Chemical Modification of Cellulose Microfibres to Reinforce Poly(methyl methacrylate) Used for Dental Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
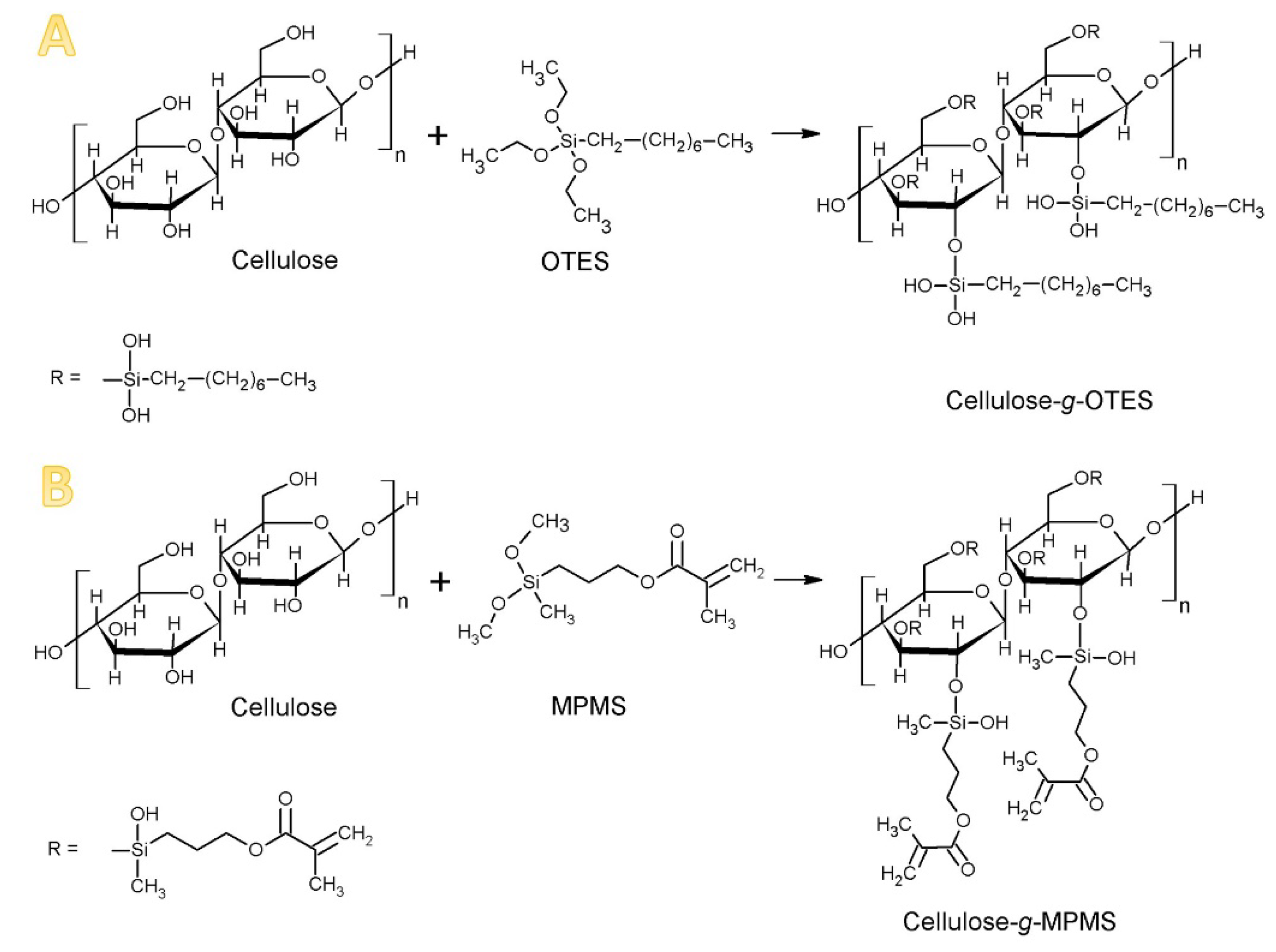
2.2. Modification of Cellulose
2.3. Preparation of Dental Composites
2.4. Methods
3. Results
3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
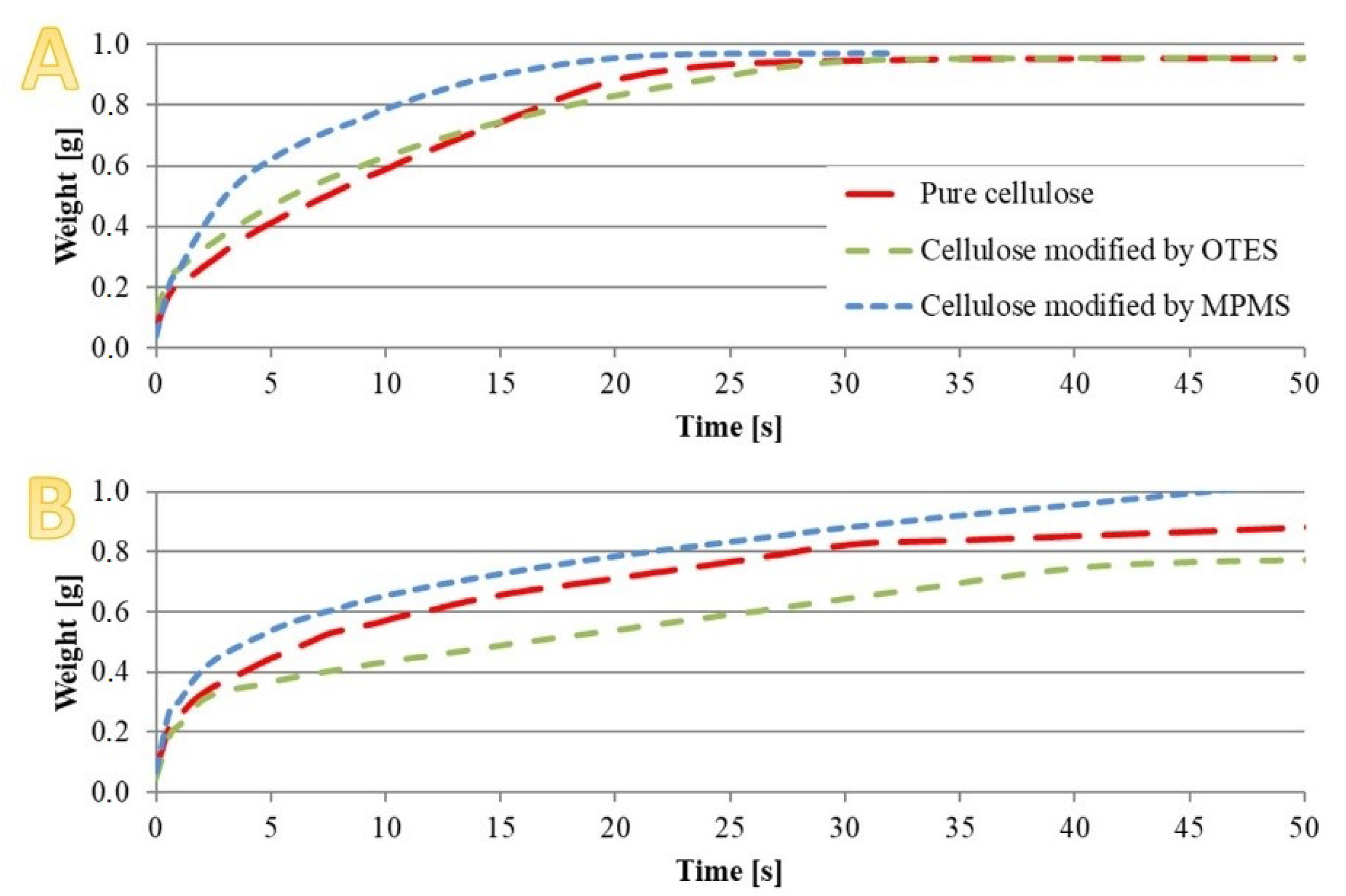
3.2. Sorption and Surface Free Energy (SFE)
3.3. Scanning Electron Microscope (SEM)
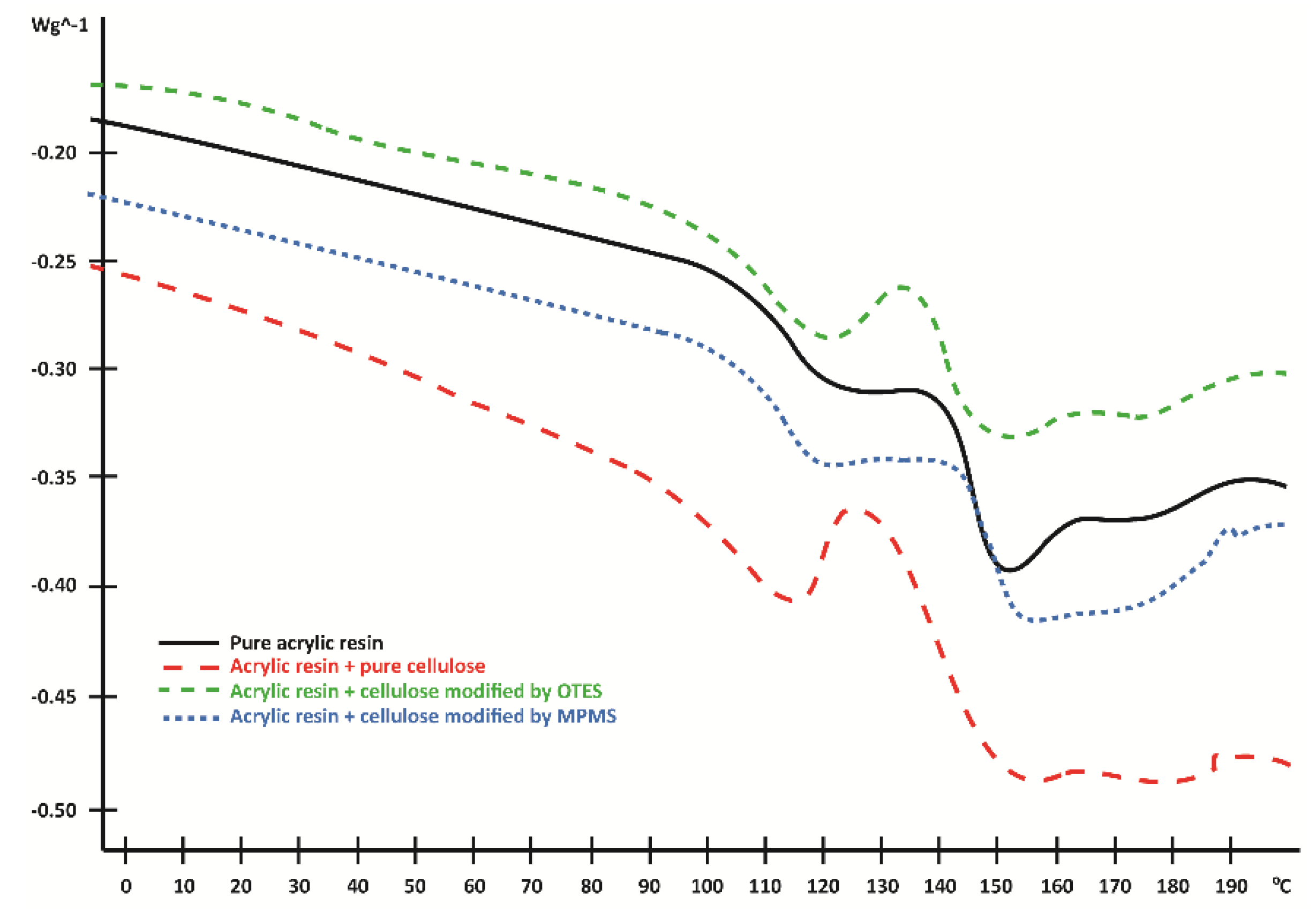
3.4. Differential Scanning Calorimetry (DSC)
3.5. Swelling Measurement
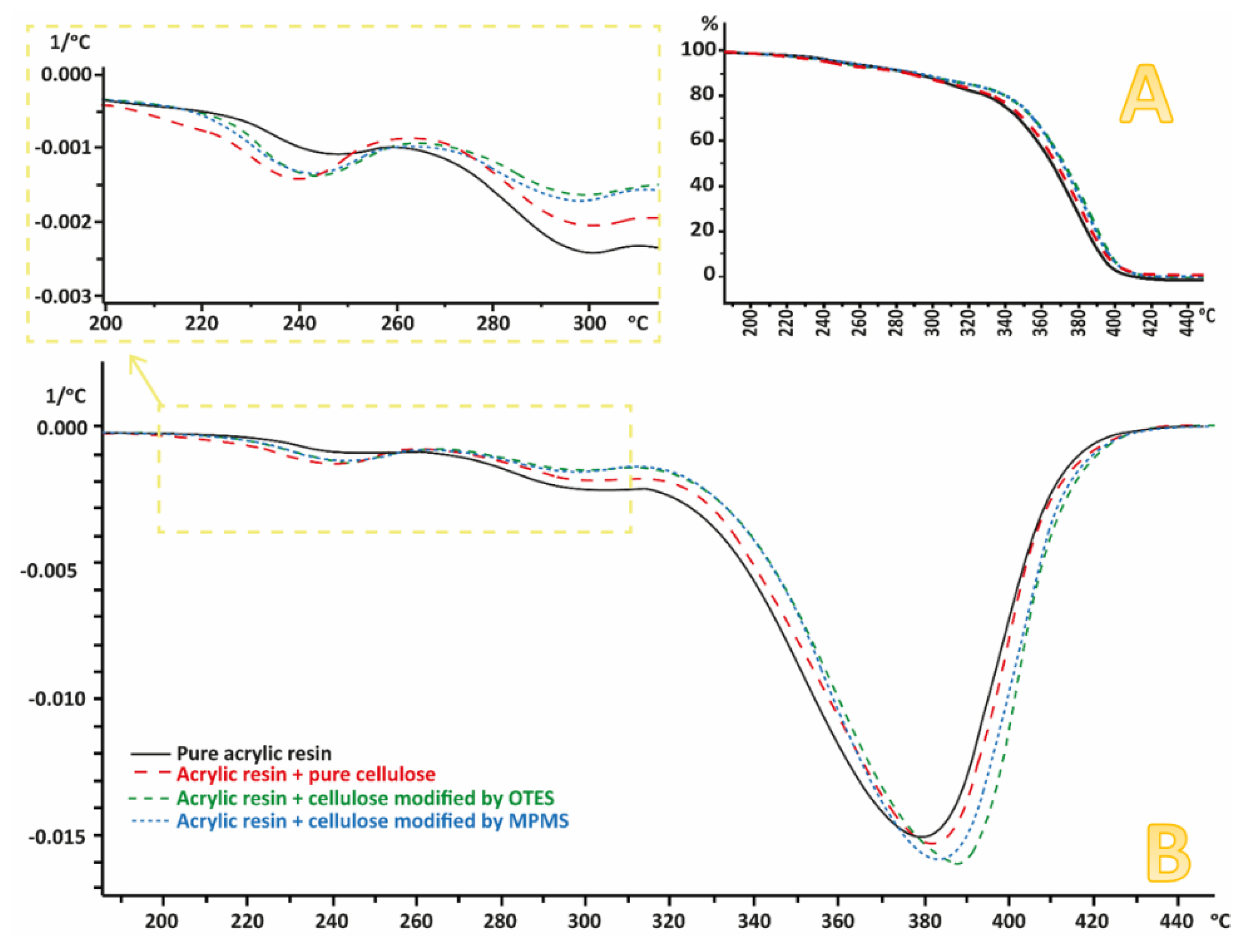
3.6. Thermogravimetric Analysis (TGA)
3.7. Three-Point Bending Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taczała, J.; Sawicki, J. Bond between single artificial teeth and the base plate in removable denture metal framework. J. Achiev. Mater. Manuf. Eng. 2018, 1, 11–21. [Google Scholar] [CrossRef]
- Sodagar, A.; Bahador, A.; Khalil, S.; Saffar Shahroudi, A.; Zaman Kassaee, M. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kul, E.; Aladağ, L.İ.; Yesildal, R. Evaluation of thermal conductivity and flexural strength properties of poly(methyl methacrylate) denture base material reinforced with different fillers. J. Prosthet. Dent. 2016, 116, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Asopa, V.; Suresh, S.; Khandelwal, M.; Sharma, V.; Asopa, S.S.; Kaira, L.S. A comparative evaluation of properties of zirconia reinforced high impact acrylic resin with that of high impact acrylic resin. Saudi J. Dent. Res. 2015, 6, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Vojdani, M.; Bagheri, R.; Khaledi, A.A.R. Effects