Study of In Situ Foamed Fly Ash Geopolymer
Abstract
:1. Introduction
2. Materials and Methods
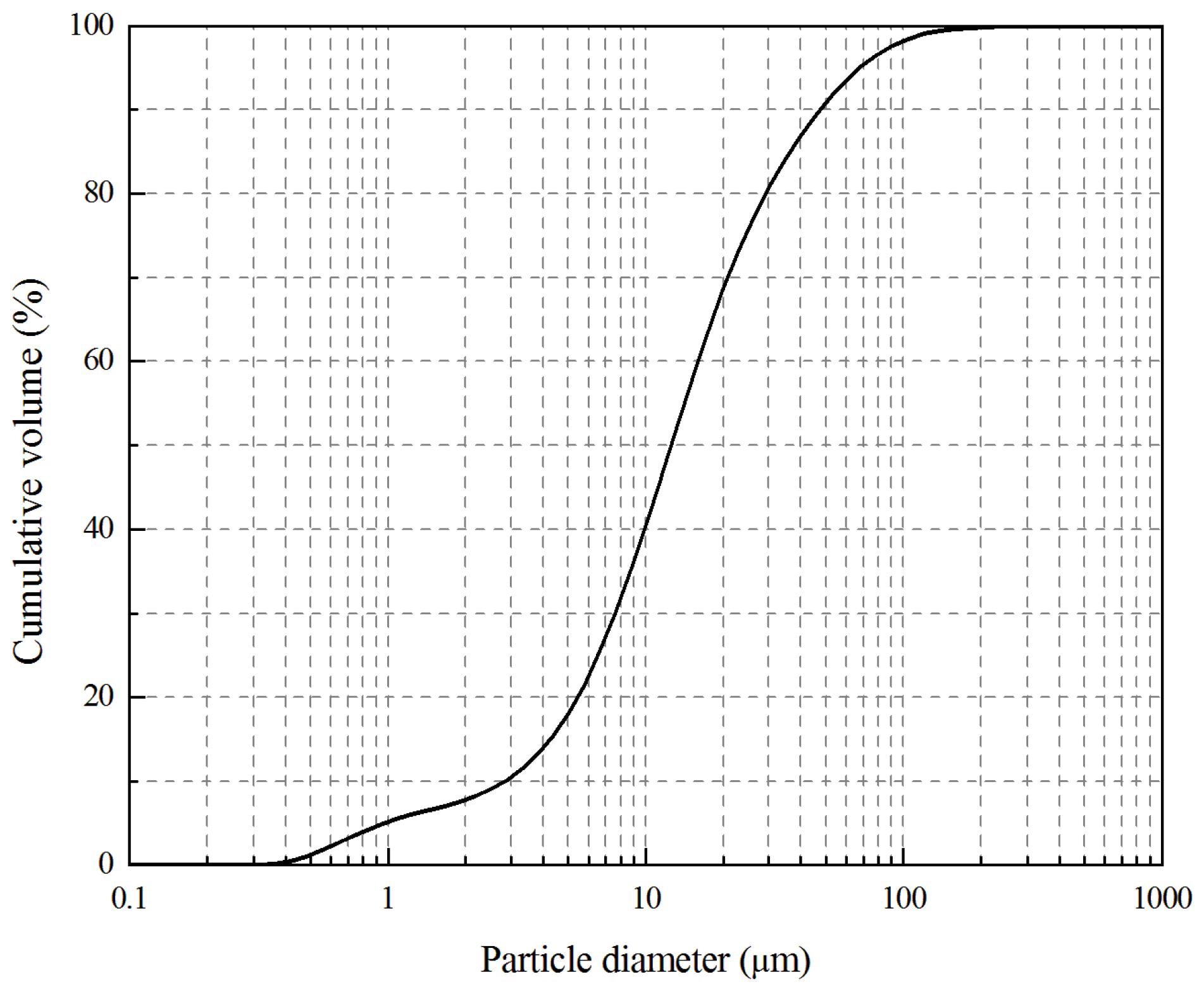
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
