Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics
Abstract
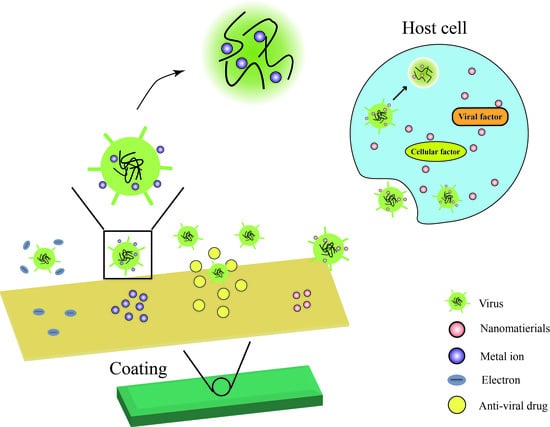
:1. Introduction
2. Coatings Empowering Antiviral and Viricidal Properties
2.1. Antiviral Polymers
2.2. Antiviral Metal Ions/Metal Oxides
2.3. Antiviral Functional Nanomaterials
3. Antiviral Products
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Howell, A.B.; D’Souza, D.H. The Pomegranate: Effects on Bacteria and Viruses That Influence Human Health. Evid.-Based Complementary Altern. Med. 2013, 2013, 606212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S. Dengue shock. J. Emergencies Trauma Shock 2011, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Parker, R.A.; Strilms, R.L. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J. Infect. Dis. 1990, 161, 640–646. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Wu, T.-Z.; Liu, D.-P.; Shao, P.-L.; Chang, L.-Y.; Lu, C.-Y.; Lee, C.-Y.; Huang, F.-Y.; Huang, L.-M. Influenza pandemics: Past, present and future. J. Formos. Med. Assoc. 2006, 105, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y. H5N1 influenza viruses: Outbreaks and biological properties. Cell Res. 2010, 20, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Cheung, T.K.W.; Poon, L.L.M. Biology of influenza a virus. Ann. N. Y. Acad. Sci. 2007, 1102, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Zhong, N.; Zheng, B.; Li, Y.; Poon, L.; Xie, Z.; Chan, K.; Li, P.; Tan, S.; Chang, Q.; Xie, J.P. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003, 362, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Moneim, A.S. Middle East respiratory syndrome coronavirus (MERS-CoV): Evidence and speculations. Springer 2014, 159, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Muyembe-Tamfum, J.-J.; Mulangu, S.; Masumu, J.; Kayembe, J.; Kemp, A.; Paweska, J.T. Ebola virus outbreaks in Africa: Past and present. Onderstepoort J. Vet. Res. 2012, 79, 6–13. [Google Scholar] [CrossRef]
- Safari, S.; Baratloo, A.; Rouhipour, A.; Ghelichkhani, P.; Yousefifard, M.J.E. Ebola hemorrhagic fever as a public health emergency of international concern; A review article. Arch. Acad. Emerg. Med. 2015, 3, 3. [Google Scholar]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020, 92, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. (Lond. Engl.) 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Wang, Y.M.; Li, X.W.; Ren, L.L.; Zhao, J.P.; Hu, Y.; Zhang, L.; Fan, G.H.; Xu, J.Y.; Gu, X.Y.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [Green Version]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respiratory Medicine 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Prompetchara, E.; Kettoy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef]
- Durmus Tekir, S.; Cakir, T.; Ulgen, K. Infection Strategies of Bacterial and Viral Pathogens through Pathogen–Human Protein–Protein Interactions. Front. Microbiol. 2012, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, S.H.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2018, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Fan, C.H.; Li, M.; Nie, H.L.; Wang, F.B.; Wang, H.; Wang, R.; Xia, J.B.; Zheng, X.; Zuo, X.L.; et al. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldar, J.; Weight, A.K.; Klibanov, A.M. Preparation, application and testing of permanent antibacterial and antiviral coatings. Nat. Protoc. 2007, 2, 2412. [Google Scholar] [CrossRef]
- Fabbri, P.; Messori, M. Surface Modification of Polymers: Chemical, Physical, and Biological Routes. Modif. Polym. Prop. 2017, 109–130. [Google Scholar] [CrossRef]
