A Review of the Mechanical Properties and Durability of Ecological Concretes in a Cold Climate in Comparison to Standard Ordinary Portland Cement-Based Concrete
Abstract
:1. Introduction
1.1. Ordinary Portland Cement (OPC)-Based Concrete
1.2. SCM-Based Concrete
2. OPC-Based Concretes
3. Composite Portland Cement-Based Concretes
4. Alkali-Activated Concretes
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korhonen, C.J. Antifreeze Admixtures for Cold Regions Concreting: A Literature Review; US Army Corps of Engineers, Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1990. [Google Scholar]
- Polat, R. The effect of antifreeze additives on fresh concrete subjected to freezing and thawing cycles. Cold Reg. Sci. Technol. 2016, 127, 10–17. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Yang, Y.; Cui, Y. Effect of low temperature on hydration performance of the complex binder of silica fume-portland cement. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 75–81. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Zhang, G. Formation of ettringite in Portland cement/calcium aluminate cement/calcium sulfate ternary system hydrates at lower temperatures. Constr. Build. Mater. 2012, 31, 347–352. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, W.; Sha, A.; Gao, J.; Han, Z.; Xu, W. Portland cement hydration behavior at low temperatures: Views from calculation and experimental study. Adv. Mater. Sci. Eng. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Yang, H.; Ju, C.; Yang, Y. Novel selection of environment-friendly cementitious materials for winter construction: Alkali-Activated slag/Portland cement. J. Clean. Prod. 2020, 258, 1–11. [Google Scholar] [CrossRef]
- Nmai, C.K. Cold weather concreting admixtures. Cem. Concr. Compos. 1998, 20, 121–128. [Google Scholar] [CrossRef]
- Barna, L.A.; Seman, P.M.; Korhonen, C.J. Energy-Efficient approach to cold-weather concreting. J. Mater. Civ. Eng. 2011, 23, 1544–1551. [Google Scholar] [CrossRef]
- Ogurtsova, Y.N.; Zhernovsky, I.V.; Botsman, L.N. Efficiency of composite binders with antifreezing agents. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Chelyabinsk, Russia, 21–22 September 2017; Institute of Physics Publishing Ltd.: Bristol, UK, 2017; Volume 262. [Google Scholar]
- Barna, L.A.; Korhonen, C.J. Extending the Season for Concrete Construction and Repair Phase III—Guidance for Optimizing Admixture Dosage Rates Cold Regions Research and Engineering Laboratory; US Army Engineering Research and Development Center, Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 2014. [Google Scholar]
- Polat, R.; Demirboǧa, R.; Karakoç, M.B.; Türkmen, I. The influence of lightweight aggregate on the physico-mechanical properties of concrete exposed to freeze-thaw cycles. Cold Reg. Sci. Technol. 2010, 60, 51–56. [Google Scholar] [CrossRef]
- Farrington, S.A.; Christensen, B.J. The use of chemical admixtures to facilitate placement of concrete at freezing temperatures. ACI Spec. Publ. 2003, 217, 71–86. [Google Scholar]
- ACI 306R Guide to Cold Weather Concreting; American Concrete Institute: Farmington Hills, MI, USA, 2010; Available online: https://www.concrete.org/Portals/0/Files/PDF/Previews/306R-16_preview.pdf (accessed on 5 August 2020).
- Demirboǧa, R.; Karagöl, F.; Polat, R.; Kaygusuz, M.A. The effects of urea on strength gaining of fresh concrete under the cold weather conditions. Constr. Build. Mater. 2014, 64, 114–120. [Google Scholar] [CrossRef]
- Suprenant, B.A. Designing cold weather concrete mixes. Aberd. Concr. Constr. 1990, 35, 882–884. [Google Scholar]
- Korhonen, C.J.; Cortez, E.R. Antifreeze admixtures for cold weather concreting. Concr. Int. 1991, 13, 38–41. [Google Scholar]
- Strokova, V.V.; Botsman, L.N.; Ogurtsova, Y.N.; Nelubova, V.V. The efficiency of using antifreezing agents in monolithic construction. In Proceedings of the ECOS 2016: The 29th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, Portoroz, Slovenia, 19–23 June 2016; University of Ljubljana Publisher: Ljubljana, Slovenia, 2016. Available online: https://pdfs.semanticscholar.org/03c4/c86f21f76ad75101d45b4eb13008a7c1e8b9.pdf (accessed on 5 August 2020).
- Kosmatka Steven, H.; Kerkhoff, B.; Panarese, W.C. Design and Control Design and Control of Concrete Mixtures—EB001, 14th ed.; Portland Cement Association: Skokie, IL, USA, 2002; ISBN 0893122173. [Google Scholar]
- ACI 306R-88 Cold Weather Concreting; ACI Committee 306: Detroit, MI, USA, 1988.
- Karagöl, F.; Demirboǧa, R.; Kaygusuz, M.A.; Yadollahi, M.M.; Polat, R. The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg. Sci. Technol. 2013, 89, 30–35. [Google Scholar] [CrossRef]
- Jokela, J.; Kivekas, L.; Kukko, H.; Ratvio, J.; Rissanen, E. Arctic Concrete Technology—Research Report 343; Technical Research Center of Finland (VTT), Concrete and Silicate Laboratory: Espoo, Finland, 1982. [Google Scholar]
- Scanlon, J.M.; Ryan, R.J. Placing cold weather concrete. Constr. Specif. 1989, 42, 58–65. [Google Scholar]
- Kessler, S.; Thiel, C.; Grosse, C.U.; Gehlen, C. Effect of freeze–thaw damage on chloride ingress into concrete. Mater. Struct. Constr. 2017, 50, 1–13. [Google Scholar] [CrossRef]
- Klieger, P. Effect of mixing and curing temperature on concrete strength. J. Proc. 1958, 54, 1063–1081. [Google Scholar]
- Franke, W.; Thiel, C.; Duran, F.; Gehlen, C. Effect of calcium nitrate on the freeze-thaw-resistance of concrete. Betonw. Fert. Plant Precast Technol. 2015, 81, 34–42. [Google Scholar]
- Sobolev, K.G.; Batrakov, V.G. Effect of a polyethylhydrosiloxane admixture on the durability of concrete with supplementary cementitious materials. J. Mater. Civ. Eng. 2007, 19, 809–819. [Google Scholar] [CrossRef]
- Myrdal, R. Accelerating Admixtures for Concrete. State of the Art; COIN Concrete Innovation Centre: Trondheim, Norway, 2007; ISBN 9788253609898. [Google Scholar]
- Mironov, S.A.; Lagoyda, A.V.; Ukhov, Y.N. Curing Concrete with Chemical Additives in Freezing Weather; U. S. Army Engineering Research and Development Centre, Cold Regions Research and Engineering Laboratory publishers: Hanover, NH, USA, 1976. [Google Scholar]
- Stormer, C.D. Cold Concrete—Technical Report 220; U. S. Army Engineering Research and Development Centre, Cold Regions Research and Engineering Laboratory publishers: Hanover, NH, USA, 1970. [Google Scholar]
- Price, W.H. Factors influencing concrete strength. ACI J. Proc. 1951, 47, 41–432. [Google Scholar] [CrossRef]
- Shideler, J.J. Calcium cholride in concrete. ACI J. Proc. 1952, 48, 537–559. [Google Scholar] [CrossRef]
- Sutter, L.; Peterson, K.; Julio-Betancourt, G.; Hooton, D.; Dam, T.V.; Smith, K. The Deleterious Chemical Effects of Concentrated Deicing Solutions on Portland Cement Concrete; Michigan Tech Transportaion Institute: Houghton, MI, USA, 2008. [Google Scholar]
- Ratinov, V.B.; Rosenberg, T.I. Antifreezing Admixtures. In Concrete Admixtures Handbook—Properties, Science and Technology; Noyes Publications: Park Ridge, NJ, USA, 1995; pp. 740–799. Available online: https://drive.google.com/file/d/1eMNAWYfluillI0xvtwTvHgDKqzEZG7-4/view (accessed on 5 August 2020).