3. Results and Discussion
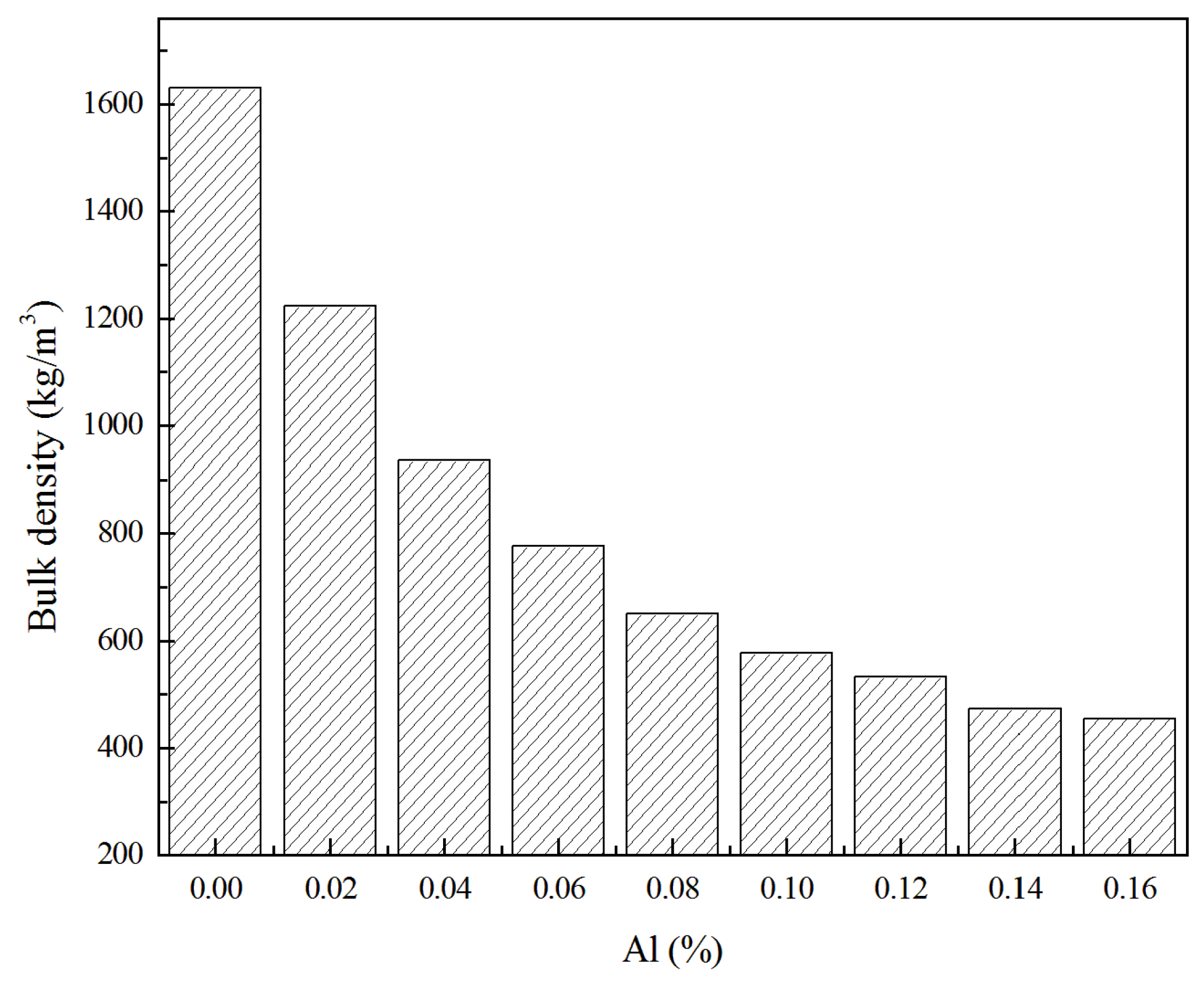
3.1. Physical Properties
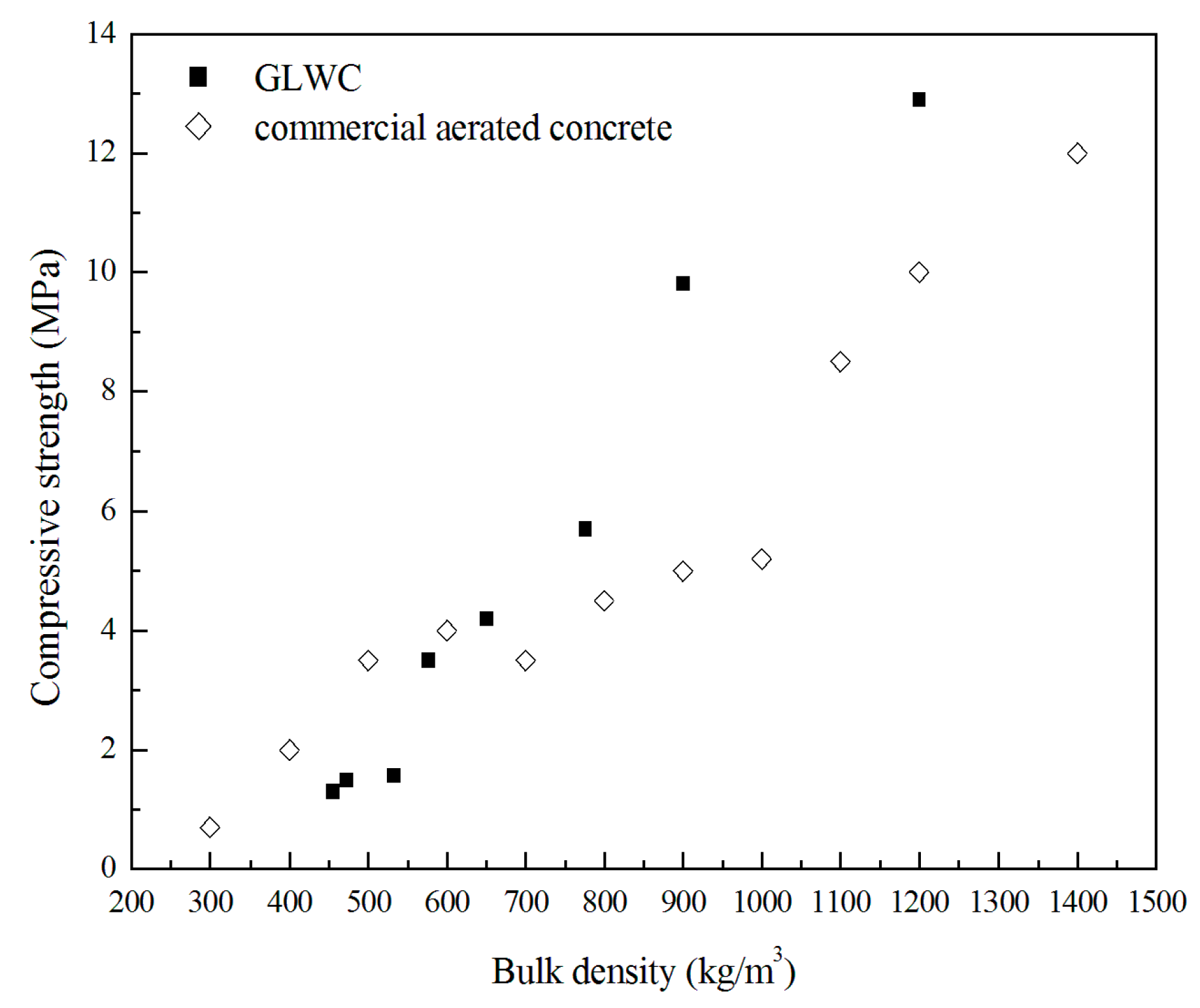