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Nanda, H.S. Surface modification of promising cerium oxide nanoparticles for nanomedicine applications. RSC Adv. 2016, 6, 111889–111894. [Google Scholar] [CrossRef]
- Nanda, H.S. Preparation and Biocompatible Surface Modification of Redox Altered Cerium Oxide Nanoparticle Promising for Nanobiology and Medicine. Bioengineering 2016, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Le, T.T.; Andreadakis, Z.; Kumar, A.; Rom?n, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Trevino, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, H.H.; Ixtepan-Turrent, L.; Garza-Treviño, E.N.; Rodriguez-Padilla, C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J. Nanobiotechnol. 2010, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Boettcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of Influenza Virus Infection by Multivalent Sialic-Acid-Functionalized Gold Nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Modak, S.M.; Sampath, L. Antiviral Glove. U.S. Patent 5,133,090, 28 July 1992. [Google Scholar]
- Yao, L. Thermoplastic Particles which Comprise an Antiviral or Antimicrobial Agent. U.S. Patent 10/408,095, 15 January 2004. [Google Scholar]
- Snyder Jr, D.E. Anti-Bacterial/Anti-Viral Coatings, Coating Process and Parameters Thereof. U.S. Patent 5,968,538, 19 October, 1999. [Google Scholar]
- Swanson, M.J. Virus Inactivating Coatings. U.S. Patent 5,783,502, 21 July 1998. [Google Scholar]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.-i.; Nakata, K.; Furudate, M. Photocatalytic, superhydrophilic, self-cleaning TiO2 coating on cheap, light-weight, flexible polycarbonate substrates. Appl. Surf. Sci 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Hill, B.R.; Watson Sr, T.F.; Triplett, B.L. Antimicrobial Microporous Coating. U.S. Patent 11/726,121, 18 October 2007. [Google Scholar]
- Gabbay, J. Antimicrobial and Antiviral Polymeric Materials and a Process for Preparing the Same. U.S. Patent 10/890,936, 9 December 2004. [Google Scholar]
- Matsushita, M.; Otsuki, K.; Takakuwa, H.; Tsunekuni, R. Antiviral Substance, Antiviral Fiber, and Antiviral Fiber Structure. U.S. Patent 12/679,211, 28 October 2010. [Google Scholar]
- Haldar, J.; Chen, J.; Tumpey, T.M.; Gubareva, L.V.; Klibanov, A.M. Hydrophobic polycationic coatings inactivate wild-type and zanamivir- and/or oseltamivir-resistant human and avian influenza viruses. Biotechnol. Lett. 2008, 30, 475–479. [Google Scholar] [CrossRef]
- Larson, A.M. Antiviral Polymeric Drugs and Surface Coatings; Massachusetts Institute of Technology: Cambridge, MA, USA, 2013. [Google Scholar]
- Lieleg, O.; Lieleg, C.; Bloom, J.; Buck, C.B.; Ribbeck, K. Mucin Biopolymers As Broad-Spectrum Antiviral Agents. Biomacromolecules 2012, 13, 1724–1732. [Google Scholar] [CrossRef] [Green Version]
- Matthews, B.R.; Holan, G. Antiviral Dendrimers. U.S. Patent 6,190,650, 20 February 2001. [Google Scholar]
- Tavakoli, A.; Ataei-Pirkooh, A.; Mm Sadeghi, G.; Bokharaei-Salim, F.; Sahrapour, P.; Kiani, S.J.; Moghoofei, M.; Farahmand, M.; Javanmard, D.; Monavari, S.H. Polyethylene glycol-coated zinc oxide nanoparticle: An efficient nanoweapon to fight against herpes simplex virus type 1. Nanomedicine 2018, 13, 2675–2690. [Google Scholar] [CrossRef]
- Poggio, C.; Colombo, M.; Arciola, C.R.; Greggi, T.; Scribante, A.; Dagna, A.J.M. Copper-Alloy Surfaces and Cleaning Regimens against the Spread of SARS-CoV-2 in Dentistry and Orthopedics. From Fomites to Anti-Infective Nanocoatings. Materials 2020, 13, 3244. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. CRC Crit. Rev. Environ. Control 1988, 18, 295–315. [Google Scholar] [CrossRef]