- Hooton, R.; Johnston, C.; Rixom, R.; Mailvaganam, N. Chemical Admixtures for Concrete, 3rd ed.; E & FN Spoon: London, UK, 1999; ISBN 041922520X. [Google Scholar]
- Sakai, K.; Watanabe, H.; Nomachi, H.; Hamabe, K. Antifreeze admixture developed in Japan. Concr. Int. 1991, 13, 26–30. [Google Scholar]
- Karagöl, F.; Demirboga, R.; Khushefati, W.H. Behavior of fresh and hardened concretes with antifreeze admixtures in deep-freeze low temperatures and exterior winter conditions. Constr. Build. Mater. 2015, 76, 388–395. [Google Scholar] [CrossRef]
- Karagöl, F.; Yegin, Y.; Polat, R.; Benli, A.; Demirboğa, R. The influence of lightweight aggregate, freezing-thawing procedure and air entraining agent on freezing-thawing damage. Struct. Concr. 2018, 19, 1328–1340. [Google Scholar] [CrossRef]
- Justnes, H. Explanation of long-term compressive strength of concrete caused by the set accelerator calcium nitrate. In Proceedings of the 11th International Congress on the Chemistry of Cement, Durba, South Africa, 11–16 May 2003; Volume 11, p. 475. [Google Scholar]
- Mason, M.R.; Schroeder, H.P. Freeze-Thaw durability of concrete cured below 0 °C using antifreeze admixtures. In Proceedings of the Eighth International Conference on Cold Regions Engineering, Fairbanks, AK, USA, 12 August 1996; American Society of Civil Engineers: New York, NY, USA, 1996; pp. 185–195. [Google Scholar]
- Mou, T.; Zhou, X.; Fan, B.; Ding, Q. Frost resistance of steel fiber reinforced micro-expansive concrete filled steel tube. Appl. Mech. Mater. 2012, 204, 3956–3960. [Google Scholar] [CrossRef]
- Korhonen, C.J.; Charles, J.; Cortex, E.R.; Durning, T.A.; Jeknavorian, A.A. Antifreeze Admixtures for Concrete—Special Report SP-97-26; U. S. Army Engineering Research and Development Centre, Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1997. [Google Scholar]
- Kivekäs, L.; Huovinen, S.; Leivo, M. Concrete under Arctic Conditions; VTT Technical Research Centre of Finland: Espoo, Finland, 1985; Volume 343, ISBN 951-38-2256-7. [Google Scholar]
- Kivekas, L.; Kukko, H. Strength development and frost resistance of concrete at low temperatures. Nord. Concr. Res. 1983, 2, 137–148. [Google Scholar]
- Reddy, P.N.; Naqash, J.A. Effect of antifreeze admixtures on cold weather concrete. Int. J. Eng. 2019, 32, 366–372. [Google Scholar] [CrossRef]
- Wise, T.; Ramachandran, V.S.; Polomark, G.M. The effect of thiocyanates on the hydration of portland cement at low temperatures. Thermochim. Acta 1995, 264, 157–171. [Google Scholar] [CrossRef]
- Arslan, M.; Çullu, M.; Durmuş, G. The effect of antifreeze admixtures on compressive strength of concretes subjected to frost action. Gazi Univ. J. Sci. 2011, 24, 299–307. [Google Scholar]
- Brook, J.W.; Factor, D.F.; Kinney Frederick, D.; Sarkar, A.K. Cold weather admixture. Concr. Int. 1988, 10, 44–49. [Google Scholar]
- Çullu, M.; Arslan, M. The effects of chemical attacks on physical and mechanical properties of concrete produced under cold weather conditions. Constr. Build. Mater. 2014, 57, 53–60. [Google Scholar] [CrossRef]
- Naik, T.R.; Kraus, R.N.; Ramme, B.W.; Chun, Y.M. Deicing salt-scaling resistance: Laboratory and field evaluation of concrete containing up to 70% Class C and Class F fly ash. J. ASTM Int. 2005, 2, 93–104. [Google Scholar] [CrossRef]