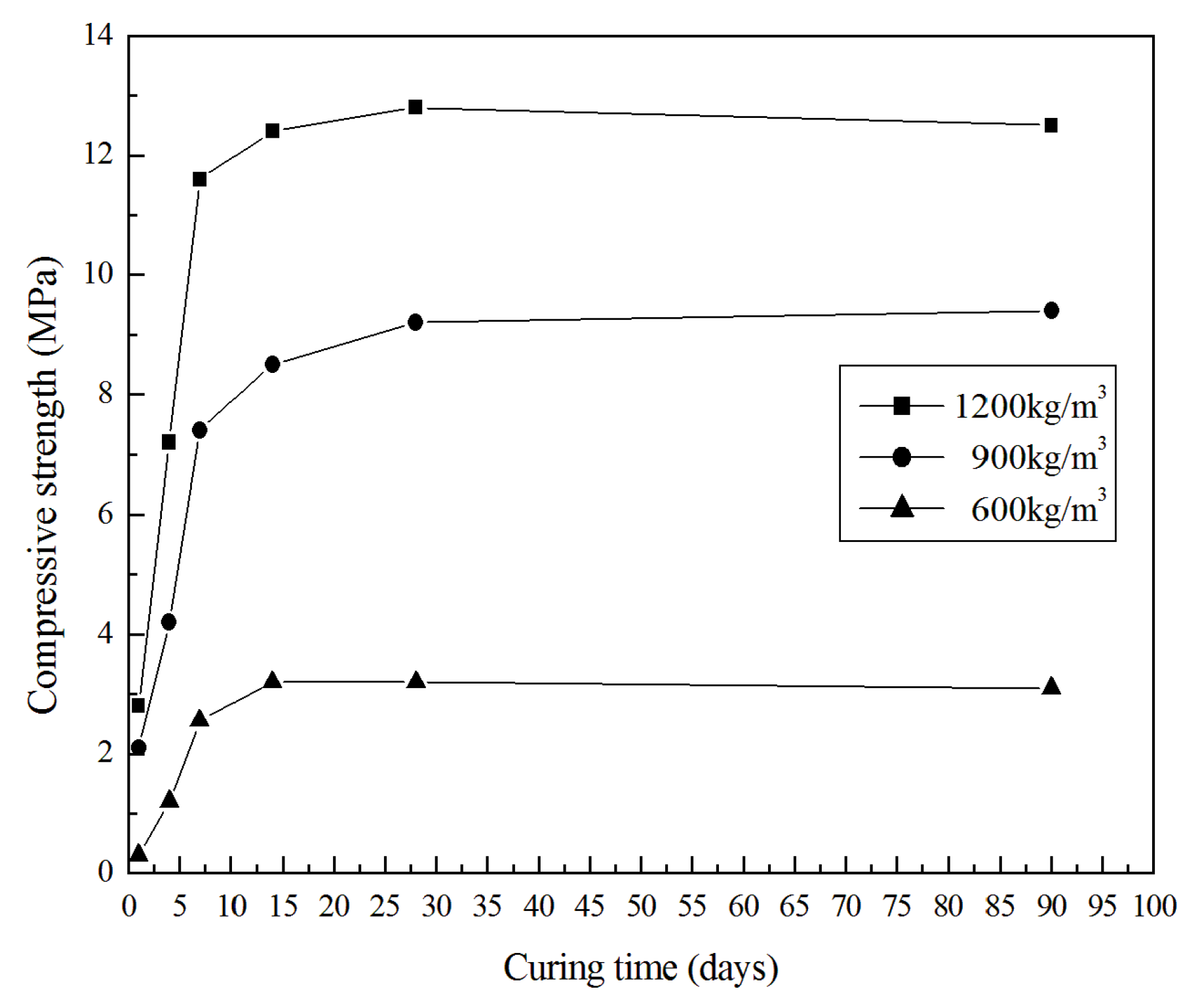
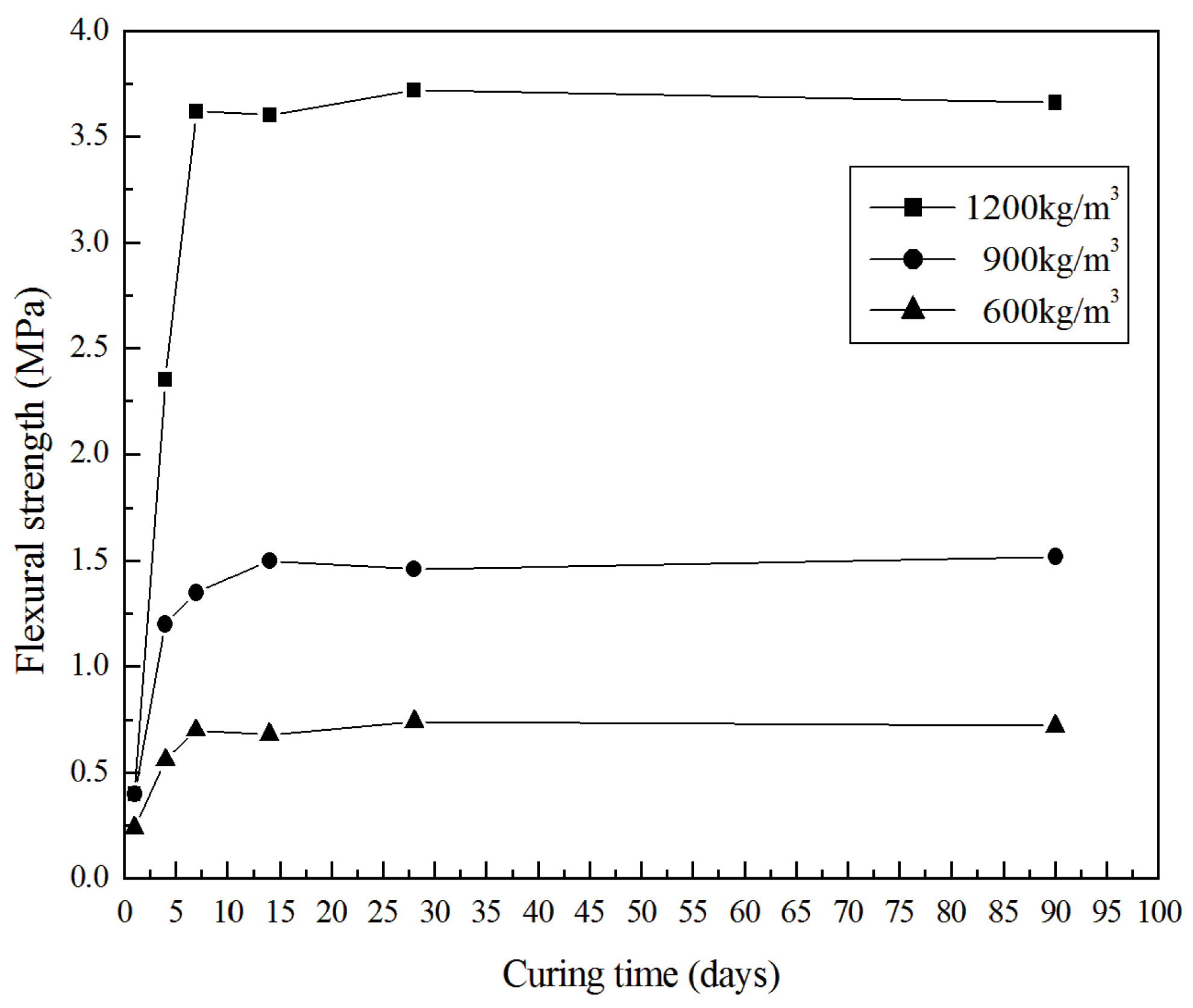
3.2. Mechanical Properties
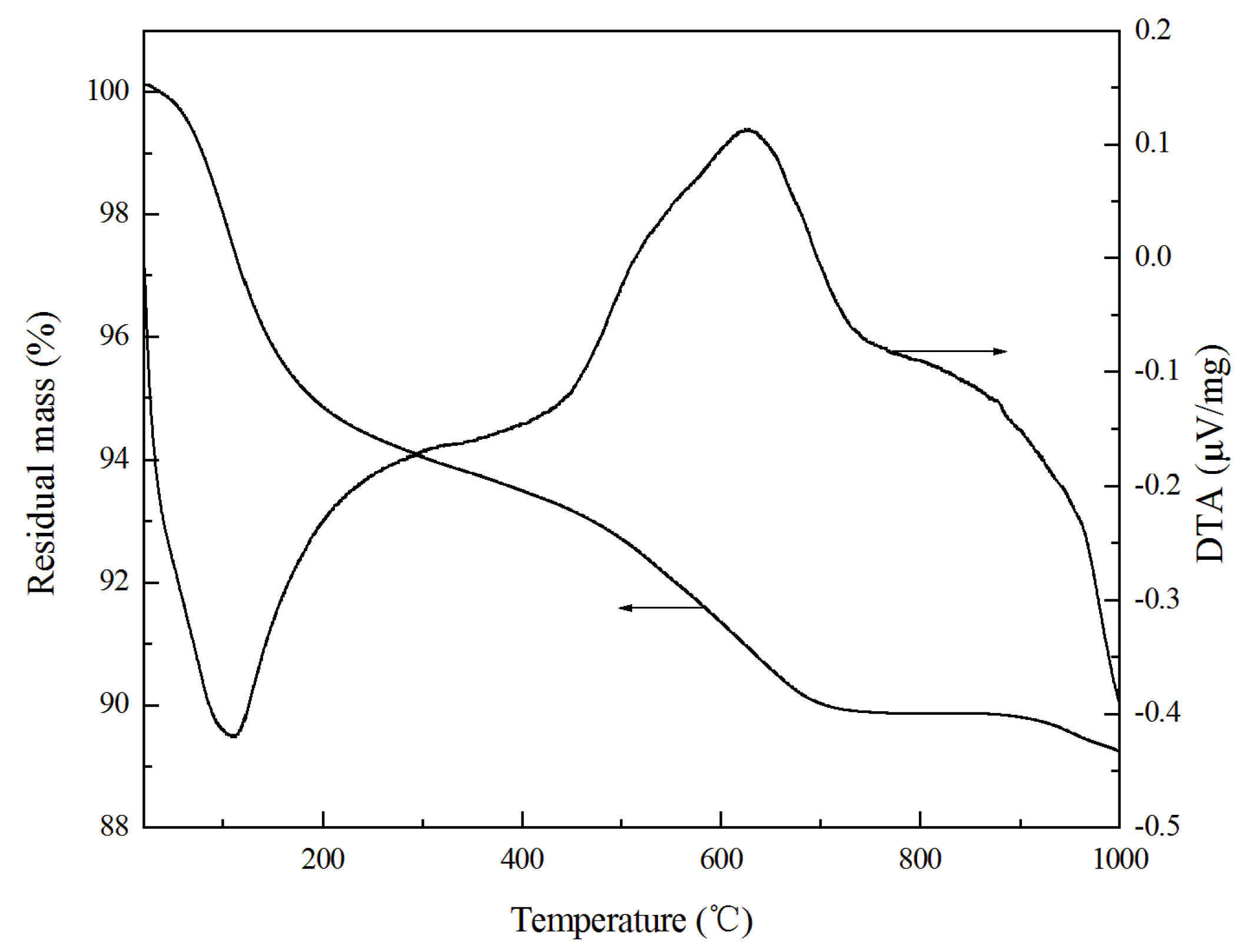
3.3. Thermogravimetric Property of GLWC