- Hodek, J.; Zajicova, V.; Lovetinska-Slamborova, I.; Stibor, I.; Muellerova, J.; Weber, J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, S.; Ueda, T.; Yamashina, D.; Kinugawa, K. Coating Agent Composition and Antibacterial/Antiviral Member. U.S. Patent 10,131,797, 20 November 2018. [Google Scholar]
- Pallenberg, A.J.; Marschner, T.M.; Barnhart, D.M. Phenanthroline complexes of the d10 metals nickel(0), zinc(II) and silver(I)—comparison to copper(I) species. Polyhedron 1997, 16, 2711–2719. [Google Scholar] [CrossRef]
- Zeedan, G.; EL-Razik, K.; Allam, A.; Abdalhamed, A.; Zeina, H.A.A. Evaluations of potential antiviral effects of green zinc oxide and silver nanoparticles against bovine herpesvirus-1. Adv. Anim. Vet. Sci. 2020, 8, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Bright, K.R.; Sicairos-Ruelas, E.E.; Gundy, P.M.; Gerba, C.P. Assessment of the Antiviral Properties of Zeolites Containing Metal Ions. Food Environ. Virol. 2008, 1, 37. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Ueda, T.; Yamashina, D.; Kinugawa, K. Copper Complex Titanium Oxide Dispersion Liquid, Coating Agent Composition, and Antibacterial/Antiviral Member. U.S. Patent 9,585,385, 7 March 2017. [Google Scholar]
- Charan, N.; Lavanya, N.; Praveen, B.; Praveen, A.; Sridevi, A.; Narasimha, G. Antiviral activity of antimony and arsenic oxides. Der. Pharma. Chem. 2012, 4, 687–689. [Google Scholar]
- Kelly, S.R.; Gbadamosi, K.A.; Seger, G.E.; Biedermann, K.A.; Morgan, J.M.; Swaile, D.F. Antiviral Compositions for Tissue Paper. U.S. Patent 6,475,501, 5 November 2002. [Google Scholar]
- Hei, R.D.; Smith, K.R.; Laugen, P.D.; Kennedy, S.P. Antimicrobial and Antiviral Compositions Containing an Oxidizing Species. U.S. Patent 6,436,445, 20 August 2002. [Google Scholar]
- Hei, R.D.; Richter, F.L.; Reinhardt, D.J.; Leafblad, B.R. Antimicrobial and Antiviral Compositions Containing an Oxidizing Species. U.S. Patent 6,855,328, 15 February 2005. [Google Scholar]
- Gabbay, J. Antimicrobial and Antiviral Polymeric Materials. U.S. Patent 7,169,402, 30 January 2007. [Google Scholar]
- Oka, H.; Tomioka, T.; Tomita, K.; Hoshino, K.; Nishino, A.; Ueda, S. Method of Disinfecting an Object and Antiviral Disinfection Liquid Therefor. U.S. Patent 5,645,846, 8 July 1997. [Google Scholar]
- Trogolo, J.A. Antiviral Methods. U.S. Patent 11/726,121, 18 October 2007. [Google Scholar]
- Zhou, J.; Hu, Z.; Zabihi, F.; Chen, Z.; Zhu, M. Progress and Perspective of Antiviral Protective Material. Adv. Fiber Mater. 2020, 2, 123–139. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Larson, A.M.; Klibanov, A.M.; Wang, Y. Antiviral and Antibacterial Polyurethanes of Various Modalities. Appl. Biochem. Biotechnol. 2013, 169, 1134–1146. [Google Scholar] [CrossRef] [Green Version]
- Park, D. Antimicrobial Polyurethane Materials and Methods of Forming and Using Same. U.S. Patent 9,949,484, 24 April 2018. [Google Scholar]
- Bignozzi, C.A.; Dissette, V.; Corallini, A.; Carra, G.; Della Valle, R. Functional Nanomaterials with Antibacterial and Antiviral Activity. U.S. Patent 8,158,137, 17 April 2012. [Google Scholar]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-Like Particles. PLoS ONE 2015, 10, e0141050. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [Green Version]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against Hepatitis C Virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Masarwa, M.; Cohen, H.; Meyerstein, D.; Hickman, D.L.; Bakac, A.; Espenson, J.H. Reactions of low-valent transition-metal complexes with hydrogen peroxide. Are they "Fenton-like" or not? 1. The case of Cu+aq and Cr2+aq. J. Am. Chem. Soc. 1988, 110, 4293–4297. [Google Scholar] [CrossRef]