- Toutanji, H.; Delatte, N.; Aggoun, S.; Duval, R.; Danson, A. Effect of supplementary cementitious materials on the compressive strength and durability of short-term cured concrete. Cem. Concr. Res. 2004, 34, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Matalkah, F.; Soroushian, P. Freeze thaw and deicer salt scaling resistance of concrete prepared with alkali aluminosilicate cement. Constr. Build. Mater. 2018, 163, 200–213. [Google Scholar] [CrossRef]
- Škvára, F.; Jílek, T.; Kopecký, L. Geopolymer materials based on fly ash. Ceram. Silik. 2005, 49, 195–204. [Google Scholar]
- Memiş, S.; Kaplan, G.; Yaprak, H.; Yilmazoğlu, M.U.; Mütevvelli Özkan, I.G. Some durability properties of alkali activated materials (AAM) produced with ceramic powder and micro calcite. Ceram. Silik. 2018, 62, 342–354. [Google Scholar] [CrossRef]
- Shahrajabian, F.; Behfarnia, K. The effects of nano particles on freeze and thaw resistance of alkali-activated slag concrete. Constr. Build. Mater. 2018, 176, 172–178. [Google Scholar] [CrossRef]
- Justnes, H.; Nygaard, E.C. Technical calcium nitrate as set accelerator for cement at low temperatures. Cem. Concr. Res. 1995, 25, 1766–1774. [Google Scholar] [CrossRef]
- Kumar, A.; Bishnoi, S.; Scrivener, K.L. Modelling early age hydration kinetics of alite. Cem. Concr. Res. 2012, 42, 903–918. [Google Scholar] [CrossRef]
- Liu, Z.; Lou, B.; Barbieri, D.M.; Sha, A.; Ye, T.; Li, Y. Effects of pre-curing treatment and chemical accelerators on Portland cement mortars at low temperature (5 °C). Constr. Build. Mater. 2020, 240, 1–9. [Google Scholar] [CrossRef]
- Ghosh, A.H.; Das, B.B. Implication of Concrete with Chemical Admixture Cured in Low Temperature on Strength, Chloride Permeability and Microstructure; Das, B.B., Neithalath, N., Eds.; Sustainable Construction and Building Materials, Lecture Notes in Civil Engineering; Springer Nature: Singapore, 2019; Volume 25, pp. 287–298. [Google Scholar] [CrossRef]
- Uchikawa, H.; Hanehara, S.; Shirasaka, T.; Sawaki, D. Effect of admixture on hydration of cement, adsorptive behavior of admixture and fluidity and setting of fresh cement paste. Cem. Concr. Res. 1992, 22, 1115–1129. [Google Scholar] [CrossRef]
- Gagné, R.; Boisvert, A.; Pigeon, M. Effect of superplasticizer dosage on mechanical properties, permeability, and freeze-thaw durability of high-strength concretes with and without silica fume. ACI Mater. J. 1996, 93, 111–120. [Google Scholar] [CrossRef]
- Vovk, A.I. Blended antifreezing admixture with extreme freezing-point. In Proceedings of the 11th International Conference on Superplasticizers and Other Chemical Admixtures in Concrete, Ottawa, ON, Canada, 10–13 July 2015; Gupta, P., Holland, T., Malhotra, V., Eds.; American Concrete Institute: Farmington Hills, MI, USA, 2015; pp. 279–287, Issue SP 302. [Google Scholar]
- Ratajczak, M.; Babiak, M.; Kulczewski, P.; Kosno, J. Diamidoamine salt as the admixture for concrete increasing the resistance to the freeze-thaw cycle. In Proceedings of the 3rd World Multidisciplinary Civil Engineering, Architecture, Urban Planning Symposium: IOP Conference Series: Materials Science and Engineering, Prague, Czech Republic, 18–22 June 2018; Yilmaz, I., Coisson, E., Segalini, A., Marschalko, M., Rybak, J., Decky, M., Drusa, M., Dabija, A.-M., Eds.; Institute of Physics Publishing Ltd.: Bristol, UK, 2019; Volume 471. [Google Scholar] [CrossRef] [Green Version]
- Barna, L.A.; Seman, P.M.; Korhonen, C.J. Cold Weather Admixture Systems Demonstration at Fort Wainwright, Alaska; US Army Engineering Research and Development Centre, Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 2010. [Google Scholar]