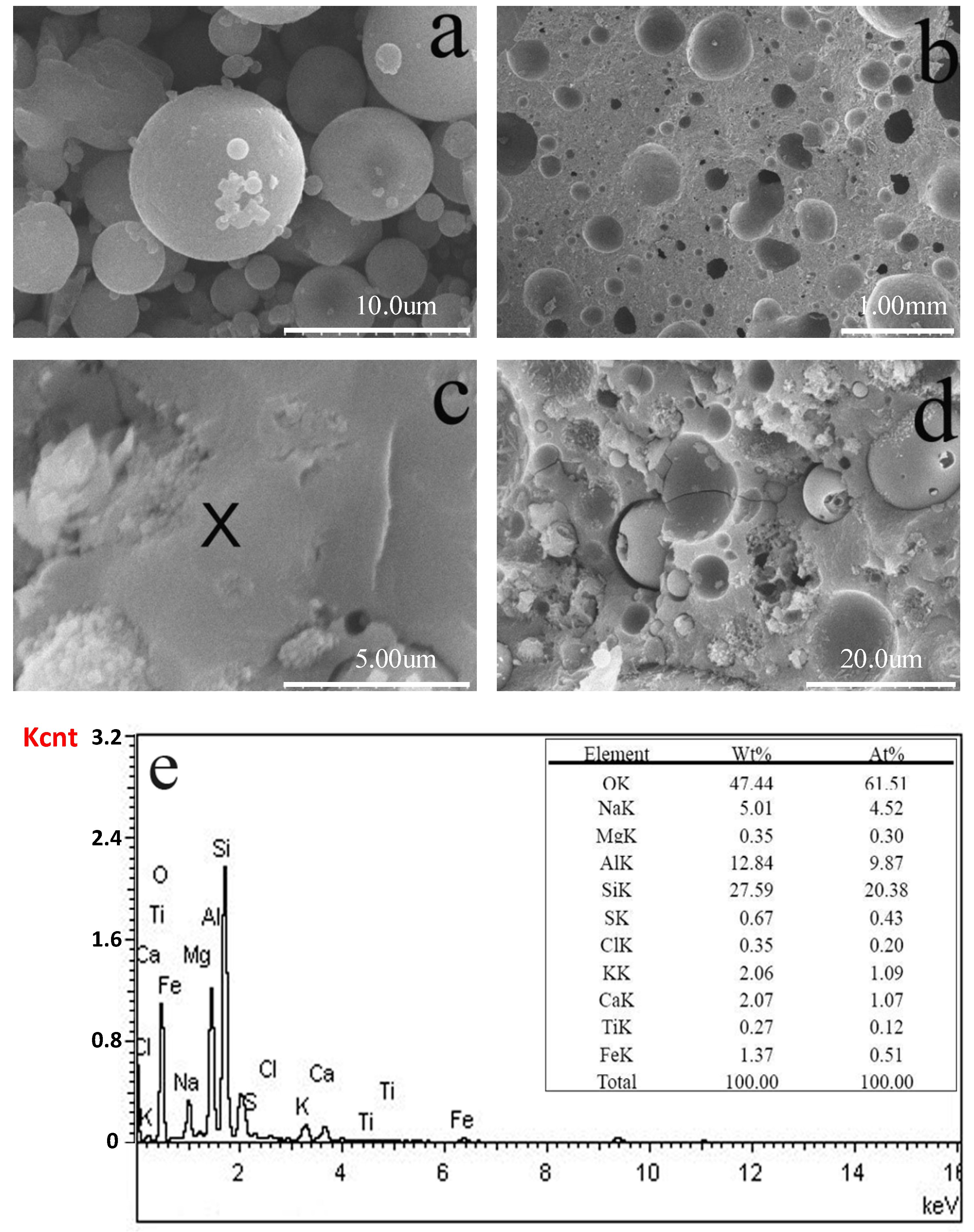
3.4. Microstructure of GLWC
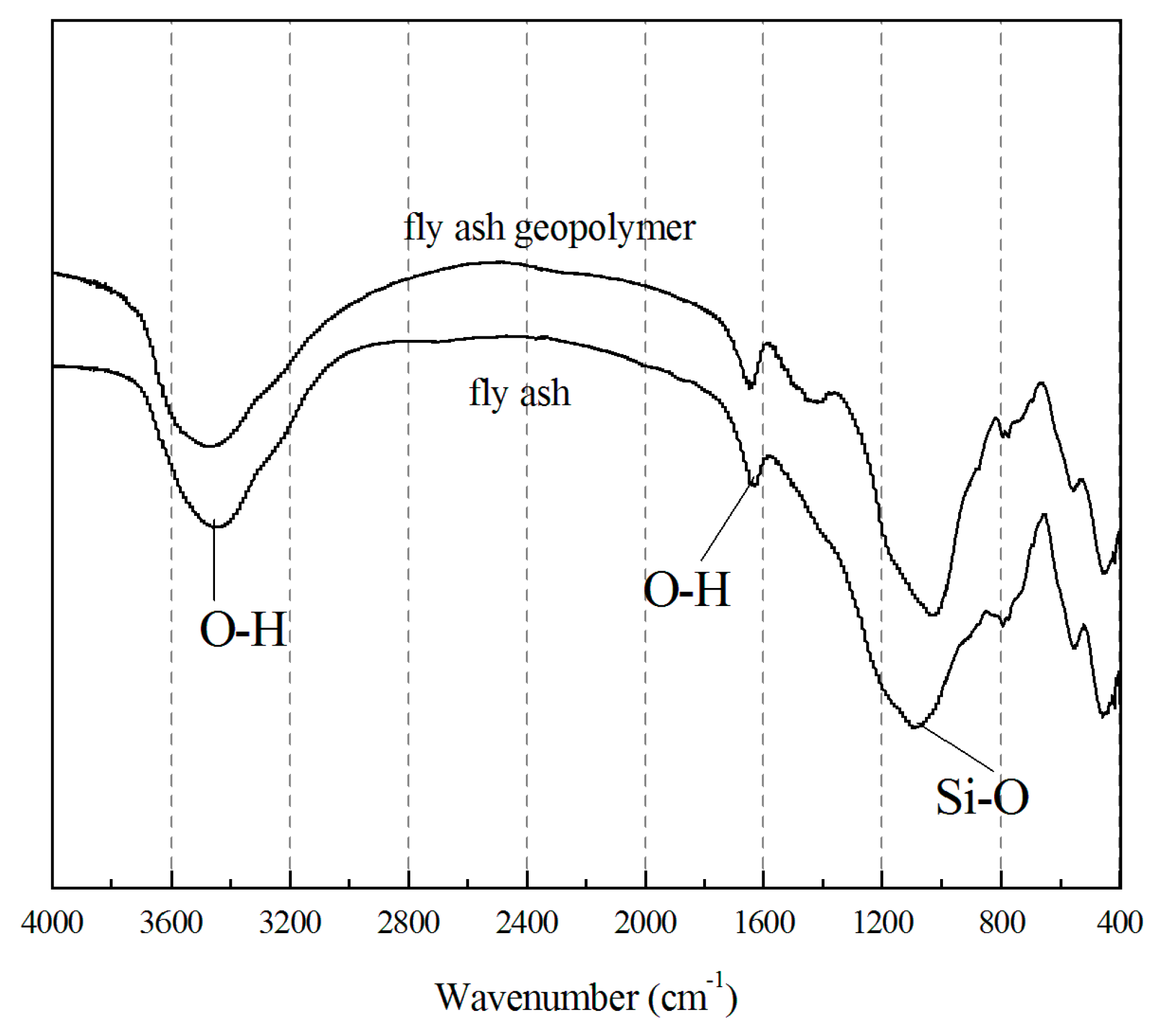
3.5. Infrared Spectroscopy