- Khandelwal, N.; Kaur, G.; Kumar, N.; Tiwari, A. Application of silver nanoparticles in viral inhibition: A new hope for antivirals. Dig. J. Nanomater. Biostruct. 2014, 9, 175–186. [Google Scholar]
- Ghosh, S.K. Anti-Viral Surface Coating to Prevent Spread of Novel Coronavirus (COVID-19) Through Touch; Nova Surface-Care Centre Pvt: Maharashtra, India, 2020. [Google Scholar]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V.; et al. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Vinh Quang, N.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Zhu, Z.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano 2020, 14, 6213–6221. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, T.; Kinugawa, K.; Yamashina, D. Cuprous Oxide Particle Dispersion Liquid, Coating Agent Composition, and Antibacterial/Antiviral Member. U.S. Patent 9,414,585, 16 August 2016. [Google Scholar]
- Watanabe, Y.; Shuto, Y.; Itoda, Y. Antibacterial/Antiviral Coating Material, and Method for Forming Antibacterial/Antiviral Coating Film. U.S. Patent 9,943,081, 17 April 2018. [Google Scholar]
- Otsuki, K.; Ito, T.; Murase, T.; Ito, H.; Wakabayashi, K.; Yakura, M.; Yamana, H. Antiviral Agent, and Fabric and Antiviral Member Supporting Antiviral Agent. U.S. Patent 10/567,986, Feb. 12, 2009. [Google Scholar]
- Sugiura, K. Antiviral Agent, Coating Composition, Resin Composition and Antiviral Product. U.S. Patent 16/078,534, 14 February 2019. [Google Scholar]
- Rossett, H. Fluid Compositions that can Form a Coating Having Antiviral Properties. U.S. Patent 13/899,902, 5 June 2014. [Google Scholar]
- Rolf, D. Inhalation Antiviral Patch. U.S. Patent 10/458,078, 15 April 2004. [Google Scholar]
- Rinehart, K.L., Jr. Didemnins A, B, C, and derivatives thereof, as antiviral agents. U.S. Patent 4,493,796, 15 January 1985. [Google Scholar]
- Nashimoto, K.; Tashiro, Y.; Kosaka, Y.; Hara, Y. Antiviral Filter Air Cleaner Impregnated with Tea Extract. U.S. Patent 5,747,053, 5 May 1998. [Google Scholar]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revathi, T.; Thambidurai, S. Synthesis of chitosan incorporated neem seed extract (Azadirachta indica) for medical textiles. Int. J. Biol. Macromol. 2017, 104, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schunemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet (Lond. Engl.) 2020. [Google Scholar] [CrossRef]
- Odeberg, J.; Wirsén, A.; Norberg, Å.; Frie, J.; Printz, G.; Lagercrantz, H.; Gudmundsson, G.H.; Agerberth, B.; Jonsson, B. A novel cysteine-linked antibacterial surface coating significantly inhibits bacterial colonization of nasal silicone prongs in a phase one pre-clinical trial. Mater. Sci. Eng. C 2018, 93, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.H. Antiviral and Antibacterial Respirator Mask. U.S. Patent 6,681,765, 27 January 2004. [Google Scholar]
- Morse, S.S.; Garwin, R.L.; Olsiewski, P.J. Public health—Next flu pandemic: What to do until the vaccine arrives? Science 2006, 314, 929. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.W.; Kwok, S.K.C.; Kwok, H.C. Protective Masks with Coating Comprising Different Electrospun Fibers Interweaved with Each Other, Formulations Forming the Same, and Method of Producing Thereof. U.S. Patent 10,201,198, 12 February 2019. [Google Scholar]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun Nanofibers-Based Face Masks. SpringerLink 2020. [Google Scholar] [CrossRef]
- Fox, P.S.; Pedersen, D.E.; Rolando, J.J.; Staub, R.K. Compositions Having a High Antiviral Efficacy. U.S. Patent 8,034,844, 11 October 2011. [Google Scholar]