- Hazaree, C.; Ceylan, H.; Wang, K. Influences of mixture composition on properties and freeze-thaw resistance of RCC. Constr. Build. Mater. 2011, 25, 313–319. [Google Scholar] [CrossRef]
- Shang, H.S.; Yi, T.H. Freeze-Thaw durability of air-entrained concrete. Sci. World J. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meddah, M.S.; Lmbachiya, M.C.; Dhir, R.K. Potential use of binary and composite limestone cements in concrete production. Constr. Build. Mater. 2014, 58, 193–205. [Google Scholar] [CrossRef]
- Grapp, A.A.; Grapp, V.B.; Kaplan, A.S. The structure and cold resistance of concretes containing antifreeze admixtures. In Proceedings of the 2nd International Symposium on Winter Concreting, Moscow, Russia, 14–16 October 1975; Stroiizdat: Moscow, Russia, 1975; pp. 60–68. [Google Scholar]
- Marchand, J.; Pigeon, M.; Bager, D.; Talbot, C. Influence of chloride solution concentration on deicer salt scaling deterioration of concrete. ACI Mater. J. 1999, 96, 429–435. [Google Scholar] [CrossRef]
- Valenza, J.J.; Scherer, G.W. A review of salt scaling: II. Mechanisms. Cem. Concr. Res. 2007, 37, 1022–1034. [Google Scholar] [CrossRef]
- Golewski, G.L. Effect of curing time on the fracture toughness of fly ash concrete composites. Compos. Struct. 2018, 185, 105–112. [Google Scholar] [CrossRef]
- Sivasundaram, V.; Malhotra, V.M. Properties of concrete incorporating low quantity of cement and high volumes of ground granulated slag. ACI Mater. J. 1992, 89, 554–563. [Google Scholar] [CrossRef]
- Hale, W.M.; Bush, T.D.; Russell, B.W.; Freyne, S.F. Effect of curing temperature on hardened concrete properties: Mixtures of ground granulated blast furnace slag, fly ash, or a combination of both. Transp. Res. Rec. 2005, 1914, 97–104. [Google Scholar] [CrossRef]
- Jiang, S.; Guan, X.; Dong, S.; Zhu, W. Application of C50 negative temperature concrete in bridge engineering. In Proceedings of the ICTE 2013–Proceedings of the 4th International Conference on Transportation Engineering, Chengdu, China, 19–20 October 2013; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 3021–3026. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Daugėla, A. Investigation into the properties of concrete modified with biomass combustion fly ash. Constr. Build. Mater. 2018, 174, 369–375. [Google Scholar] [CrossRef]
- Dong, S.; Feng, D.; Jiang, S.; Zhu, W. Effect of a new type antifreeze agent on the mechanical behavior of negative temperature concrete. In Proceedings of the ICTE 2013–Proceedings of the 4th International Conference on Transportation Engineering, Chengdu, China, 19–20 October 2013; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 3027–3032. [Google Scholar] [CrossRef]
- Shi, X.; Fay, L.; Peterson, M.M.; Berry, M.; Mooney, M. A FESEM/EDX investigation into how continuous deicer exposure affects the chemistry of Portland cement concrete. Constr. Build. Mater. 2011, 25, 957–966. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, D.; Yuan, L.; Fei, Q. Durability of concrete under sulfate attack exposed to freeze-thaw cycles. Cold Reg. Sci. Technol. 2015, 112, 112–117. [Google Scholar] [CrossRef]
- Niu, D.; Jiang, L.; Fei, Q. Deterioration mechanism of sulfate attack on concrete under freeze-thaw cycles. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 1172–1176. [Google Scholar] [CrossRef]
- Mu, R.; Miao, C.; Liu, J.; Sun, W. Effect of NaCl and Na2SO4 solution on the frost resistance of concrete and its mechanism. Kuei Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2001, 29, 523–529. [Google Scholar]
