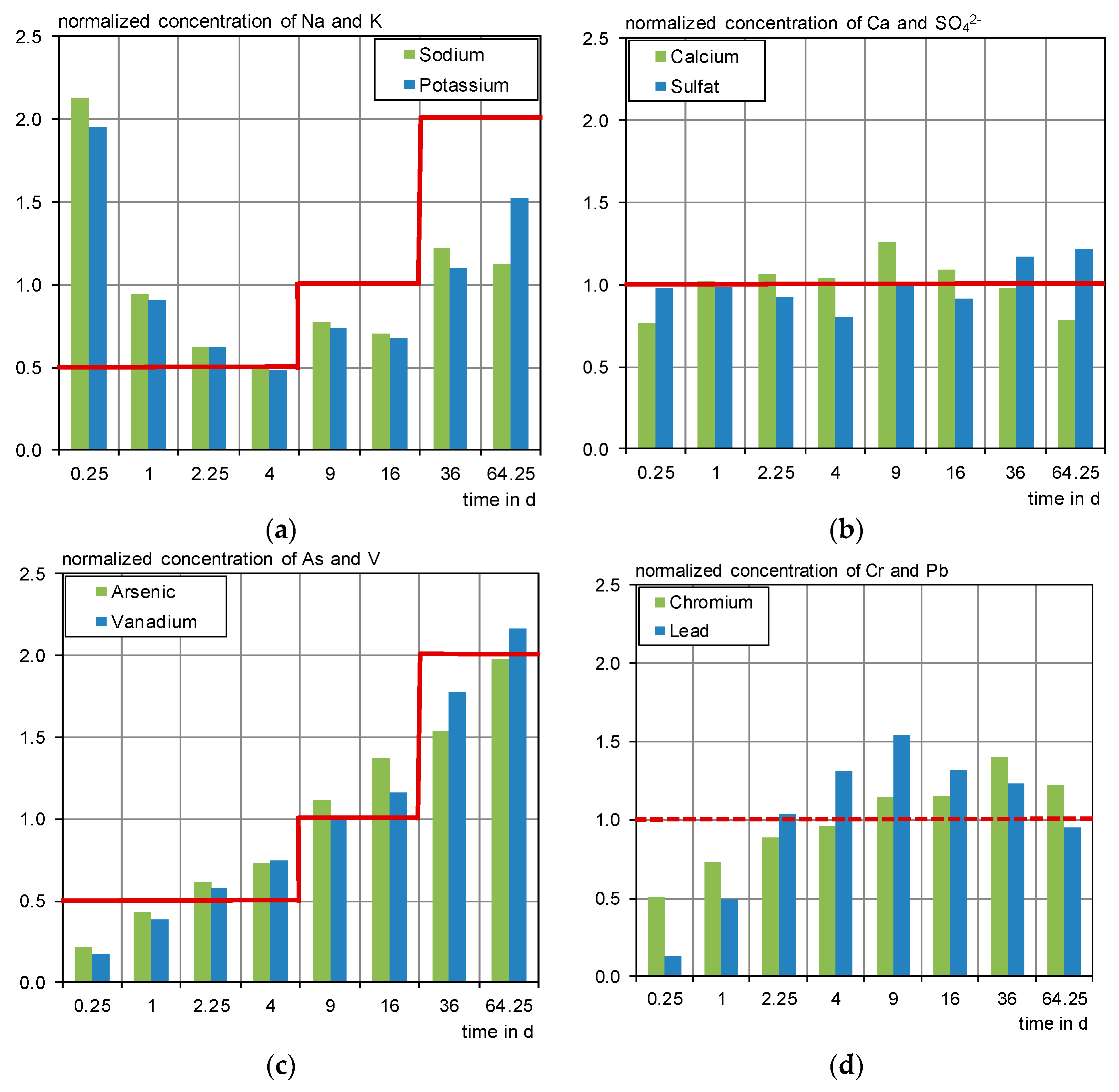
3.2. Overview of Eluate Concentrations in the Different Tests
- Cadmium and thallium (determination limit: 0.1 µg/L) could not be detected in any eluate in the laboratory irrigation and were detected in maximum 10% of the leachates due to the background concentration outdoor. They were at no time leached from the specimens.
- Cobalt (determination limit: 0.1 µg/L) could not be detected in any eluate in the laboratory irrigation and was found in 92% of the outdoor leachates, always in the range of the background concentrations. A slightly increasing tendency was observed outdoor.
- In the laboratory mercury was detected in concentrations close to the detection limit (0.01 < c ≤ 0.023 µg/L) in 29% of the eluates. Five of eight test specimens (L 4-2 A + B, L 1-2 A, and L 1-20 C + D) and the outdoor experiments showed no detectable mercury leaching at all.
- Outdoor antimony (detected in 74% of the eluates), lead (93%) and zinc (100%) were released from C3 in only 6 to 10% of the samples.
- Chloride (determination limit: 0.1 mg/L) was detected in concentrations of 0.1 < c ≤ 0.4 mg/L in only 13% of the laboratory eluates. In 94% of the outdoor leachates, emissions with an average concentration of 2.28 mg/L were measured.
- Copper and zinc showed relatively high releases in the laboratory irrigations’ blind testing, which has to be considered in the interpretation of the results. It is assumed that zinc as a ubiquitous element is introduced through several pathways. Copper could be leached from the cannulae that are indicated as stainless steel. This steel might have copper residues or is alloyed with it to enhance corrosion and acid resistance [39].