- Torkelson, A.A.; da Silva, A.K.; Love, D.C.; Kim, J.Y.; Alper, J.P.; Coox, B.; Dahm, J.; Kozodoy, P.; Maboudian, R.; Nelson, K.L. Investigation of quaternary ammonium silane-coated sand filter for the removal of bacteria and viruses from drinking water. J. Appl. Microbiol. 2012, 113, 1196–1207. [Google Scholar] [CrossRef]




| Type of Coating | Coating Materials | Mechanism | Effectiveness Conditions | Average Time Duration to Destroy Viruses | References |
|---|---|---|---|---|---|
| Polymer | Polymers containing both quaternary ammonium groups and hydrocarbon chains | Creating localized surfactancy | Inactivation of lipid-enveloped viruses | −NA− | [39] |
| Polymer | Microbicidal polycation N,N-dodecyl, methyl-polyethylenimine | −NA− | Destruction of human bacteria pathogens (Staphylococcus aureus and Escherichia coli) and two common strains of influenza virus | Up to 5 days | [23] |
| Polymer | An anti-infective agent selected from the group consisting of chlorhexidine and pharmaceutically acceptable salts of chlorhexidine | −NA− Blocking adsorption sites of anti-infective agent | −NA− | −NA− | [36] |
| Polymer | Methyl-polyethylenimine (N,N-dodecyl,methyl-PEI) | −NA− | Highly lethal to waterborne Influenza A viruses, including wild-type human and avian strains | Up to 5 days | [44] |
| Polymer | Polyethylene (preferred polyolefin) | −NA− | Porous plastic materials with antiviral agent | 2−3 days | [37] |
| Polymer | Polyethylenimine | −NA− | Antiviral surface coating that can detoxify aqueous solutions containing various viruses | −NA− | [45] |
| Polymer | PVP-I and N-9 | −NA− | Dual or multilayered format antiviral coating that imparts antipathogenic properties to substrate | −NA− | [38] |
| Polymer | A dendrimer such as a polyamidoamine or polyly sine having a plurality of terminal groups | Attachment of Ionic moieties | Antiviral activity against human immunodeficiency (HIV) and other enveloped viruses | −NA− | [47] |
| Polymer | Porcine gastric mucin polymers | Shielding effect | Protect an underlying cell layer from infection by small viruses like human papillomavirus (HPV), Merkel cell polyomavirus (MCV), or a strain of influenza A virus | −NA− | [46] |
| Polymer | A polymeric component selected from the group consisting of a polyamide, a polyester, an acrylic, a polyalkylene, and mixtures thereof | Precipitation and reduction | Action of Cu++ particles as antiviral agents | Less than 4 h | [42] |
| Polymer | A polymer containing a maleic acid component as a monomer unit in a polymer chain thereof | Action of metallic particles with copolymerized fiber | Effective against an avian influenza virus | −NA− | [43] |
| Metal ions/metal oxides | Copper ion, silver ion or both | Ion exchange type | Action of single source of both Ag++ and Cu++ ions in treating virally contaminated surfaces | Less than 4 h | [64] |
| Metal ions/metal oxides | Cu in powder | Electrolytic Plating | Action of Cu++ ions | Less than 4 h | [62] |
| Metal ions/metal oxides | Silver, copper and zinc | Washing with PBS | Action of Ag++, Zn++ and Cu++ ions showed viricidal activity against (HIV-1), and other enveloped viruses | Less than 4 h | [51] |
| Metal ions/metal oxides | Reduction/oxidation reaction on surfaces of photocatalytic particles | Inactivating Influenza viruses and Norovirus | Less than 4 h | [52] | |
| Metal ions/metal oxides | A copper complex titanium oxide dispersion liquid | Dispersion | −NA− | Less than 4 h | [57] |
| Metal ions/metal oxides | Cuprous oxide particle dispersion liquid and a binder resin | Coupling effect | −NA− | Less than 4 h | [82] |