- Yang, Q.; Zhu, P.; Wu, X.; Huang, S. Properties of concrete with a new type of saponin air-entraining agent. Cem. Concr. Res. 2000, 30, 1313–1317. [Google Scholar] [CrossRef]
- Munteanu, C.; Georgescu, M. The influence of fly ash addition (of CET Govora origin) on some properties of concretes. Univ. Politeh. Buchar. Sci. Bull. Ser. B Chem. Mater. Sci. 2013, 75, 43–52. [Google Scholar]
- Zhang, P.; Li, Q.F. Freezing-thawing durability of fly ash concrete composites containing silica fume and polypropylene fiber. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2014, 228, 241–246. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Andiç-Çakir, Ö.; Ramyar, K. Freeze-Thaw resistance and transport properties of high-volume fly ash roller compacted concrete designed by maximum density method. Cem. Concr. Compos. 2013, 37, 259–266. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Surface scaling of CO2-cured concrete exposed to freeze-thaw cycles. J. CO2 Util. 2018, 27, 137–144. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, X.; Shao, Y. Carbonation curing of precast fly ash concrete. J. Mater. Civ. Eng. 2016, 28. [Google Scholar] [CrossRef]
- Farnam, Y.; Villani, C.; Washington, T.; Spence, M.; Jain, J.; Jason Weiss, W. Performance of carbonated calcium silicate based cement pastes and mortars exposed to NaCl and MgCl2 deicing salt. Constr. Build. Mater. 2016, 111, 63–71. [Google Scholar] [CrossRef]
- Sadowski, Ł.; Stefaniuk, D.; Różańska, M.; Usydus, I.; Szymanowski, J. The effect of waste mineral powders on the structure of air voids in low-strength air-entrained concrete floor screeds. Waste Biomass Valorization 2020, 11, 2211–2225. [Google Scholar] [CrossRef] [Green Version]
- Yamato, T.; Emoto, Y.; Soeda, M. Strength and freezing-and-thawing resistance of concrete incorporating condensed silica fume. Publ. SP Am. Concr. Inst. 1986, 91, 1095–1117. [Google Scholar]
- Sabir, B.B. Mechanical properties and frost resistance of silica fume concrete. Cem. Concr. Compos. 1997, 19, 285–294. [Google Scholar] [CrossRef]
- Feldman, R.F. Influence of condensed silica fume and sand/cement ratio on pore structure and frost resistance of portland cement mortars. Publ. SP Am. Concr. Inst. 1986, 91, 973–990. [Google Scholar]
- Karakurt, C.; Bayazit, Y. Freeze-Thaw resistance of normal and high strength concretes produced with fly ash and silica fume. Adv. Mater. Sci. Eng. 2015, 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Siddique, R. Utilization of silica fume in concrete: Review of durability properties. Resour. Conserv. Recycl. 2011, 57, 30–35. [Google Scholar] [CrossRef]
- Fediuk, R.; Timokhin, R.; Mochalov, A.; Otsokov, K.; Lashina, I. Performance properties of high-density impermeable cementitious paste. J. Mater. Civ. Eng. 2019, 31, 04019013. [Google Scholar] [CrossRef]
- Chung, C.W.; Shon, C.S.; Kim, Y.S. Chloride ion diffusivity of fly ash and silica fume concretes exposed to freeze-thaw cycles. Constr. Build. Mater. 2010, 24, 1739–1745. [Google Scholar] [CrossRef]
- Cohen, M.D.; Yixia, Z.; Dolch, W.L. Non-Air-Entrained high-strength concrete—Is it frost resistant? ACI Mater. J. 1992, 89, 406–415. [Google Scholar] [CrossRef]
- Sorensen, E.V. Freezing and thawing resistance of condensed silica fume (Microsilica) concrete exposed to deicing chemicals. Publ. SP Am. Concr. Inst. 1983, 79, 709–718. [Google Scholar]
- Svintsov, A.P.; Shchesnyak, E.L.; Galishnikova, V.V.; Fediuk, R.S.; Stashevskaya, N.A. Effect of nano-modified additives on properties of concrete mixtures during winter season. Constr. Build. Mater. 2020, 237, 1–13. [Google Scholar] [CrossRef]