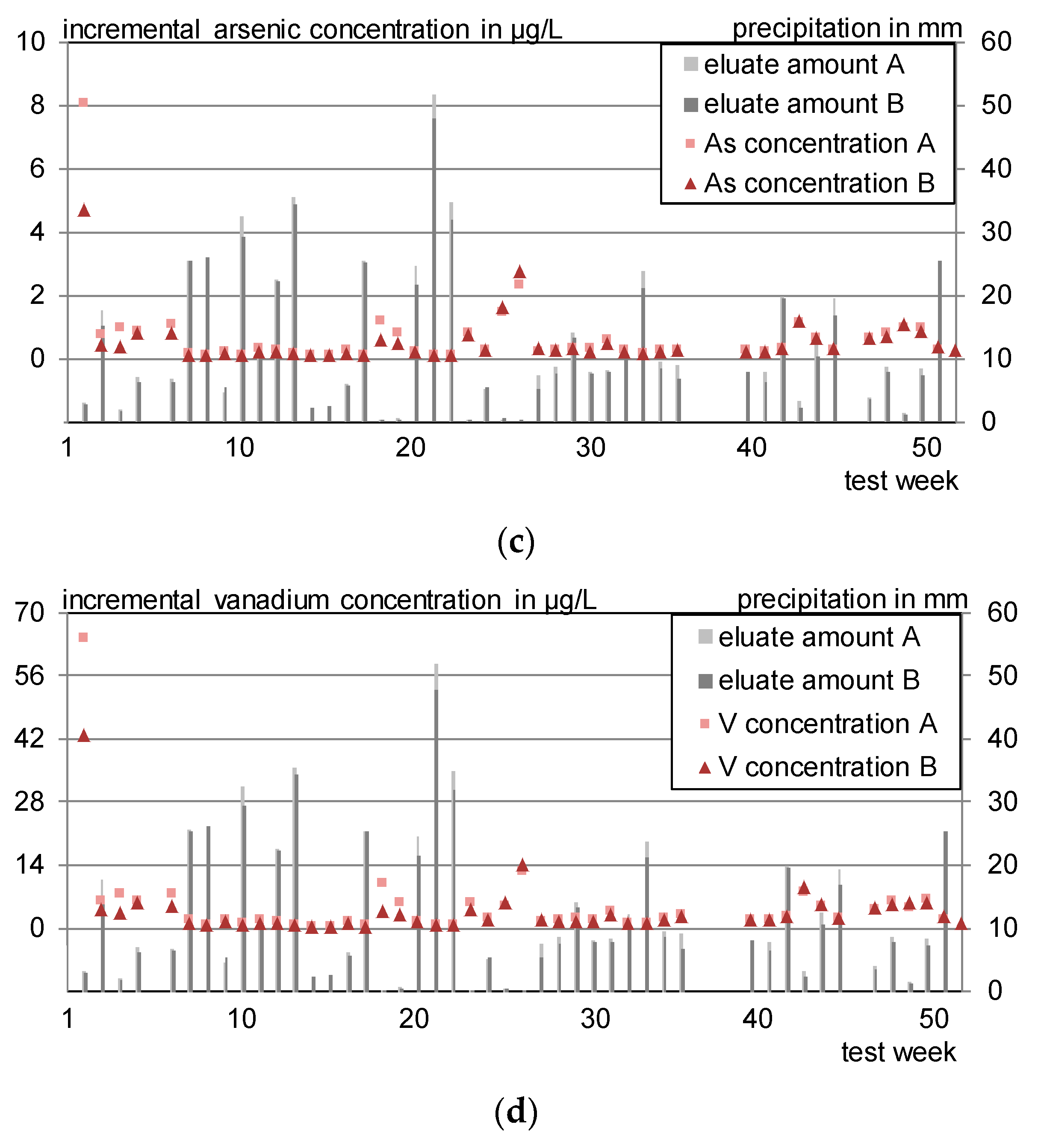
- Arsenic, lead and selenium were detected in 18% (As, Se), respectively, 7% (Pb) of the laboratory eluates in concentrations of 0.1 < c < 0.3 µg/L. Outdoors, arsenic is leached and lead is adsorbed. Selenium is mainly leached in concentrations below 0.1 µg/L.
- Sodium, potassium, calcium, barium, boron, chromium, and vanadium were leached in 70 to 100% of the outdoor samples and detected in 100% of the laboratory eluates.
3.3. Laboratory Irrigation
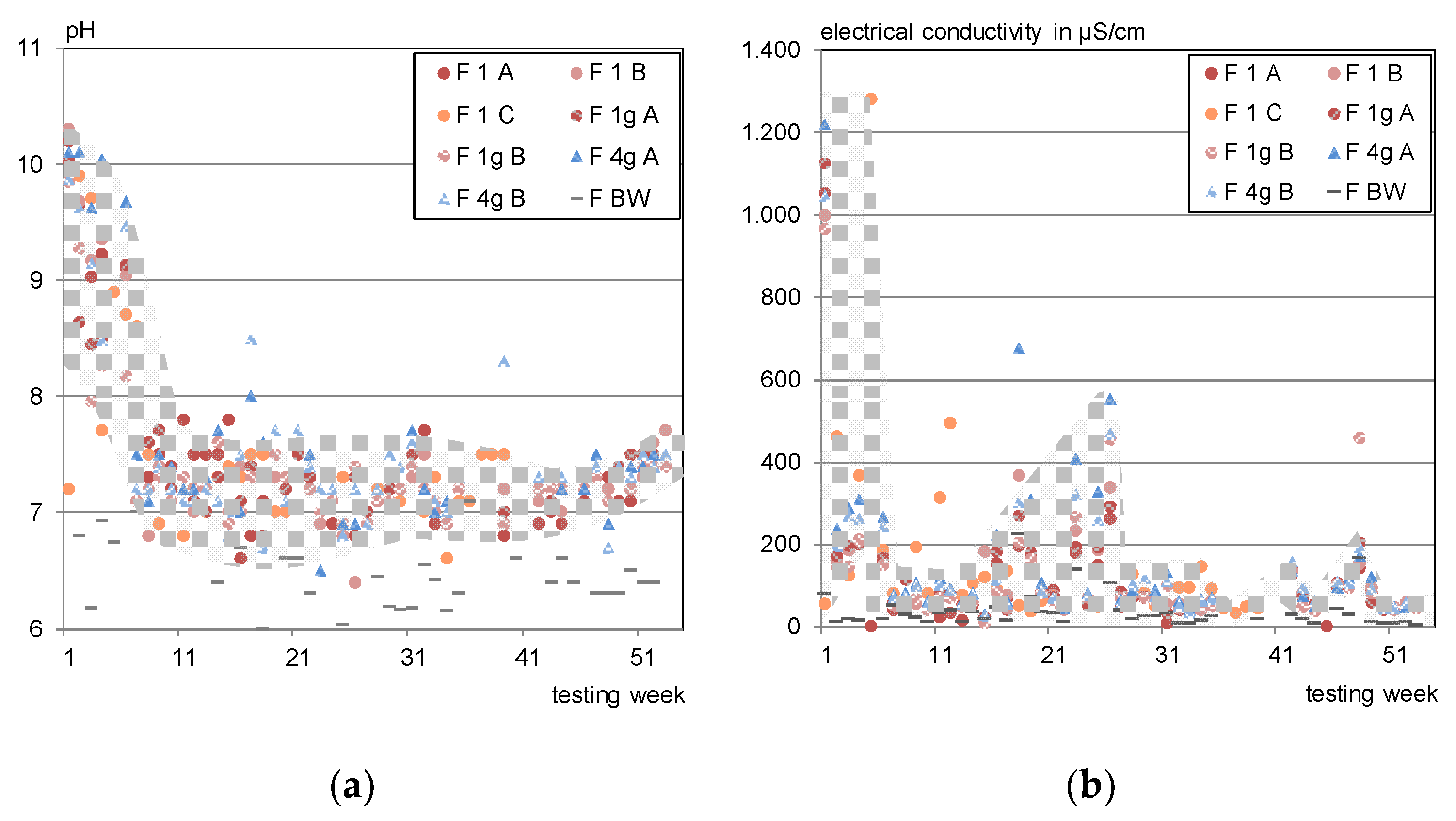
3.4. Outdoor Testing
3.5. Standardized Leaching Tests
- Sodium leaches strongest under outdoor conditions, which confirms the findings concerning the influence of dry phases from the laboratory irrigation. It is assumed that capillary transport mechanisms transport dissolved substances to the surface as described in [11,13] and therefore cause an increased availability of sodium on the test specimens’ surface.
- Sulphate also shows higher outdoor concentrations but considering the blank, the net concentrations of sulphate were mainly below the DSLT and in the range of the lab data.