| Metal ions/metal oxides | Water soluble metal ions include aluminum, copper, and mixtures thereof | Hydroxide formation | Water soluble metal ion has the ability to kill certain strains of viruses | 4−8 h | [59] |
| Metal ions/metal oxides | An antiviral composition consisting of a thiosulfate complex salt coated with a material layer like Silicon dioxide of a metal like silver, copper and zinc | Releasing salts and by attaching chemotherapeutic agents to complex | The composition releases its salts into the contaminated sites | −NA− | [63] |
| Metal ions/metal oxides | Metal oxides or metalloid oxides, such as, e.g., TiO2, ZrO2, SnO2, ZnO, and SiO2 | oligodynamic effect | −NA− | 4−8 h | [69] |
| Metal ions/metal oxides | Antiviral activity of Arsenic oxide (As2O3) and Antimony Oxide (Sb2O3) | Hydroxyl radical oxidation, diffusion of disinfectant | Excellent viricidal property on viral strain bacteriophage | −NA− | [58] |
| Nanomaterials | Nanosized copper (I) iodide (CuI) | Hydroxyl radical formation | CuI particles showed antiviral activity against influenza A virus of swine origin | Less than 4 h | [31] |
| Nanomaterials | Silver nanoparticles | Blocking interaction | inactivating many viral strains from lipid envelope viruses | Less than 4 h | [73,79] |
| Nanomaterials | (GO-Ag) Nanocomposites | Washing | Antiviral activity of nanomaterials on non-enveloped viruses | Less Than 4 h | [81] |
| Other | A quaternary ammonium salt and a polyhydric carboxylic acid having a C6 or more hydrocarbon group and two or more carboxyl groups | Preventing elution of salts in water thus maintaining antiviral effect | Water resistant antiviral coating | 2−3 days | [83] |
| Other | An antiviral agent contains a powder obtained by baking (calcining) dolomite and hydrating a part thereof | Filtration | An antiviral coating effective for Coronavirus | −NA− | [84] |
| Other | An antiviral agent includes an inorganic solid acid having an acid site concentration of more than 0.005 mmol/g | Dispersion | Excellent in heat resistance and maintains the inactivating effect on viruses | −NA− | [85] |
| Other | The fluid compositions consist of at least one viricide like lauric acid and essential oils like laurel essential oil, soybean oil | −NA− | −NA− | 2−3 days | [86] |
| Other | A therapeutically effective amount of an essential oil | Presence of different functional groups in essential oils. | This method prevents a respiratory infection in a mammal | −NA− | [87] |
| Other | Novel antibiotics didemnin A, B, C, D, and E (didemnins) | Drug delivery system (DDS) | Inactivate variety of DNA and RNA viruses. | −NA− | [88] |
| Other | Antiviral Filter air Cleaner impregnated with tea extract | Filtration | Disable influenza virus | −NA− | [89] |
| Other | Tea extract, other herbals, and phytochemicals like Curcumin | Filtration | Acting against Influenza virus, hepatitis C Virus, HIV | −NA− | [90] |
| Other | Chitosan incorporated neem seed extract (Azadirachta indica) | Higher antibacterial activity against gram-positive and gram-negative bacteria | −NA− | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials 2020, 13, 4041. https://doi.org/10.3390/ma13184041
Pemmada R, Zhu X, Dash M, Zhou Y, Ramakrishna S, Peng X, Thomas V, Jain S, Nanda HS. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials. 2020; 13(18):4041. https://doi.org/10.3390/ma13184041
Chicago/Turabian StylePemmada, Rakesh, Xiaoxian Zhu, Madhusmita Dash, Yubin Zhou, Seeram Ramakrishna, Xinsheng Peng, Vinoy Thomas, Sanjeev Jain, and Himansu Sekhar Nanda. 2020. "Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics" Materials 13, no. 18: 4041. https://doi.org/10.3390/ma13184041