- Jang, Y.I.; Park, W.S.; Kim, S.W.; Yun, S.H.; Yun, H.D.; Khil, B.S.; Kim, D.G. Influence of cold weather on compressive strength in high performance with silica fume. Key Eng. Mater. 2015, 627, 445–448. [Google Scholar] [CrossRef]
- Cwirzen, A.; Penttala, V. Aggregate-Cement paste transition zone properties affecting the salt-frost damage of high-performance concretes. Cem. Concr. Res. 2005, 35, 671–679. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Wallevik, O.H.; Fjällberg, L. Microstructure and microchemistry of the paste-aggregate interfacial transition zone of high-performance concrete. Adv. Cem. Res. 1998, 10, 30–40. [Google Scholar] [CrossRef]
- Alkaysi, M.; El-Tawil, S.; Liu, Z.; Hansen, W. Effects of silica powder and cement type on durability of ultra high performance concrete (UHPC). Cem. Concr. Compos. 2016, 66, 47–56. [Google Scholar] [CrossRef]
- Hooton, R.D. Influence of silica fume replacement of cement on physical properties and resistance to sulfate attack, freezing and thawing, and alkali-silica reactivity. ACI Mater. J. 1993, 90, 143–151. [Google Scholar] [CrossRef]
- Tosun, K.; Baradan, B. Effect of ettringite morphology on DEF-related expansion. Cem. Concr. Compos. 2010, 32, 271–280. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Inan Sezer, G.; Ramyar, K. Comparison of fly ash, silica fume and metakaolin from mechanical properties and durability performance of mortar mixtures view point. Constr. Build. Mater. 2014, 70, 17–25. [Google Scholar] [CrossRef]
- Liu, Z.; El-Tawil, S.; Hansen, W.; Wang, F. Effect of slag cement on the properties of ultra-high performance concrete. Constr. Build. Mater. 2018, 190, 830–837. [Google Scholar] [CrossRef]
- Tavasoli, S.; Nili, M.; Serpoosh, B. Effect of GGBS on the frost resistance of self-consolidating concrete. Constr. Build. Mater. 2018, 165, 717–722. [Google Scholar] [CrossRef]
- Shang, H.; Song, Y.; Ou, J. Behavior of air-entrained concrete after freeze-thaw cycles. Acta Mech. Solida Sin. 2009, 22, 261–266. [Google Scholar] [CrossRef]
- Raczkiewicz, W.; Grzmil, W.; Zapała-Sławeta, J. Impact of the air-entrained concrete with the blast-furnace slag cement on the intensity of reinforcement corrosion process. In Proceedings of the MATEC Web of Conferences—MATBUD’2018—8th Scientific-Technical Conference on Material Problems in Civil Engineering, Cracow, Poland, 25–27 June 2018; EDP Sciences: Les Ulis, Paris, France, 2018; Volume 163, pp. 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Hansen, W. Freezing characteristics of air-entrained concrete in the presence of deicing salt. Cem. Concr. Res. 2015, 74, 10–18. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Enhancing microstructure and durability of concrete from ground granulated blast furnace slag and metakaolin as cement replacement materials. J. Mater. Res. Technol. 2013, 2, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Evju, C.; Hansen, S. The kinetics of ettringite formation and dilatation in a blended cement with β-hemihydrate and anhydrite as calcium sulfate. Cem. Concr. Res. 2005, 35, 2310–2321. [Google Scholar] [CrossRef]
- Turk, K.; Kina, C. Freeze-Thaw resistance and sorptivity of self-compacting mortar with ternary blends. Comput. Concr. 2018, 21, 149–156. [Google Scholar] [CrossRef]
- Benli, A.; Turk, K.; Kina, C. Influence of silica fume and class F fly ash on mechanical and rheological properties and freeze-thaw durability of self-compacting mortars. J. Cold Reg. Eng. 2018, 32. [Google Scholar] [CrossRef]