- Calcium concentrations show a good concordance between laboratory and outdoor irrigation.
- Arsenic, chromium and vanadium leach more strongly in the outdoor irrigation compared to the laboratory, especially for higher pH values.
4. Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lupsea, M.; Tiruta-Barna, L.; Schiopu, N. Leaching of hazardous substances from a composite construction product—An experimental and modelling approach for fibre-cement sheets. J. Hazard. Mater. 2014, 264, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, N.; Jayr, E.; Méhu, J.; Barna, L.; Moszkowicz, P. Horizontal environmental assessment of building products in relation to the construction products directive (CPD). Waste Manag. (N. Y.) 2007, 27, 1436–1443. [Google Scholar] [CrossRef]
- Wicke, D.; Matzinger, A.; Rouault, P. Relevanz Organischer Spurenstoffe im Regenwasserabfluss Berlins—OgRe. (Relevance of Organic Trace Substances in the Rainwater Discharge of Berlin); Kompetenzzentrum Wasser Berlin GmbH: Berlin, Germany, 2017. [Google Scholar]
- Persson, D.; Kucera, V. Release of Metals from Buildings, Constructions and Products during Atmospheric Exposure in Stockholm. Water Air Soil Pollut. Focus 2001, 1, 133–150. [Google Scholar] [CrossRef]
- Björklund, K. Substance flow analyses of phthalates and nonylphenols in stormwater. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2010, 62, 1154–1160. [Google Scholar] [CrossRef]
- European Parliament. Directive 2013/39/EU of the European Parliament and of the Council on Environmental Quality Standards in the Field of Water Policy; European Parliament: Strasbourg, France, 2013. [Google Scholar]
- Clara, M.; Ertl, T.; Giselbrecht, G.; Gruber, G.; Hofer, T.F.; Humer, F.; Kretschmer, F.; Kolla, L.; Scheffknecht, C.; Weiß, S.; et al. SCHTURM—Spurenstoffemissionen Aus Siedlungsgebieten Und Von Verkehrsflächen: Studie Im Auftrag Des Bundesministeriums Für Land- Und Forstwirtschaft, Umwelt Und Wasserwirtschaft. (Trace Substances Emissions from Residential and Traffic Areas); BMLFUW: Wien, Austria, 2014. [Google Scholar]
- Gasperi, J.; Sebastian, C.; Ruban, V.; Delamain, M.; Percot, S.; Wiest, L.; Mirande, C.; Caupos, E.; Demare, D.; Kessoo, M.D.K.; et al. Micropollutants in urban stormwater: Occurrence, concentrations, and atmospheric contributions for a wide range of contaminants in three French catchments. Environ. Sci. Pollut. Res. Int. 2014, 21, 5267–5281. [Google Scholar] [CrossRef] [Green Version]
- Vollpracht, A.; Brameshuber, W. Investigations on the leaching behaviour of irrigated construction elements. Environ. Sci. Pollut. Res. Int. 2010, 17, 1177–1182. [Google Scholar] [CrossRef]
- CEN European Committee for Standardization. EN 16105 Paints and Varnishes—Laboratory Method for Determination of Release of Substances from Coatings in Intermittent Contact with Water; CEN European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- Hecht, M. Quellstärke ausgewählter Betone in Kontakt mit Sickerwasser. (Source Strength of Selected Concretes in Contact with Leachates). Beton Und Stahlbetonbau 2005, 100, 85–88. [Google Scholar] [CrossRef]