of aluminum oxide addition on the flexural strength, surface hardness, and roughness of heat-polymerized acrylic resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Yang, C.; Zeng, Q.H.; Xiang, Y. Morphological and mechanical properties of graphene-reinforced PMMA nanocomposites using a multiscale analysis. Comput. Mater. Sci. 2018, 150, 107–120. [Google Scholar] [CrossRef]
- Jagger, D.C.; Harrison, A.; Jandt, K.D. The reinforcement of dentures. J. Oral Rehabil. 1999, 26, 185–194. [Google Scholar] [CrossRef]
- Narva, K.K.; Lassila, L.V.; Vallittu, P.K. The static strength and modulus of fiber reinforced denture base polymer. Dent. Mater. 2005, 21, 421–428. [Google Scholar] [CrossRef]
- Sun, W.; Cai, Q.; Li, P.; Deng, X.; Wei, Y.; Xu, M.M.; Yang, X. Post-draw PAN-PMMA nanofiber reinforced and toughened Bis-GMA dental restorative composite. Dent. Mater. 2010, 26, 873–880. [Google Scholar] [CrossRef]
- Vidotti, H.A.; Manso, A.P.; Leung, V.; Do Valle, A.L.; Ko, F.; Carvalho, R.M. Flexural properties of experimental nanofiber reinforced composite are affected by resin composition and nanofiber/resin ratio. Dent. Mater. 2015, 31, 1132–1141. [Google Scholar] [CrossRef]
- Keyf, F.; Uzun, G. The effect of glass fibre-reinforcement on the transverse strength, deflection and modulus of elasticity of repaired acrylic resins. Int. Dent. J. 2000, 50, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kanie, T.; Fujii, K.; Arikawa, H.; Inoue, K. Flexural properties and impact strength of denture base polymer reinforced with woven glass fibers. Dent. Mater. 