- Yazici, H. The effect of silica fume and high-volume Class C fly ash on mechanical properties, chloride penetration and freeze-thaw resistance of self-compacting concrete. Constr. Build. Mater. 2008, 22, 456–462. [Google Scholar] [CrossRef]
- Gebler, S.; Klieger, P. Effect of fly ash on the air-void stability of concrete. In Proceedings of the International Conference on Using Fly Ash, Silica Fume, Slag and Other Mineral By-products in Concrete, Montebello, QC, Canada, July–August 1983; American Concrete Institute: Detroit, MI, USA, 1983; Volume 79, pp. 103–142. [Google Scholar]
- Shon, C.S.; Abdigaliyev, A.; Bagitova, S.; Chung, C.W.; Kim, D. Determination of air-void system and modified frost resistance number for freeze-thaw resistance evaluation of ternary blended concrete made of ordinary Portland cement/silica fume/class F fly ash. Cold Reg. Sci. Technol. 2018, 155, 127–136. [Google Scholar] [CrossRef]
- Sobolev, K.G.; Soboleva, S.V. High-Performance concrete mixture proportioning. Spec. Publ. 1998, 179, 421–438. [Google Scholar]
- Sahmaran, M.; Yildirim, G.; Erdem, T.K. Self-Healing capability of cementitious composites incorporating different supplementary cementitious materials. Cem. Concr. Compos. 2013, 35, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.K.; Rehman, S.; Al-Amoudi, O.S.B. Literature review on cement kiln dust usage in soil and waste stabilization and experimental investigation. J. Res. Rev. Appl. Sci. 2011, 7, 77–87. [Google Scholar]
- Siddique, R. Utilization of cement kiln dust (CKD) in cement mortar and concrete—An overview. Resour. Conserv. Recycl. 2006, 48, 315–318. [Google Scholar] [CrossRef]
- Najim, K.B.; Mahmod, Z.S.; Atea, A.K.M. Experimental investigation on using Cement Kiln Dust (CKD) as a cement replacement material in producing modified cement mortar. Constr. Build. Mater. 2014, 55, 5–12. [Google Scholar] [CrossRef]
- Abbaszadeh, R.; Modarres, A. Freeze-Thaw durability of non-air-entrained roller compacted concrete designed for pavement containing cement kiln dust. Cold Reg. Sci. Technol. 2017, 141, 16–27. [Google Scholar] [CrossRef]
- Solomahin, A.; Svatovskaya, L.; Kamenev, Y. The acceleration of hardening of non-autoclaved foam concrete with the mechano-activated binder when constructing in the arctic and cold regions. In Transportation Soil Engineering in Cold Regions, Lecture Notes in Civil Engineering 50; Petriaev, A., Konon, A., Eds.; Springer Nature: Singapore, 2020; Volume 2, pp. 487–494. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag—A guide for civil engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S. Alkali Avtivated Materials: State of Art Report, Rilem Tc 224-AAM; Springer: Dordrecht, The Netherlands, 2013; Volume 13, ISBN 9789400776715. [Google Scholar] [CrossRef]
- Gifford, P.M.; Gillott, J.E. Freeze-Thaw durability of activated blast furnace slag cement concrete. ACI Mater. J. 1996, 93, 242–245. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Gu, Y.M.; Fang, Y.H.; You, D.; Gong, Y.F.; Zhu, C.H. Properties and microstructure of alkali-activated slag cement cured at below—And about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, L.; Yonggen, W. Freeze-Thaw cycle test and damage mechanics models of alkali-activated slag concrete. Constr. Build. Mater. 2011, 25, 3144–3148. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, L.; Fu, Y. Durability of green high performance alkali-activated slag pavement concrete. Appl. Mech. Mater. 2011, 99, 158–161. [Google Scholar] [CrossRef]