- Burkhardt, M.; Junghans, M.; Zuleeg, S.; Boller, M.; Schoknecht, U.; Lamani, X.; Bester, K.; Vonbank, R.; Simmler, H. Biozide in Gebäudefassaden—Ökotoxikologische Effekte, Auswaschung und Belastungsabschätzung für Gewässer. (Biocides in facades of buildings—Ecotoxicologic effects, wash off and pollution assessment for water bodies). Environ. Sci. Eur. 2009, 21, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Schoknecht, U.; Gruycheva, J.; Mathies, H.; Bergmann, H.; Burkhardt, M. Leaching of biocides used in façade coatings under laboratory test conditions. Environ. Sci. Technol. 2009, 43, 9321–9328. [Google Scholar] [CrossRef]
- Bruntsch, S.; Nebel, H. Untersuchung Des Auslaugverhaltens Von Metallischen Werkstoffen. Bachelor’s Thesis, RWTH Aachen University, Aachen, 2011. [Google Scholar]
- Nebel, H.; Vollpracht, A.; Brameshuber, W. Auslaugverhalten von Putzen und Mörteln. (Leaching behaviour of renderings and mortars). Mauerwerk 2012, 16, 2–9. [Google Scholar] [CrossRef]
- Schoknecht, U.; Töpfer, A.; Uhlig, S.; Baldauf, H. Characterisation of Leaching of Biocidal Active Substances of Main Group 2 ‘Preservatives’ from Different Materials under Weathering Conditions; UBA: Dessau-Roßlau, Germany, 2012; Project No. (FKZ) 3708 65 404, Report No. (UBA-FB) 001641/E), UBA-Texte No. 6. [Google Scholar]
- Nebel, H.; Scherer, C.; Schwitalla, C.; Schwerd, R.; Vollpracht, A.; Brameshuber, W. Leaching Behaviour of Renderings and Mortars. In Proceedings of the Eighth International Masonry Conference, MASONRY 11, Dresden, Germany, 4–7 July 2010; Jäger, W., Haseltine, B., Fried, A., Eds.; International Masonry Society: Dresden, Germany, 2010; pp. 1801–1808. [Google Scholar]
- Rougelot, T.; Burlion, N.; Bernard, D.; Skoczylas, F. About microcracking due to leaching in cementitious composites: X-ray microtomography description and numerical Approach. Cem. Concr. Res. 2010, 40, 271–283. [Google Scholar] [CrossRef]
- Garrabrants, A.; Sanchez, F.; Gervais, C.; Moszkowicz, P.; Kosson, D. The effect of storage in an inert atmosphere on the release of inorganic constituents during intermittent wetting of a cement-based material. J. Hazard. Mater. 2002, 91, 159–185. [Google Scholar] [CrossRef]
- Hendriks, I.C.F.; Raad, J.S. Report—Principles and background of the building materials decree in the Netherlands. Mat. Struct. 1997, 30, 3–10. [Google Scholar] [CrossRef]
- Scherer, C. Umwelteigenschaften Mineralischer Werkmörtel. (Environmental Properties of Mineral Mortars) Forschungsergebnisse Aus Der Bauphysik 12; Fraunhofer Verlag: Stuttgart, Germany, 2013. [Google Scholar]
- van Gerven, T.; van Baelen, D.; Dutré, V.; Vandecasteele, C. Influence of carbonation and carbonation methods on leaching of metals from mortars. Cem. Concr. Res. 2004, 34, 149–156. [Google Scholar] [CrossRef]
- Vega-Garcia, P.; Schwerd, R.; Scherer, C.; Schwitalla, C.; Johann, S.; Rommel, S.H.; Helmreich, B. Influence of façade orientation on the leaching of biocides from building façades covered with mortars and plasters. Sci. Total Environ. 2020, 734, 139465. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, N.; Jayr, E.; Méhu, J.; Moszkowicz, P. Environmental impact of construction products: Leaching Behaviour During Service Life. In Proceedings of the 2008 World Sustainable Building Conference, World SB08 Melbourne, Melbourne Convention Centre, Melbourne, Australia, 21–25 September 2008; Foliente, G., Lützkendorf, T., Newtron, P., Paevere, P., Eds.; ASN Events: Balnarring, Australia, 2008. [Google Scholar]