2000, 16, 150–158. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Yu, T.; Cong, L. Reinforcement of denture base resin with short vegetable fiber. Dent. Mater. 2013, 29, 1273–1279. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef]
- Erbas Kiziltas, E.; Kiziltas, A.; Bollin, S.C.; Gardner, D.J. Preparation and characterization of transparent PMMA–cellulose-based nanocomposites. Carbohydr. Polym. 2015, 127, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.F.; Chou, M.Y.; Lian, H.Y.; Hsu, L.R.; Chen-Wei, S.M. Highly transparent and impact-resistant PMMA nanocomposites reinforced by cellulose nanofibers of pineapple leaves modified by eco-friendly methods. Express Polym. Lett. 2018, 12, 844–854. [Google Scholar] [CrossRef]
- Huang, T.; Kuboyama, K.; Fukuzumi, H.; Ougizawa, T. PMMA/TEMPO-oxidized cellulose nanofiber nanocomposite with improved mechanical properties, high transparency and tunable birefringence. Cellulose 2018, 25, 2393–2403. [Google Scholar] [CrossRef]
- Banerjee, M.; Sain, S.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ray, D. Surface treatment of cellulose fibers with methylmethacrylate for enhanced properties of in situ polymerized PMMA/cellulose composites. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Rol, F.; Naceur, M.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Wolski, K.; Zaborski, M. Universal approach of cellulose fibres chemical modification result analysis via commonly used techniques. Polym. Bull. 2019, 76, 2147–2162. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.K.; Gupta, R.K.; Thakur, V.K. Surface modification of cellulose using silane coupling agent. Carbohydr. Polym. 