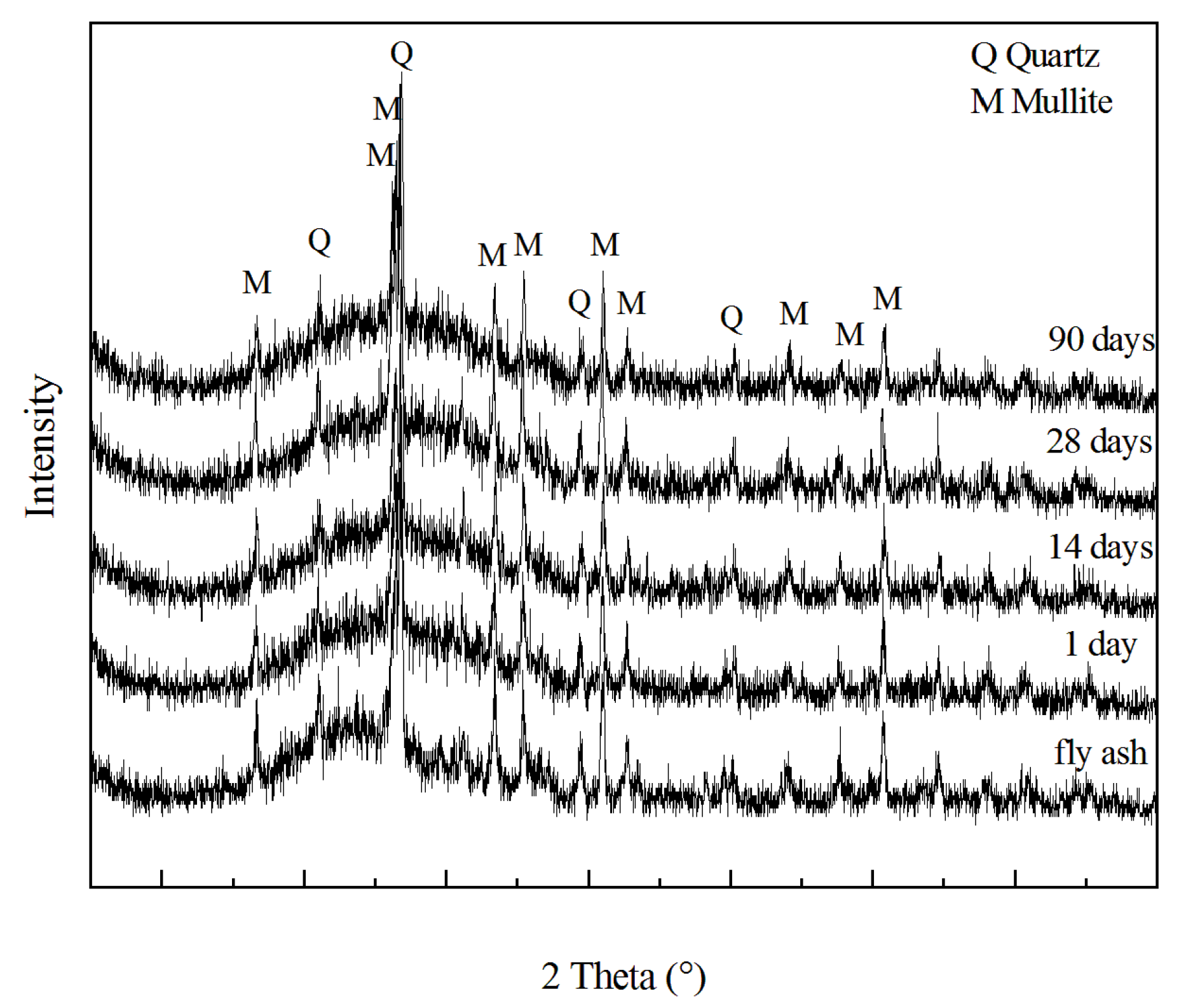
3.6. Mineralogical Analyses
4. Summary and Conclusions
- (1)
- GLWC provides a better performance than traditional cement-based LWC, especially in terms of mechanical strength. Geopolymers can provide high compressive strengths when the density is reduced, and their mechanical properties are acceptable.