- Schneider, K.; Butler, M.; Mechtcherine, V. Carbon Concrete Composites C 3—Nachhaltige Bindemittel und Betone für die Zukunft. (Carbon Concrete Composites C 3—Sustainable binders and concretes for the future). Beton und Stahlbetonbau 2017, 112, 784–794. [Google Scholar] [CrossRef]
- DIN e.V. Construction Products—Assessment of Release of Dangerous Substances—Part. 2: Horizontal Dynamic Surface Leaching Test; German Version; Beuth Verlag: Berlin, Germany, 2014; (DIN CEN/TS 16637-2). [Google Scholar]
- Weiler, L.; Vollpracht, A.; Jesse, F.; Müller, C.; Reiners, J.; Spanka, G. Umweltverträglichkeit von Carbonbeton: Ergebnisse eines Forschungsvorhabens. (Environmental Compatibility of Carbon Reinforced Concrete—Results of a Research Project). Beton 2020, 70, 166–175. [Google Scholar]
- VDZ e.V. Tätigkeitsbericht 2003–2005: VI Umweltverträglichkeit Von Zement Und Beton. (Activity Report of the Association of German Cement Factories for the Years of 2003 to 2005)); Verlag Bau+Technik GmbH: Düsseldorf, Germany, 2005. [Google Scholar]
- Spanka, G. DSLT Analysedaten Email [Excel Dataset]; Düsseldorf, Germany, 2018. [Google Scholar]
- Jesse, F. Fertigungsstand Mit Frischbetonkennwerten, Email [PDF]; Bautzen, Germany, 2018. [Google Scholar]
- DIN e.V. DIN EN 14429:2015, Characterization of Waste –Leaching Behaviour Test—Influence of PH on Leaching with Initial Acid/Base Addition German Version; Beuth: Berlin, Germany, 2015; (DIN EN 14429:2015). [Google Scholar]
- Baumgartner, A.; Liebscher, H.-J. Lehrbuch Der Hydrologie: Quantitative Hydrologie. (Textbook of Hydrology—Quantitative Hydrology); Borntraeger: Stuttgart, Germany, 1996. [Google Scholar]
- Marshall, J.S.; Palmer, W.M.K. The distribution of raindrops with size. J. Meteor. 1948, 5, 165–166. [Google Scholar] [CrossRef]
- German Meteorological Service. Wetterlexikon Des DWD: Weather Encyclopaedia of the German Meteorological Service. Available online: https://www.dwd.de/DE/service/lexikon/Functions/glossar.html?lv2=101812&lv3=101906 (accessed on 1 October 2020).
- Weiler, L.; Vollpracht, A. Environmental Compatibility of Carbon Reinforced Concrete: Irrigated Construction Elements. KEM 2019, 809, 314–319. [Google Scholar] [CrossRef]
- Schneider, C.; Ketzler, G. Climate Data Logger Aachen-Hörn—Data of Annual Report 2018; Datensatz (Dataset); RWTH Aachen University: Aachen, Germany, 2018; ISSN of summarized version 1861-3993. [Google Scholar]
- Schneider, C.; Ketzler, G. Climate Data Logger Aachen-Hörn—Data of Annual Report 2019; Datensatz (Dataset); RWTH Aachen University: Aachen, Germany, 2019; ISSN of summarized version 1861-3993. [Google Scholar]
- ISO International Organization for Standardization. Hygrothermal Performance of Buildings—Calculation and Presentation of Climatic Data—Part. 3: Calculation of a Driving Rain Index for Vertical Surfaces from Hourly Wind and Rain Data; ISO International Organization for Standardization: Genf, Switzerland, 2009; (ISO 15927-3:2009). [Google Scholar]
- Davis, J.R. Stainless Steels, ASM Specialty Handbook; ASM International: Materials Park, OH, USA, 2010. [Google Scholar]
- Vollpracht, A.; Brameshuber, W. Binding and leaching of trace elements in Portland cement pastes. Cem. Concr. Res. 2016, 79, 76–92. [Google Scholar] [CrossRef]
- Bund-/Länderarbeitsgemeinschaft Wasser (LAWA); Ministerium für Umwelt; Klima und Energiewirtschaft Baden-Württemberg. Ableitung Von Geringfügigkeitsschwellenwerten Für Das Grundwasser: Aktualisierte und überarbeitete Fassung 2016; Bund-/Länderarbeitsgemeinschaft Wasser (LAWA): Stuttgart, Germany, 2017. [Google Scholar]