2014, 111, 849–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, B.P.; Durkin, D.P.; Caudill, E.R.; Zhu, L.; White, D.H.; Curry, M.L.; Pedersen, J.A.; Fairbrother, D.H. Impact of Silanization on the Structure, Dispersion Properties, and Biodegradability of Nanocellulose as a Nanocomposite Filler. ACS Appl. Nano Mater. 2018, 1, 7025–7038. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Tan, Y.; Du, J.; Wang, J. Synthesis and characterization of a porous and hydrophobic cellulose-based composite for efficient and fast oil–water separation. Carbohydr. Polym. 2016, 140, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Nevesa, R.M.; Ornaghi, H.L., Jr.; Zattera, A.J.; Campos, A. The influence of silane surface modification on microcrystalline cellulose characteristics. Carbohydr. Polym. 2020, 230, 115595. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.; Hashimoto, K.; Miyazaki, K.; Terano, M. Cellulose/syndiotactic polypropylene composites: Effects of maleated polypropylene as a compatibilizer and silanized cellulose on the morphology and tensile properties. J. Appl. Polym. Sci. 2009, 113, 2022–2029. [Google Scholar] [CrossRef]
- Shang, S.M.; Li, Z.; Xing, Y.; Xin, J.H.; Tao, X.M. Preparation of durable hydrophobic cellulose fabric from water glass and mixed organosilanes. Appl. Surf. Sci. 2010, 257, 1495–1499. [Google Scholar] [CrossRef]
- Raquez, J.M.; Murena, Y.; Goffin, A.L.; Habibi, Y.; Ruelle, B.; DeBuyl, F.; Dubois, P. Surface-modification of cellulose nanowhiskers and their use as nanoreinforcers into polylactide: A sustainably-integrated approach. Compos. Sci. Technol. 2012, 72, 544–549. [Google Scholar] [CrossRef]