- (2)
- Apart from possessing relatively higher thermal conductivity than OPC-based LWC, the thermal conductivity and strength of GLWC synthesized in this study also reached a better balance.
- (3)
- The optimal GLWC produced in this study is the one with a density of 900 kg/m3; its 28 days compressive strength is 10 MPa, and its thermal conductivity is 0.52 W/mK, both of which are higher than those of its OPC counterpart of similar density.
- (4)
- In contrast to OPC, the main mineralogical phases of GLWC contain various amorphous and semicrystalline phases with a decent microstructure.
Author Contributions
Funding
Conflicts of Interest
References
- Gennaro, R.D.; Langella, A.; D′Amore, M.; Dondi, M.; Colella, A.; Cappelletti, P.; Gennaro, A.d. Use of zeolite-rich rocks and waste materials for the production of structural lightweight concretes. Appl. Clay Sci. 2008, 41, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Demirboga, R.; Gul, R. The effects of expanded perlite aggregate, silica fume and fly ash on the thermal conductivity of lightweight concrete. Cem. Concr. Res. 2003, 33, 723–727. [Google Scholar] [CrossRef]
- Karakurt, C.; Kurama, H.; Topcu, I.B. Utilization of natural zeolite in aerated concrete production. Cem. Concr. Compos. 2010, 32, 1–8. [Google Scholar] [CrossRef]
- Gadea, J.; Rodríguez, A.; Campos, P.L.; Garabito, J.; Calderón, V. Lightweight mortar made with recycled polyurethane foam. Cem. Concr. Compos. 2010, 32, 672–677. [Google Scholar] [CrossRef]
- Kan, A.; Demirboga, R. A novel material for lightweight concrete production. Cem. Concr. Compo. 2009, 31, 489–495. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO2-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef]
- Narayanan, N.; Ramamurthy, K. Microstructural investigations on aerated concrete. Cem. Concr. Res. 2000, 30, 457–464. [Google Scholar] [CrossRef]
- Little, M.R.; Adell, V.; Boccaccini, A.R.; Cheeseman, C.R. Production of novel ceramic materials from coal fly ash and metal finishing wastes. Resour. Conserv. Recycl. 2008, 52, 1329–1335. [Google Scholar] [CrossRef]
- Kearsley, E.P.; Wainwright, P.J. The effect of high fly ash content on the compressive strength of foamed concrete. Cem. Concr. Res. 2001, 31, 105–112. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Characteristics of lightweight fly ash aggregates produced with different binders and heat treatments. Cem. Concr. Compos. 2011, 33, 61–67. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Effects of lightweight fly ash aggregate properties on the behavior of lightweight concretes. J. Hazard Mater. 2010, 179, 954–965. [Google Scholar] [CrossRef]
- Alzaza, A.; Mastali, M.; Kinnunen, P.; Korat, L.; Abdollahnejad, Z.; Ducman, V.; Illikainen, M. Production of Lightweight Alkali Activated Mortars Using Mineral Wools. Materials 2019, 12, 1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Shuai, Q.; Li, H.; Ding, Q.; Gu, Y.; Cheng, C.J.; Xu, Z.H. Fabrication and Fireproofing Performance of the Coal Fly Ash-Metakaolin-Based Geopolymer Foams. Materials 2020, 13, 1750. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer Cements to minimize carbon-dioxide greenhousewarming. In Cementbased Materials: Present, Future and Environmental Aspects; Moukwa, M., Sarkar, S.L., Luke, K., Grutzeck, M.W., Eds.; American Ceramic Society, Ceramic Transactions; Geopolymer Institute: Saint Quentin, France, 1993; Volume 37, pp. 165–182. [Google Scholar]
- Williams, L.O. Fossil fuels. In An End to Global Warming; Elsevier: Amsterdam, The Netherlands, 2002; pp. 8–16. ISBN 0679438416. [Google Scholar]
- Ramachandran, V.S. (Ed.) Concrete admixtures handbook. In Properties, Science, and Technology, 1st ed.; Noyes Publications: Park Ridge, NJ, USA, 1984; pp. 1–2, 276. [Google Scholar]
- Davidovits, J. SPE PACTEC ’79; Society of Plastic Engineers: Brookfield Center, NY, USA, 1979; p. 151. [Google Scholar]
- Davidovits, J. Geopolymers and Geopolymeric Materials. J. Thermal Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The potential use of geopolymeric materials to immobilise toxic metals. Part I. Theory and applications. Miner Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer chemistry and sustainable development. The Poly(sialate) terminology: A very useful and simple model for the promotion and understanding of green chemistry. In Proceedings of the World Congress Geopolymer; Davidovits, J., Ed.; Geopolymer Institute: Saint Quentin, France, 2005; pp. 9–15. [Google Scholar]