- Wenzel, A.; Schlich, K.; Shemotyuk, L.; Nendza, M. Revision der Umweltqualitätsnormen der Bundes-Oberflächengewässerverordnung: Nach Ende der Übergangsfrist für Richtlinie 2006/11/EG und Fortschreibung der europäischen Umweltqualitätsziele für prioritäre Stoffe. (Revision of the Environmental Quality Standards of the Federal German Surface Water Body Directive); UBA: Berlin, Germany, 2014; Volume 2015. [Google Scholar]
- Brameshuber, W.; Vollpracht, A. Umweltverträglichkeit von mineralischen Baustoffen. (Environmental compatibility of mineral building materials). Mauerwerk 2009, 13, 190–194. [Google Scholar] [CrossRef]
- CONE SCHIOPU, N. Caracterisation des Emssions dans l’eau des Produits de Construction Pendant Leur vie en Oeuvre. Ph.D. Thesis, L’Institut National des Sciences Appliquées de Lyon, Lyon, France, 2007. [Google Scholar]
- Dijkstra, J.J.; van der Sloot, H. Dossier of Information Justifying That Concrete Qualifies for the Potential Release of Regulated Dangerous Substances without-Further-Testing (WFT) by the Producer); ECN: Petten, Netherlands, 2013. [Google Scholar]
- Bandow, N.; Aitken, M.D.; Geburtig, A.; Kalbe, U.; Piechotta, C.; Schoknecht, U.; Simon, F.-G.; Stephan, I. Using Environmental Simulations to Test the Release of Hazardous Substances from Polymer-Based Products: Are Realism and Pragmatism Mutually Exclusive Objectives? Materials 2020, 13, 2709. [Google Scholar] [CrossRef] [PubMed]
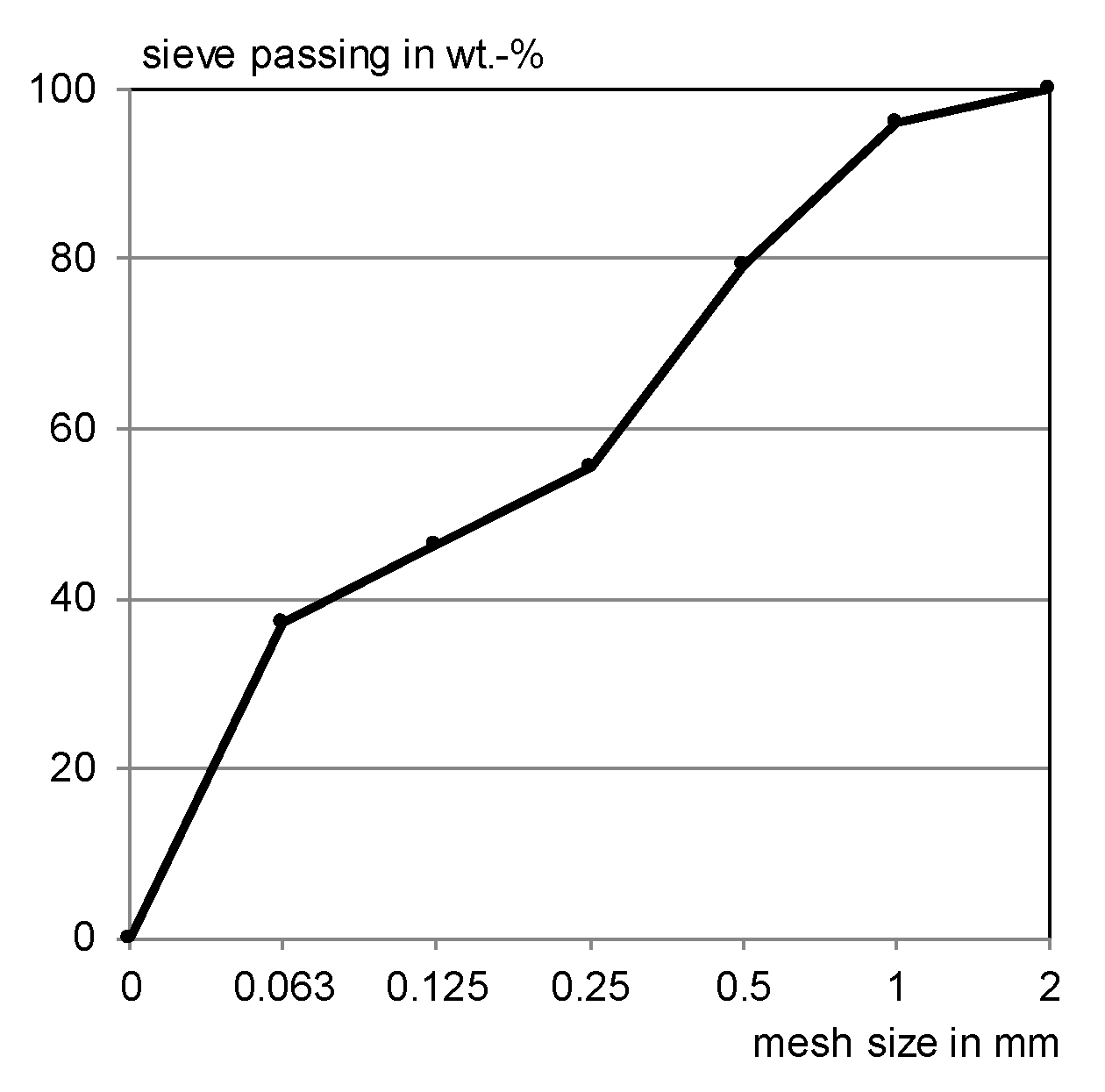

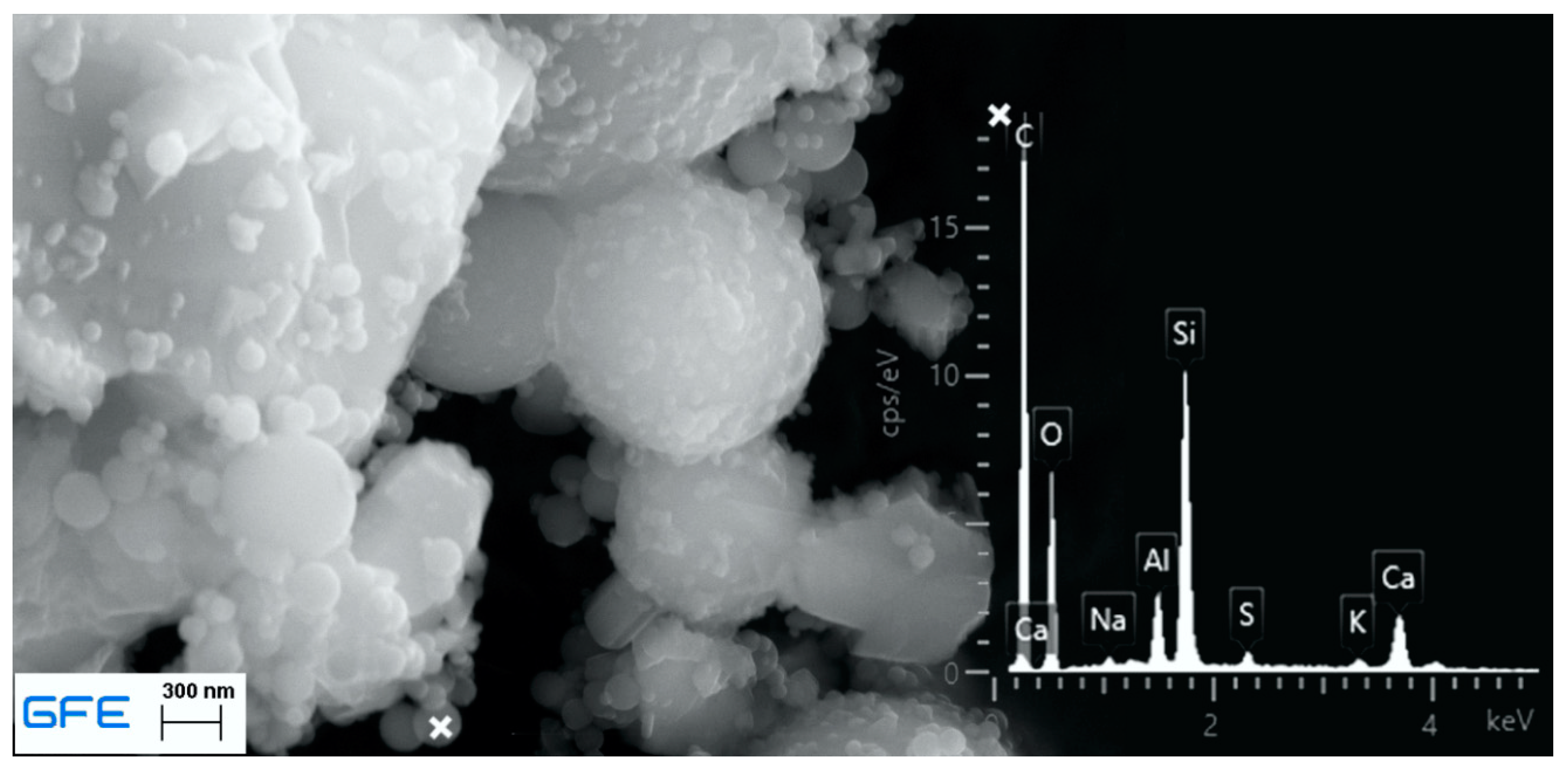
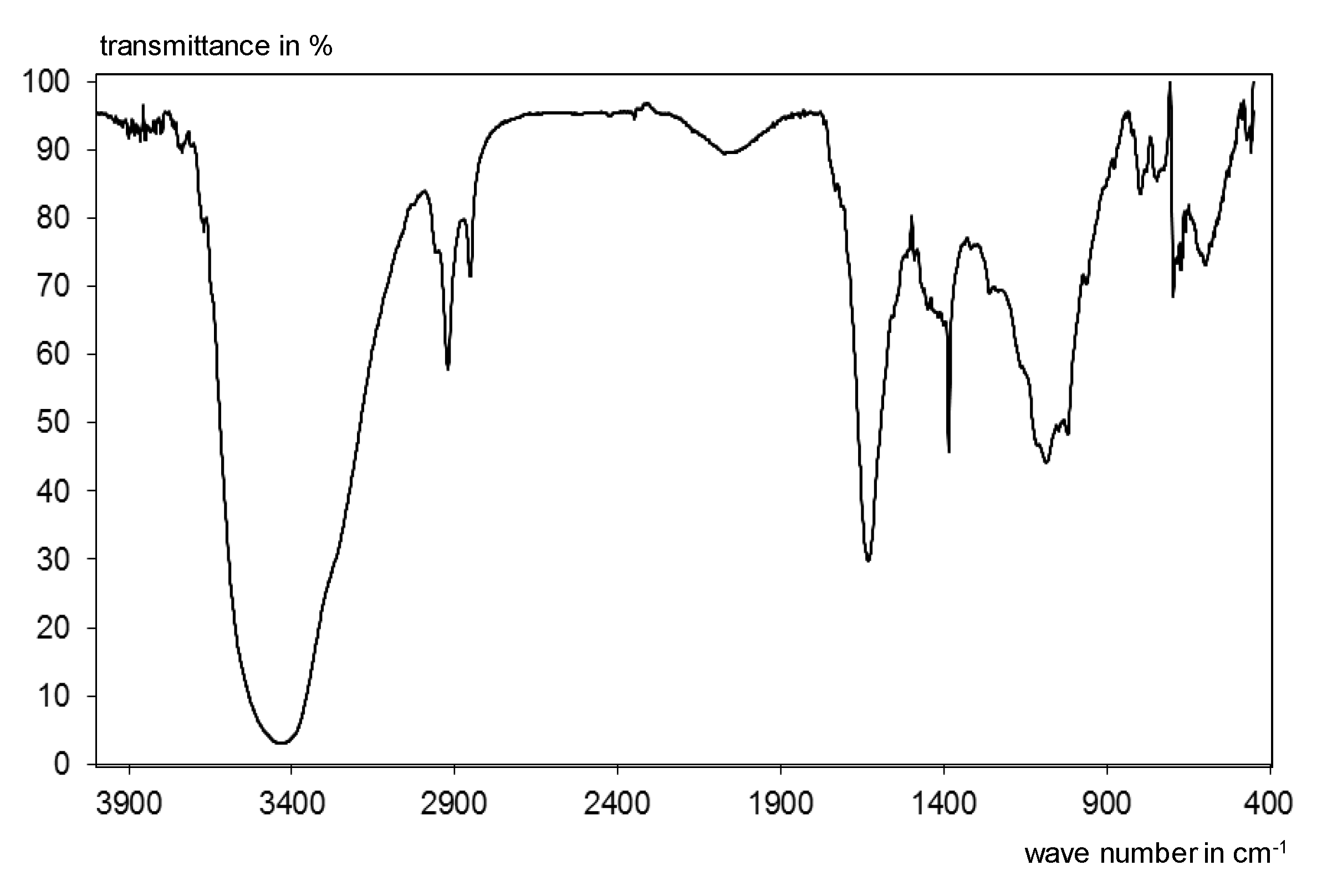
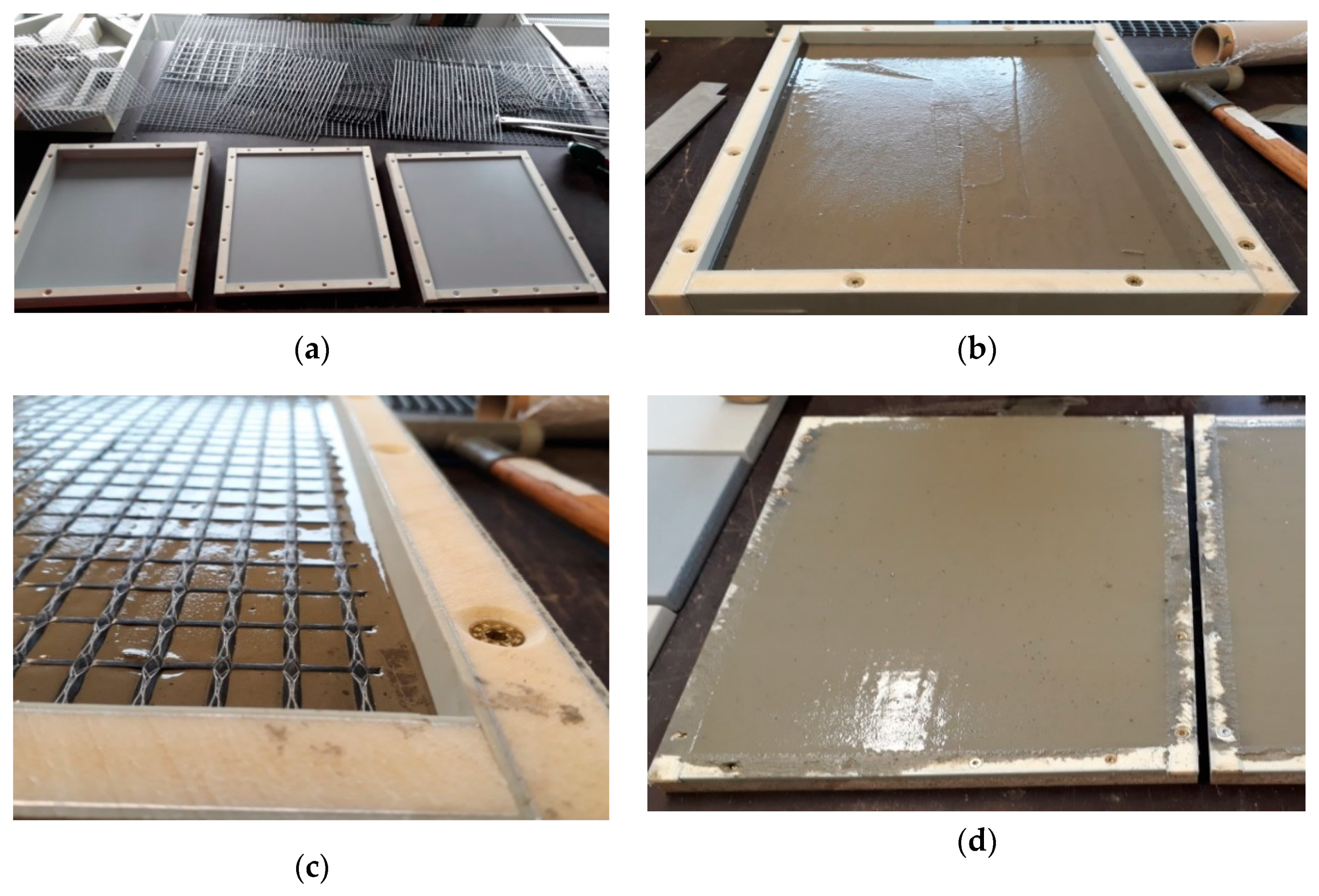








| Leached Material | Investigated Emissions | Irrigation Unit | Intensity and Irrigation Procedure | Reference |
|---|---|---|---|---|
| concrete façades/exposed concrete | chromium, zinc | plastic tray with perforated bottom | 25 mm/h,1.5 h rain, 2 h break, 1.5 h rain, 37 h drying time, total: 650 mm | 2005 [11] |
| mineral construction materials | sulfate, chloride, fluoride, cyanide, 16 trace elements | spray mechanism (compressed air + nozzle) | 0.7–5 mm/h, finely dispersed droplets (mist) | 2008 [9] |
| synthetic resin render with biocides | biocides | weathering chamber | 85 mm/h, 20 irrigation intervals of 1 h in 5 d, T = 50 °C–60 °C, total: 6800 mm | 2009 [12] |
| renders/render-paint-systems with biocides | biocides | “water pressure and flow rate were controlled” | 75 mm/h, 2 min/d | 2009 [13] |
| metal building materials (stainless steel, copper, zinc) | heavy metals, trace elements | spray mechanism (compressed air + nozzle) | 0.7 mm/h–3.5 mm/h, finely dispersed droplets (mist) | 2011 [14] |
| renders, mortars | sodium, potassium, sulfate, chloride, 10 trace elements | spray mechanism (compressed air + nozzle) | 0.7–5 mm/h, total: 60 mm, finely dispersed droplets (mist) | 2012 [15] |
| Component | Content of the Sieving Fractions in wt.% | |||||
|---|---|---|---|---|---|---|
| 0–0.063 mm | 0.063–0.125 mm | 0.125–0.25 mm | 0.25–0.5 mm | 0.5–1 mm | 1–2 mm | |
| C3S | 30 | 31.4 | – | – | – | – |
| C2S | 13.8 | 20 | – | – | – | – |
| C3A | 5.8 | 6.7 | – | – | – | – |
| Brownmillerite | 1.3 | 1.2 | – | – | – | – |
| Quartz | 1.8 | 25.6 | 85.3 | 90.9 | 91.9 | 93.9 |