- Pucci, M.F.; Liotier, P.J.; Drapier, S. Capillary wicking in a fibrous reinforcement-Orthotropic issues to determine the capillary pressure components. Compos. Part A Appl. Sci. Manuf. 2015, 77, 133–141. [Google Scholar] [CrossRef]
- Thakker, M.; Shukla, P.; Shah, D.O. Surface and colloidal properties of chalks: A novel approach using surfactants to convert normal chalks into dustless chalks. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 236–244. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Taczala, J.; Fu, C.; Sawicki, J.; Pietrasik, J. Influence different amount of cellulose on the mechanical strength of dental acrylic resin. IOP Conf. Ser. Mater. Sci. Eng. 2020, 743, 012044. [Google Scholar] [CrossRef] [Green Version]
- Hoeger, I.C. Microscopis analysis of cellulose nanofibril (CNF)- and cellulose nanocrystal (CNC)- based nanocomposites. In Handbook of Nanocellulose Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 365–392. [Google Scholar] [CrossRef]
- Sain, S.; Ray, D.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ennis, C.J.; Rahman, P.K.S.M. Synthesis and characterization of PMMA-cellulose nanocomposites by in situ polymerization technique. J. Appl. Polym. Sci. 2012, 126, E127–E134. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation, 2nd ed.; Elsevier: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Murigi, M.K.; Madivoli, E.S.; Mathenyu, M.M.; Kareru, P.G.; Gachanja, A.N.; Njenga, P.K.; Nowsheen, G.; Githira, P.N.; Mercy, G. Comparison of physicochemical characteristics of microcrystalline cellulose from four abundant Kenyan biomasses. IOSR J. Polym. Text. Eng. 2014, 1, 53–63. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Reiner, R.S.; Ralph, S.A. Cellulose I crystallinity determination using FT-Raman spectroscopy: Univariate and multivariate methods. Cellulose 2010, 17, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Hettegger, H.; Beaumont, M.; Potthast, A.; Rosenau, T. Aqueous modification of nano- and microfibrillar cellulose with a click synthon. ChemSusChem 2016, 9, 75–79. [Google Scholar] [CrossRef]
- Brochier Salon, M.C.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Gandini, A. Silane adsorption onto cellulose fibers: Hydrolysis and condensation reactions. J. Colloid Interface Sci. 2005, 289, 249–261. [Google Scholar] [CrossRef]
- Sigma-Aldrich. IR Spectrum Table & Chart by Sigma-Aldrich n.d. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html (accessed on 4 August 2020).
- Schindler, W.D.; Hauser, P.J. Chemical Finishing of Textiles; Woodhead Publishing Limited: Cambridge, UK, 2004. [Google Scholar]
- Cazes, J. Encyclopedia of Chromatography, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Melnyk, I.V.; Vaclavikova, M.; Seisenbaeva, G.A.; Kessler, V.G. Biocompatible Hybrid Oxide Nanoparticles for Human Health: From Synthesis to Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Sherma, J.; Fried, B. Handbook of Thin-Layer Chromatography, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- J. Rettenmaier USA LP. The Arbocel UFC100 Ultrafine Cellulose for Paper and Board Coating. Available online: https://www.tappi.org/content/events/11papercon/documents/JRS%20USA%20pptx.pdf (accessed on 4 August 2020).