- Su, L.J.; Fu, G.S.; Wang, Y.L.; Yao, G.C.; Zhang, J.N.; Xu, X.C.; Jia, B.X. Preparation and Performance of a Low-Carbon Foam Material of Fly-Ash-Based Foamed Geopolymer for the Goaf Filling. Materials 2020, 13, 841. [Google Scholar] [CrossRef] [Green Version]
- Vaou, V.; Panias, D. Thermal insulating foamy geopolymers from perlite. Miner Eng. 2010, 23, 1146–1151. [Google Scholar] [CrossRef]
- Liu, L.P.; Cui, X.M.; Qiu, S.H.; Yu, J.L.; Zhang, L. Preparation of phosphoric acid-based porous geopolymers. Appl. Clay Sci. 2010, 50, 600–603. [Google Scholar] [CrossRef]
- Arellano, A.R.; Burciaga, D.O.; Escalante, G.J.I. Lightweight concretes of activated metakaolin-fly ash binders, with blast furnace slag aggregates. Constr. Build Mater. 2010, 24, 1166–1175. [Google Scholar] [CrossRef]
- ASTM C618-94a. Standard specification for coal fly ash and raw calcined natural pozzolan for the use as a mineral admixture in Portland cement concrete. In Annual Book of ASTM Standards; Bernhard, L., Furcola, N.C., Gutman, E.L., Kauffman, S.L., Kramer, J.G., Leinweber, C.M., Eds.; ASTM: Philadelphia, PA, USA, 1995; Volume 4, pp. 304–306. ISBN 080312208X. [Google Scholar]
- Test Method for Bulk Density, Water Absorption of Aerated Concrete, Administration of Quality Supervision; GB/T 11970-1997; Inspection and Quarantine of the PRC: Beijing, China, 1997.
- Thermal Insulation-Determination of Steady-State Thermal Resistance and Related Properties-Guarded Hot Plate Apparatus, Administration of Quality Supervision; GB 10294-88; Inspection and Quarantine of the PRC: Beijing, China, 1988.
- Szechynska-Hebda, M.; Marczyk, J.; Ziejewska, C.; Hordynska, N.; Mikula, J.; Hebda, M. Optimal Design of pH-neutral Geopolymer Foams for Their Use in Ecological Plant Cultivation Systems. Materials 2019, 12, 2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerated Concrete Products Handbooks. Available online: http://www.litebuilt.com (accessed on 11 September 2020).
- Zhao, Y.L.; Ye, J.W.; Lu, X.B.; Liu, M.; Lin, Y.; Gong, W.T.; Ning, G.L. Preparation of sintered foam materials by alkali-activated coal fly ash. J. Hazard Mater. 2010, 174, 108–112. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Kunhanandan, N.E.K.; Indu, S.R.G. A classification of studies on properties of foam concrete. Cem. Concr. Compos. 2009, 31, 388–396. [Google Scholar] [CrossRef]
- Saradhi, B.D.; Ganesh, B.K.; Wee, T.H. Properties of lightweight expanded polystyrene aggregate concretes containing fly ash. Cem. Concr. Res. 2005, 35, 1218–1223. [Google Scholar] [CrossRef]
- Aldridge, D. Introduction to Foamed Concrete: What, Why, How; Dhir, R.K., Newlands, M.D., McCarthy, A., Eds.; Use of foamed concrete in construction; Tomas Telfod: London, UK, 2005; pp. 1–14. ISBN 0727734067. [Google Scholar]
- Lyon, R.E.; Balaguru, P.N.; Foden, A.; Sorathia, U.; Davidovits, J. Fire-resistant aluminosilicate. Compos. Fire Mater. 1997, 21, 67–73. [Google Scholar] [CrossRef]
- Skvara, F.; Kopecky, L.; Smilauer, V.; Bittna, Z. Material and structural characterization of alkali activated low-calcium brown coal fly ash. J. Hazard Mater. 2009, 168, 711–720. [Google Scholar] [CrossRef]
- Subaer van Riessen, A. Thermo-mechanical and microstructural characterisation of sodium-poly(sialate-siloxo) (Na-PSS) geopolymers. J. Mater. Sci. 2007, 42, 3117–3123. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U.; Jaturapitakkul, C. Utilization of fly ash blends from pulverized coal and fluidized bed combustions in geopolymeric materials. Cem. Concr. Compos. 2011, 33, 55–60. [Google Scholar] [CrossRef]


| Component | Composition (mass %) |
|---|---|
| Al2O3 | 28.62 |
| SiO2 | 53.18 |
| CaO | 3.83 |
| Fe2O3 | 5.64 |
| K2O | 1.60 |
| MgO | 0.84 |
| Na2O | 0.65 |
| P2O5 | 0.18 |
| TiO2 | 1.37 |
| LOI a | 3.41 |
| Specimen Density (kg/m3) | Thermal Conductivity (W/mK) | |
|---|---|---|
| GLWC | Commercial LWC [25] | |
| 600 | 0.22 | 0.115 |
| 900 | 0.52 | 0.175 |
| 1200 | 0.70 | 0.270 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Hou, W.; Sun, Z.; Lv, W. Study of In Situ Foamed Fly Ash Geopolymer. Materials 2020, 13, 4059. https://doi.org/10.3390/ma13184059
Su Z, Hou W, Sun Z, Lv W. Study of In Situ Foamed Fly Ash Geopolymer. Materials. 2020; 13(18):4059. https://doi.org/10.3390/ma13184059
Chicago/Turabian StyleSu, Zijian, Wei Hou, Zengqing Sun, and Wei Lv. 2020. "Study of In Situ Foamed Fly Ash Geopolymer" Materials 13, no. 18: 4059. https://doi.org/10.3390/ma13184059