| Ca-langbeinite | 0.5 | – | – | – | – | – |
| Anhydrite | 4.3 | 2.5 | – | – | – | – |
| Hemihydrate | 0.6 | 0.3 | – | – | – | – |
| Gypsum | 0.8 | 0.9 | – | – | – | – |
| Calcite | 1 | 1.4 | – | – | – | – |
| Portlandite | 0.2 | 0.4 | – | – | – | – |
| Mullite | 3 | 6.3 | – | – | – | – |
| Hematite | 0.4 | 0.9 | – | – | – | – |
| Microcline | – | 2.3 | 9.7 | 7.2 | 5.8 | 4.1 |
| Fluorphlogopite | – | 0.3 | 0.3 | 0.5 | 0.3 | – |
| Anorthite | – | – | 3.8 | 1.4 | 2.1 | 2 |
| Clinochlore | – | – | 0.8 | – | – | – |
| Pseudobrookite | – | – | 0.1 | – | – | – |
| Amorphous | 36.5 | – | – | – | – | – |
| Parameter | Content in wt.% |
|---|---|
| loss on ignition | 2.47 |
| insoluble in HCl | 26.24 |
| SO3 | 2.08 |
| Na2O | 0.39 |
| K2O | 1.13 |
| chloride | 0.036 |
| MgO | 0.96 |
| Al2O3 | 9.59 |
| SiO2 | 36.57 |
| P2O5 | 0.29 |
| CaO | 44.37 |
| TiO2 | 0.48 |
| MnO | 0.05 |
| Fe2O3 | 2.63 |
| Parameter | Concrete Mixture [27] | Fine Fraction (<125 µm) | Reinforcement Textile Incl. Coating [27] | Average of German Cements [28] |
|---|---|---|---|---|
| mg/kg | ||||
| antimony | 1.1 | 1.50 | 0.9 | 2.9 |
| arsenic | 7.2 | 13.0 | 2.1 | 7 |
| barium | 252 | 295 | 11.0 | - |
| lead | 26.5 | 19.9 | 0.9 | 17 |
| cadmium | 0.3 | 0.226 | <0.1 | 0.4 |
| chromium | 64.4 | 27.2 | 206.0 | 41 |
| cobalt | 6.9 | 6.84 | 2.4 | 8.7 |
| copper | 32.1 | 15.5 | 45.7 | 31 |
| molybdenum | 3.7 | 4.39 | 4.8 | - |
| nickel | 38.9 | 20.5 | 57.9 | 23 |
| mercury | 0.1 | 0.068 | <0.02 | 0.06 |
| thallium | 1.5 | 0.275 | <0.05 | 0.4 |
| vanadium | 52.8 | 60.7 | 1.25 | 50 |
| zinc | 63.1 | 44.4 | 93.8 | 192 |
| Testing Method | Label * | Width/ Length | Thickness | Layers of Reinforce-Ment | Concrete Cover | Room Temp | Concrete Temp | Flow Spread |
|---|---|---|---|---|---|---|---|---|
| mm | mm | n | mm | °C | °C | mm | ||
| DSLT | D1-1-2 ** | 150/150 | 5.50 | 1 | 2 | 20.2 | 16.5 | 270 |
| D1-4-2 ** | 150/150 | 16.0 | 4 | 2 | 21.3 | 17.3 | 260 | |
| D1-1-20 ** | 150/150 | 40.0 | 1 | 20 | 22.0 | 17.8 | 260 | |
| lab irrigation | L 1-2 A | 300/400 | 5.50 | 1 | 2 | 20.2 | 16.8 | 275 |
| L 1-2 B | 300/400 | 5.50 | 1 | 2 | 19.8 | 16.6 | 250 | |
| L 4-2 A | 300/400 | 16.0 | 4 | 2 | 21.6 | 17.0 | 255 | |
| L 4-2 B | 300/400 | 16.0 | 4 | 2 | 21.3 | 17.3 | 260 | |
| L 1-20 A | 300/400 | 40.0 | 1 | 20 | 22.3 | 17.5 | 270 | |
| L 1-20 B | 300/400 | 40.0 | 1 | 20 | 22.8 | 17.1 | 265 | |
| L 1-20 C | 300/400 | 40.0 | 1 | 20 | 22.5 | 24.2 | 230 | |
| L 1-20 D | 300/400 | 40.0 | 1 | 20 | 23.1 | 23.3 | 237.5 | |
| outdoor testing | F 1 A | 1000/600 | 20.0 | 1 | 2 | 20.2 | 21.9 | 205 |
| F 1 B | 1000/600 | 20.0 | 1 | 2 | 21.7 | 24.2 | 210 | |
| F 1 C | 1000/600 | 20.0 | 1 | 2 | n. d. | n. d. | n. d. | |
| F 1g A | 1000/600 | 20.0 | 1 | 2 | 22.0 | 23.5 | 247.5 | |
| F 1g B | 1000/600 | 20.0 | 1 | 2 | 21.9 | 23.1 | 242.5 | |
| F 4g A | 1000/600 | 20.0 | 4 | 2 | 21.9 | 23.2 | 235 | |
| F 4g B | 1000/600 | 20.0 | 4 | 2 | 21.2 | 23.4 | 230 |
| Characteristic | DSLT | Laboratory Irrigation | Outdoor Testing |
|---|---|---|---|
| blank | empty leaching vessel | irrigated glass panel | irrigated glass panel |
| duration | 64 d | 28 d (+12 d) | 365 d |
| total amount of water | 240 to 380 L/m2 | 344 L/m2 | 663 L/m2 * |
| conditions | permanent water contact | scheduled irrigation and drying phases; drop size: about 2.2 mm; intensities: 1, 2, 5 mm/h | outdoor conditions 45° angle to ground facing west |