- Madivoli, E.S.; Kareru, P.G.; Gachanja, A.N.; Mugo, S.; Murigi, M.K.; Kairigo, P.K.; Kipyegon, C.; Mutembei, J.K.; Njonge, F.K. Adsorption of selected heavy metals on modified nano cellulose. Int. Res. J. Pure Appl. Chem. 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Visakh, P.M.; Lüftl, S. Polyethylene-Based Biocomposites and Bionanocomposites; Scrivener Publishing LLC: Beverly, MA, USA, 2016. [Google Scholar] [CrossRef]
- Hussein, M.A.; Albeladi, H.K.; Al-Romaizan, A.N. Role of cross-linking process on the performance of PMMA. Int. J. Biosens. Bioelectron. 2017, 3, 279–284. [Google Scholar] [CrossRef]
- Doitrand, A.; Henry, R.; Chevalier, J.; Meille, S. Revisiting the strength of micron-scale ceramic platelets. J. Am. Ceram. Soc. 2020, 1–10. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taczała, J.; Sawicki, J.; Pietrasik, J. Chemical Modification of Cellulose Microfibres to Reinforce Poly(methyl methacrylate) Used for Dental Application. Materials 2020, 13, 3807. https://doi.org/10.3390/ma13173807
Taczała J, Sawicki J, Pietrasik J. Chemical Modification of Cellulose Microfibres to Reinforce Poly(methyl methacrylate) Used for Dental Application. Materials. 2020; 13(17):3807. https://doi.org/10.3390/ma13173807
Chicago/Turabian StyleTaczała, Joanna, Jacek Sawicki, and Joanna Pietrasik. 2020. "Chemical Modification of Cellulose Microfibres to Reinforce Poly(methyl methacrylate) Used for Dental Application" Materials 13, no. 17: 3807. https://doi.org/10.3390/ma13173807