| Substance | Concen-Tration | pHstat | DSLT | Laboratory Irrigation | Outdoor Irrigation | Outdoor Background | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | ||
| Na | mg/L | 68 | 83 | 1.46 | 10.6 | <0.2 | 12.7 | 0.4 | 334 | <0.1 | 18.4 |
| K | 136 | 174 | 4.69 | 41.2 | 0.8 | 35.4 | 1.0 | 302 | <0.1 | 2.2 | |
| Ca | 4830 | 12,700 | 32.0 | 88.7 | 1.6 | 17.6 | 1.1 | 17.2 | <0.1 | 9.4 | |
| Cl− | 0.2 | 14.3 | <0.1 | 11.3 | <0.1 | 0.4 | <0.1 | 117 | <0.1 | 39.4 | |
| SO42− | 1.4 | 742 | 2.1 | 5.3 | 1.2 | 10.9 | <0.1 | 245 | <0.1 | 19.0 | |
| Sb | µg/L | n.d. | n.d. | <0.1 | 11.8 | <0.1 | 2.58 | <0.1 | 1.52 | <0.1 | 1.03 |
| As | <2 | 26.9 | <0.02 | 1.33 | <0.1 | 0.361 | <0.1 | 8.04 | <0.1 | 1.25 | |
| Ba | 408 | 2178 | 12.8 | 74.1 | 0.350 | 14.3 | 0.520 | 20.9 | 0.560 | 20.8 | |
| Pb | <1 | 172 | <0.05 | 10.3 | 0.046 | 0.270 | 0.079 | 7.46 | <0.1 | 15.8 | |
| B | <5 | 2,532 | n.d. | n.d. | <1 | 11.4 | <1 | 147 | 0.62 | 15.7 | |
| Cd | <0.1 | 6.76 | <0.01 | 0.080 | <0.1 | <0.1 | <0.1 | 0.210 | <0.1 | 0.660 | |
| Cr | 3.21 | 320 | 0.960 | 5.02 | <0.1 | 4.71 | <0.1 | 23.3 | <0.1 | 2.26 | |
| Co | <1 | 153 | <0.01 | 0.050 | <0.1 | <0.1 | <0.1 | 3.88 | <0.1 | 0.980 | |
| Cu | <1 | 451 | <0.04 | 2.19 | 0.046 | 27.5 | <0.1 | 32.0 | 0.290 | 29.1 | |
| Mo | 5.10 | 90.4 | <0.2 | 1.50 | <0.1 | 1.44 | <0.1 | 6.20 | <0.1 | 1.55 | |
| Ni | <0.5 | 486 | <0.04 | 2.75 | <0.2 | 8.11 | <0.1 | 419 | <0.1 | 6.96 | |
| Hg | n.d. | n.d. | <0.02 | <0.02 | <0.01 | 0.023 | <0.01 | 0.028 | <0.01 | 0.020 | |
| Se | 5.87 | 30.9 | n.d. | n.d. | 0.079 | 0.331 | <0.1 | 3.19 | <0.1 | 1.14 | |
| Tl | <1 | 8.44 | <0.01 | 0.110 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| V | 2.31 | 240 | <0.7 | 14.5 | 0.358 | 5.55 | <0.1 | 64.2 | <0.1 | 2.19 | |
| Zn | <10 | 1423 | <0.7 | 11.9 | <1 | 15.7 | 0.578 | 90.0 | 3.02 | 154 | |
| Source | Examined Material | Eluate/Leachate Concentration in µg/L | |||||
|---|---|---|---|---|---|---|---|
| Cr | V | ||||||
| Outdoor | Lab. Irr. | DSLT | Outdoor | Lab. Irr. | DSLT | ||
| [15,21] | reinforcement fiber plaster | <0.08–68.9 | <0.5–26.4 | <0.5–2.8 | 0.86–29.4 | <0.5–8.3 | <0.5–6.4 |
| lime-cement plaster | <0.08–141 | <0.5–30.1 | <0.5–5 | <0.08–8.81 | 0.6–47.6 | <0.5–5.1 | |
| face masonry mortar | <0.08–93.2 | <0.5–42.4 | <0.5–7.8 | <0.08–35.0 | 0.8–44.3 | <0.5–8.4 | |
| [4] | concrete new | 16–39 | - | - | - | - | - |
| concrete old | <1–6 | - | - | - | - | - | |
| [43] | unspecified mineral building materials | - | 0.1–150 | <1–49 | - | <0.2–132 | 1–128 |
| [44] | concrete slabs | <2–104 | - | <2 * | - | - | - |
| [45] | ECRICEM mortar statistics | - | - | 0.45–14 | - | - | 0.15–50 |
| this work | C3 | <0.1–13.4 | <0.1–4.71 | 0.96–5.02 | <0.1–64.2 | 0.358–5.55 | 0.7–14.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiler, L.; Vollpracht, A. Leaching of Carbon Reinforced Concrete—Part 1: Experimental Investigations. Materials 2020, 13, 4405. https://doi.org/10.3390/ma13194405
Weiler L, Vollpracht A. Leaching of Carbon Reinforced Concrete—Part 1: Experimental Investigations. Materials. 2020; 13(19):4405. https://doi.org/10.3390/ma13194405
Chicago/Turabian StyleWeiler, Lia, and Anya Vollpracht. 2020. "Leaching of Carbon Reinforced Concrete—Part 1: Experimental Investigations" Materials 13, no. 19: 4405. https://doi.org/10.3390/ma13194405