- Barnett, S.J.; Soutsos, M.N.; Millard, S.G.; Bungey, J.H. Strength development of mortars containing ground granulated blast-furnace slag: Effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 2006, 36, 434–440. [Google Scholar] [CrossRef]
- Douglas, E.; Bilodeau, A.; Malhotra, V.M. Properties and durability of alkali-activated slag concrete. ACI Mater. J. 1992, 89, 509–516. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars—Part, I. Strength, hydration and microstructure. Cem. Concr. Res. 2002, 32, 865–879. [Google Scholar] [CrossRef]
- Mohamed, O.A. A review of durability and strength characteristics of alkali-activated slag concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Yang, C.; Zhang, J.; Pan, Q.; Yu, L.; Bai, Y. First structural use of site-cast, alkali-activated slag concrete in China. Proc. Inst. Civ. Eng. Struct. Build. 2018, 171, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Brykov, A.S.; Danilov, V.V.; Korneev, V.I.; Larichkov, A.V. Effect of hydrated sodium silicates on cement paste hardening. Russ. J. Appl. Chem. 2002, 75, 1577–1579. [Google Scholar] [CrossRef]
- Acevedo-Martinez, E.; Gomez-Zamorano, L.Y.; Escalante-Garcia, J.I. Portland cement-blast furnace slag mortars activated using waterglass: Part 1: Effect of slag replacement and alkali concentration. Constr. Build. Mater. 2012, 37, 462–469. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, S.; Wang, Q.; Shah, S.P. Effects of nano-kaolinite clay on the freeze-thaw resistance of concrete. Cem. Concr. Compos. 2015, 62, 1–12. [Google Scholar] [CrossRef]
- Al-Shether, B.S.; Al-Attar, T.S.; Hassan, Z.A. Concrete, effect of curing system on metakaolin based geopolymer. J. Babylon Univ. 2016, 24, 569–576. [Google Scholar]
- Tosheva, L.; Valtchev, V.P. Nanozeolites: Synthesis, crystallization mechanism, and applications. Chem. Mater. 2005, 17, 1494–2513. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of alkali-activated mortars containing high volume waste ceramic powder and fly ash replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Fu, Y. Freeze-Thaw resistance of alkali-slag concrete based on response surface methodology. Constr. Build. Mater. 2013, 49, 70–76. [Google Scholar] [CrossRef]
- Sun, P.; Wu, H.C. Chemical and freeze-thaw resistance of fly ash-based inorganic mortars. Fuel 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Ionescu, I.; Ispas, T. Properties and durability of some concretes containing binders based on slag and activated ashes. Publ. SP Am. Concr. Inst. 1986, 91, 1475–1494. [Google Scholar]
- Puertas, F.; Amat, T.; Fernández-Jiménez, A.; Vázquez, T. Mechanical and durable behaviour of alkaline cement mortars reinforced with polypropylene fibres. Cem. Concr. Res. 2003, 33, 2031–2036. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Tognonvi, M.T.; Tagnit-Hamou, A.; Puertas, F. Durability of alkali-activated slag concretes prepared using waste glass as alternative activator. ACI Mater. J. 2015, 112, 791–800. [Google Scholar] [CrossRef]
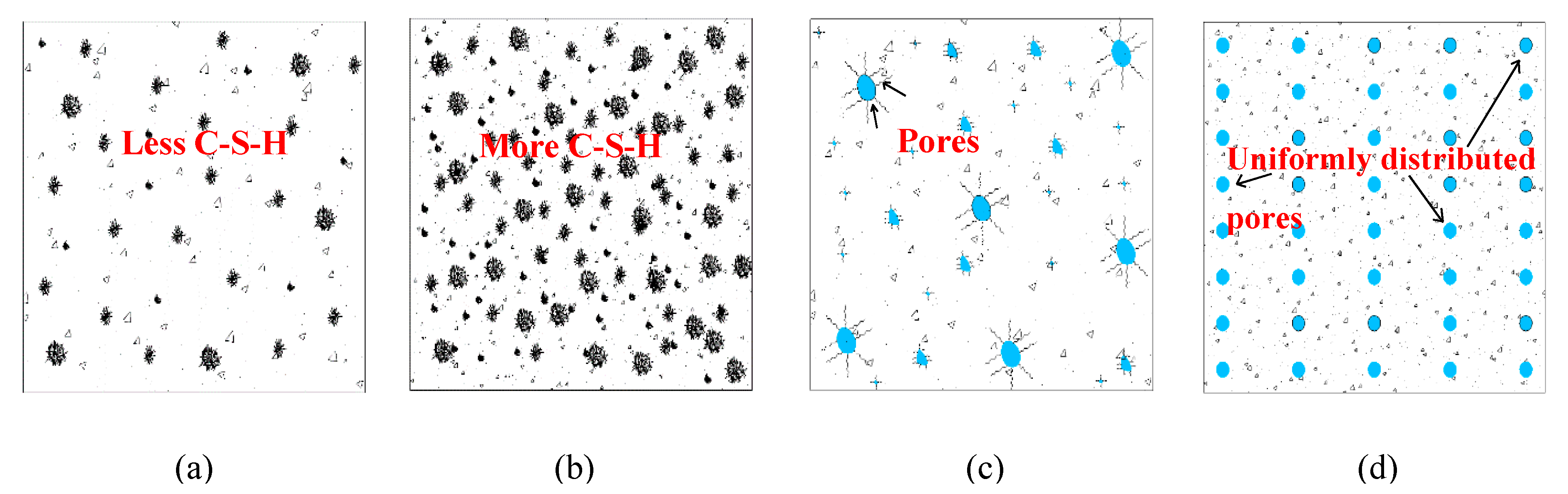



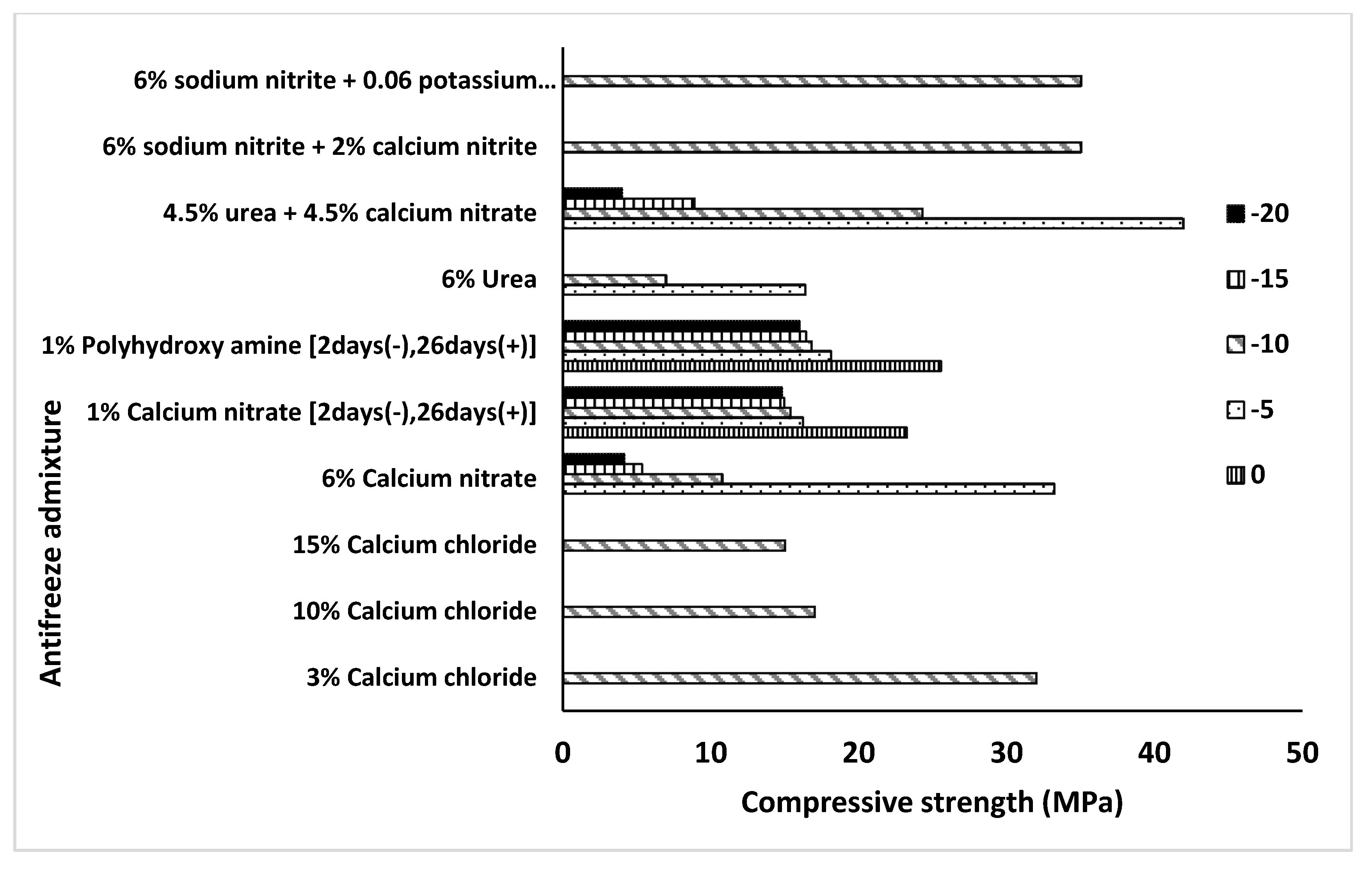
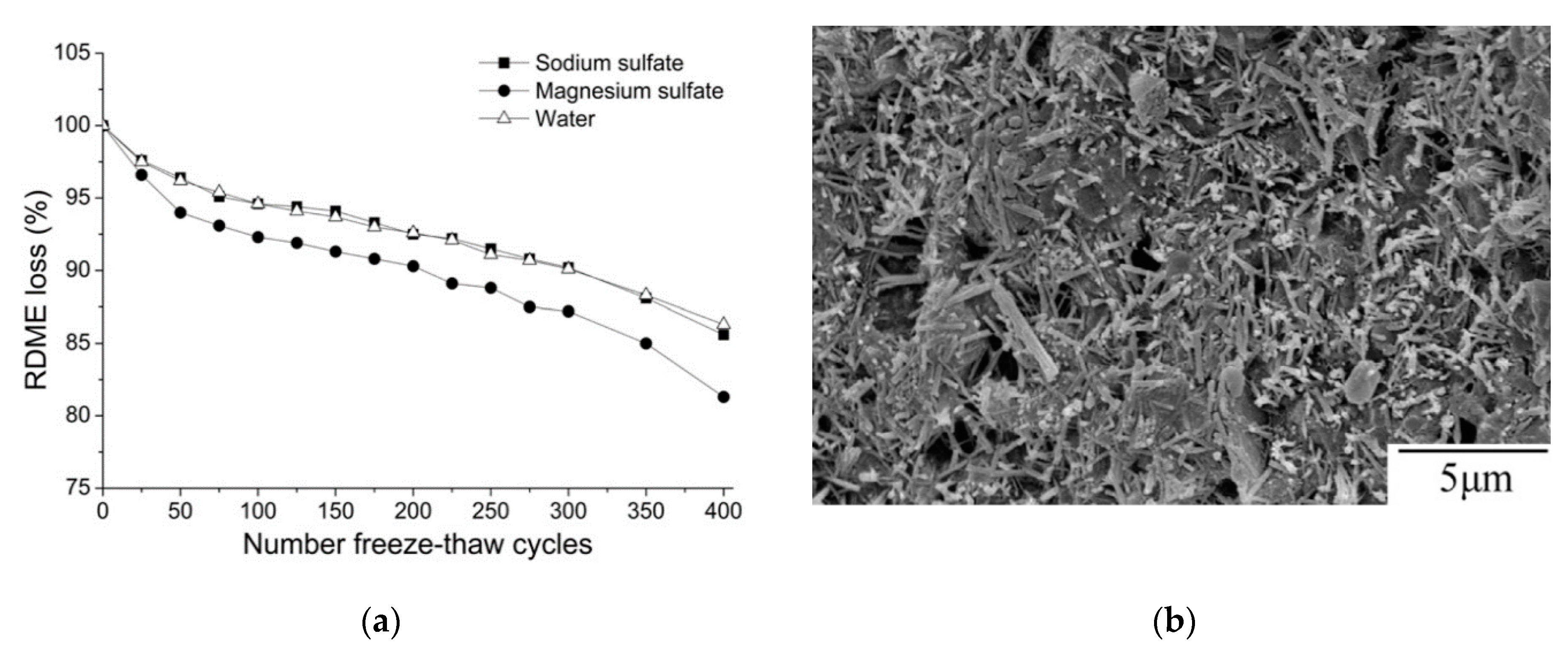
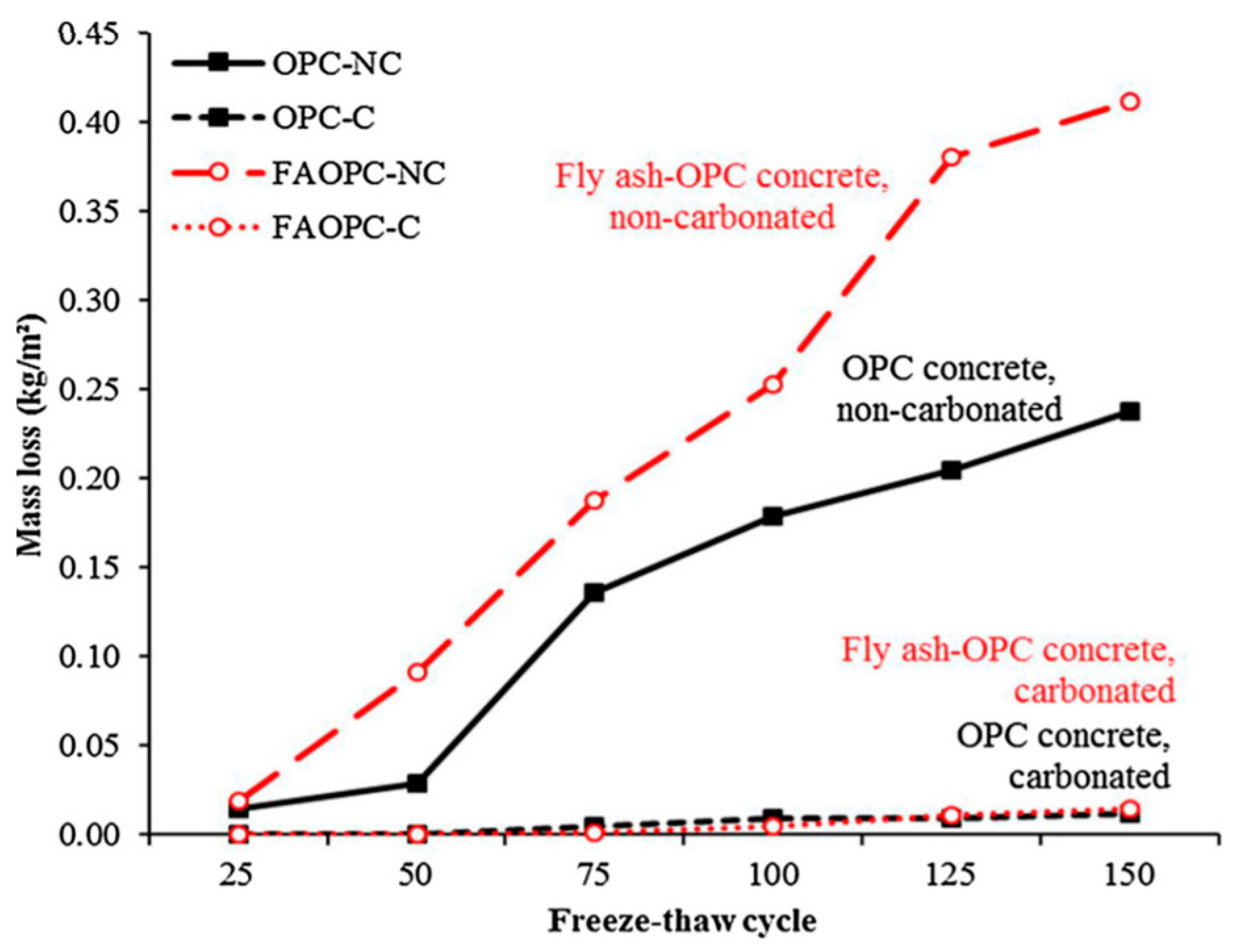
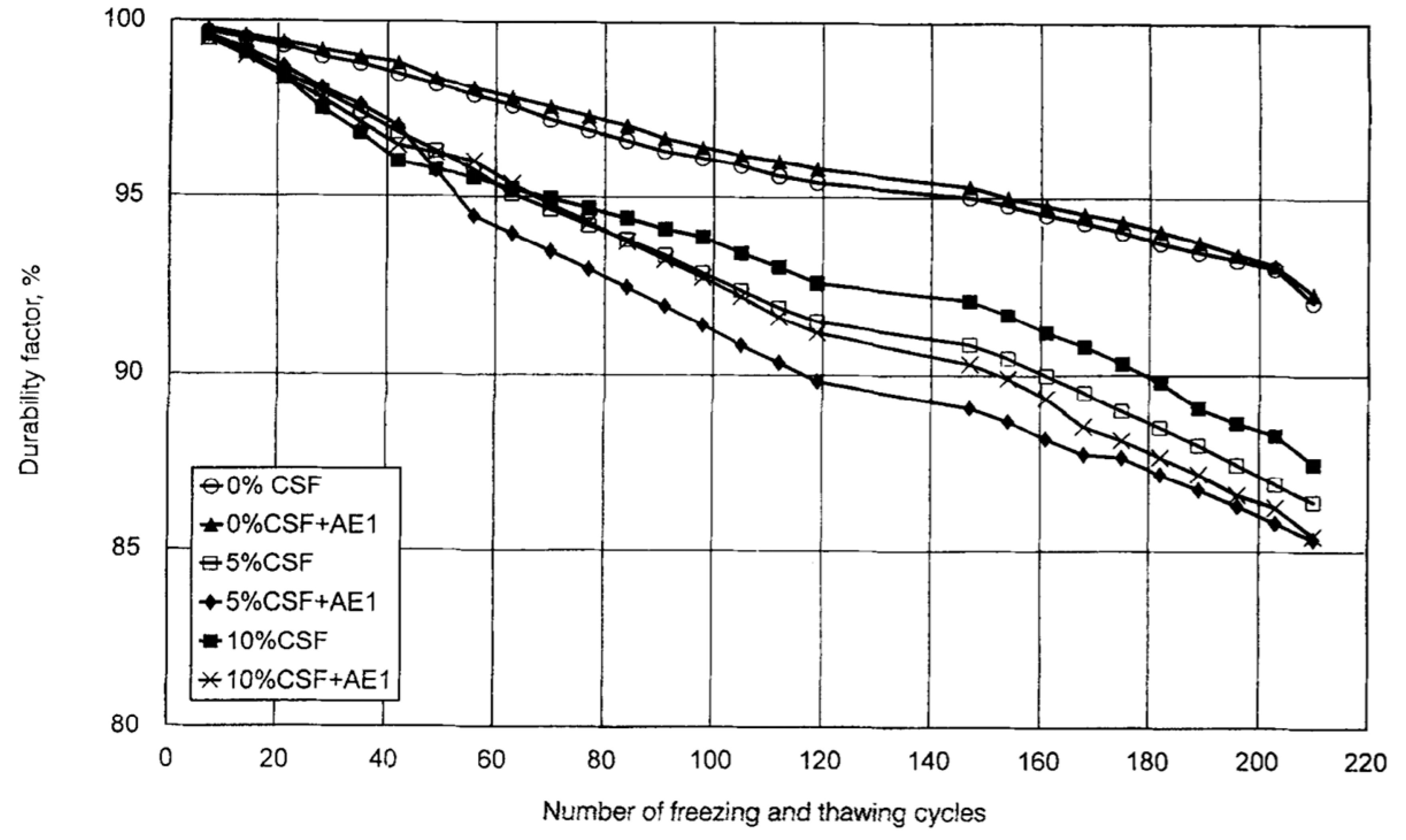
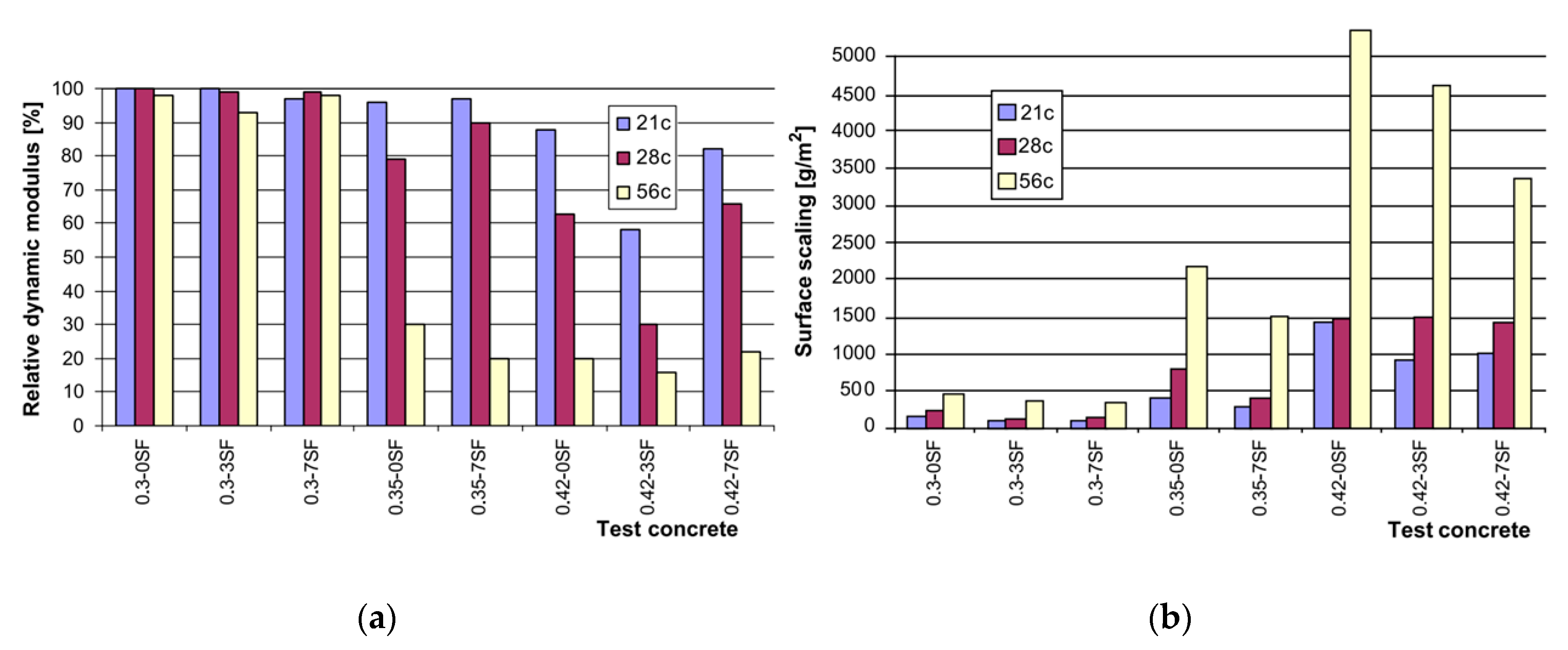

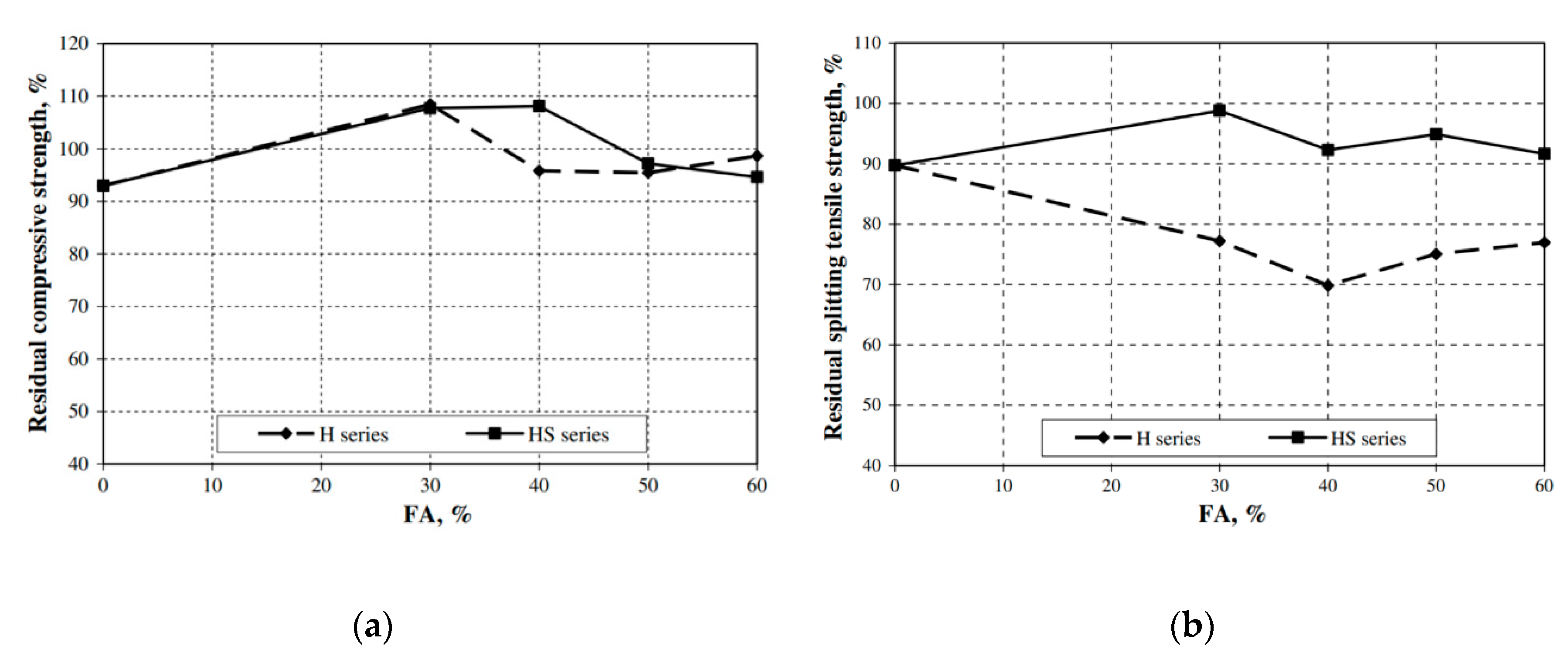
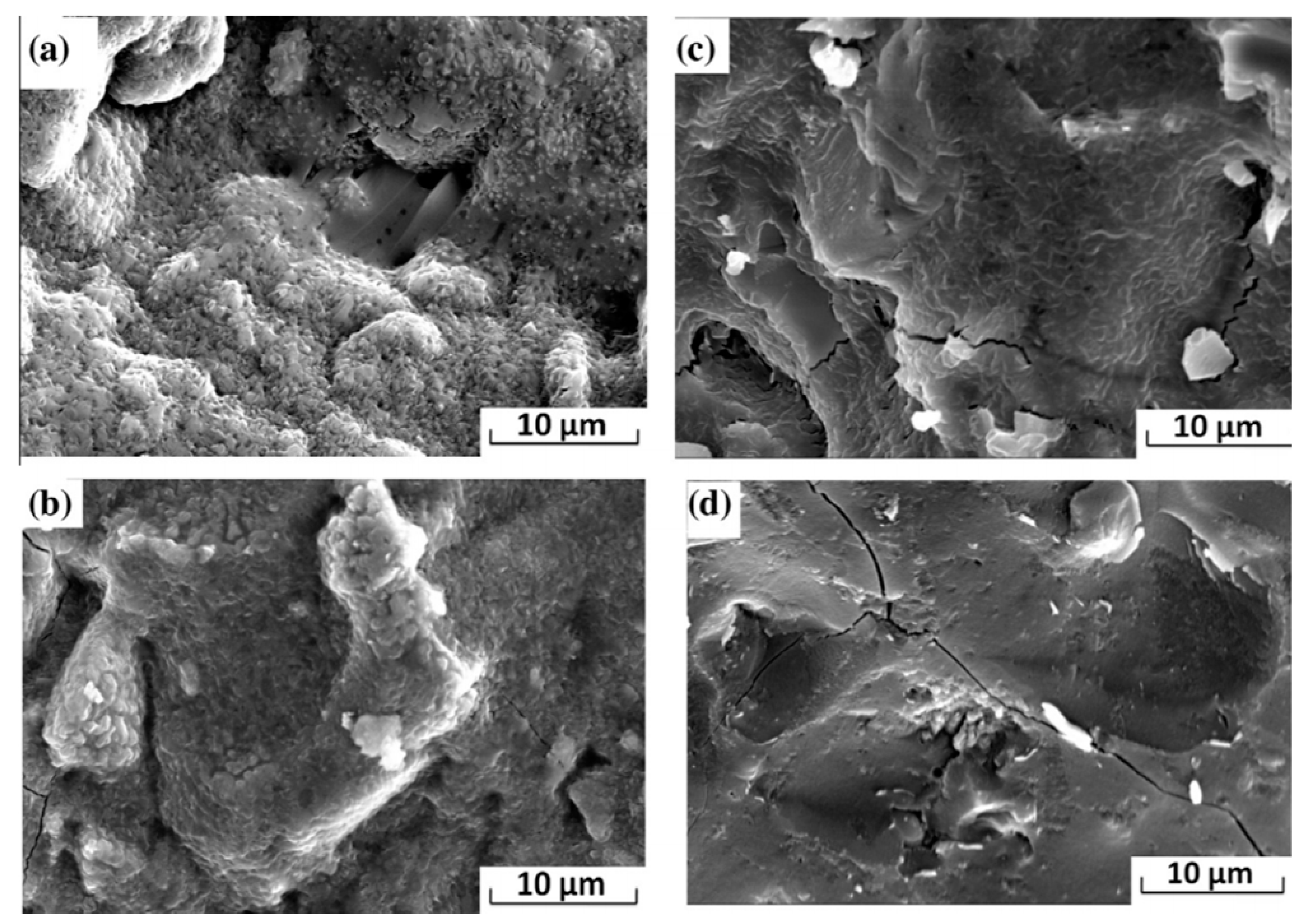
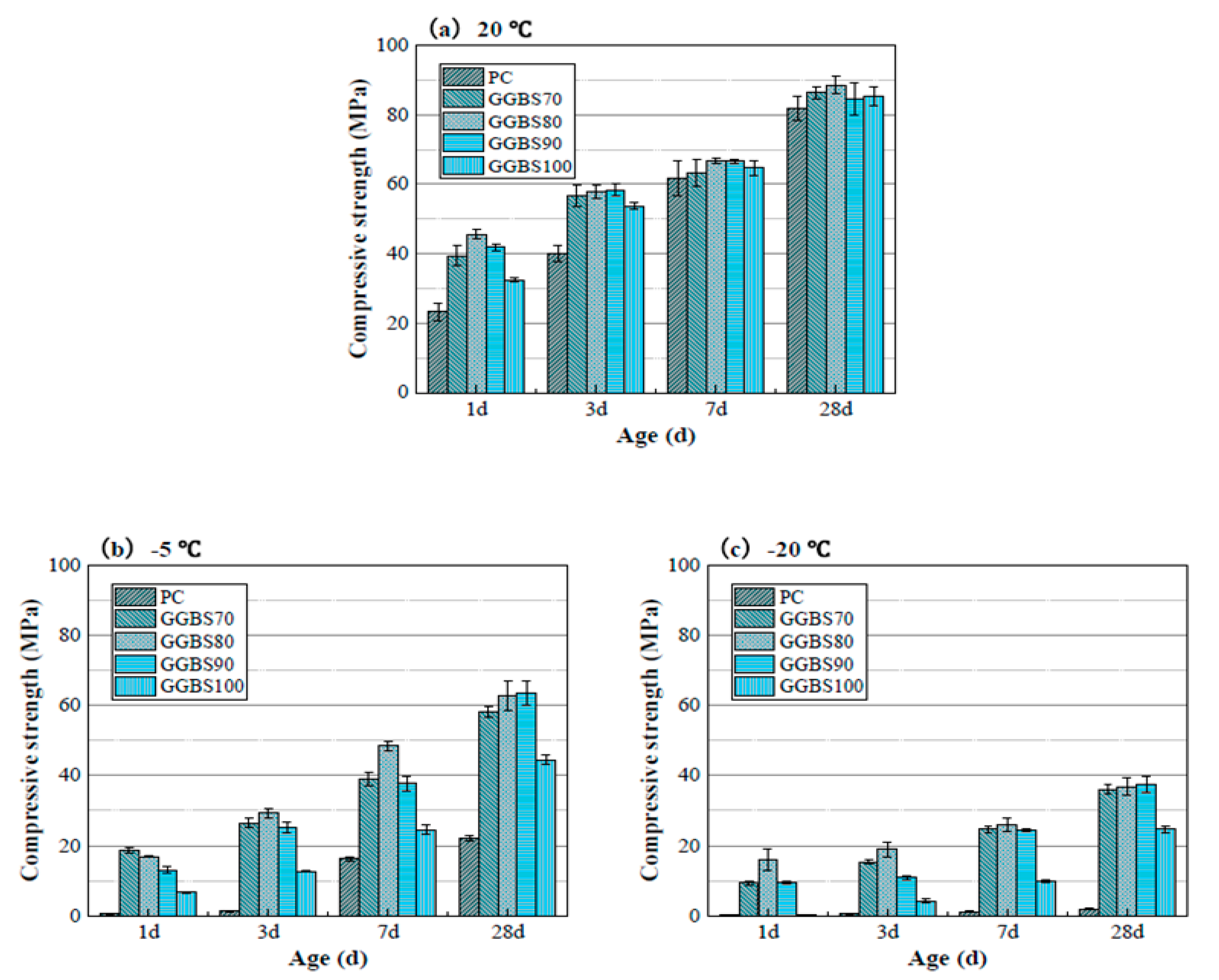
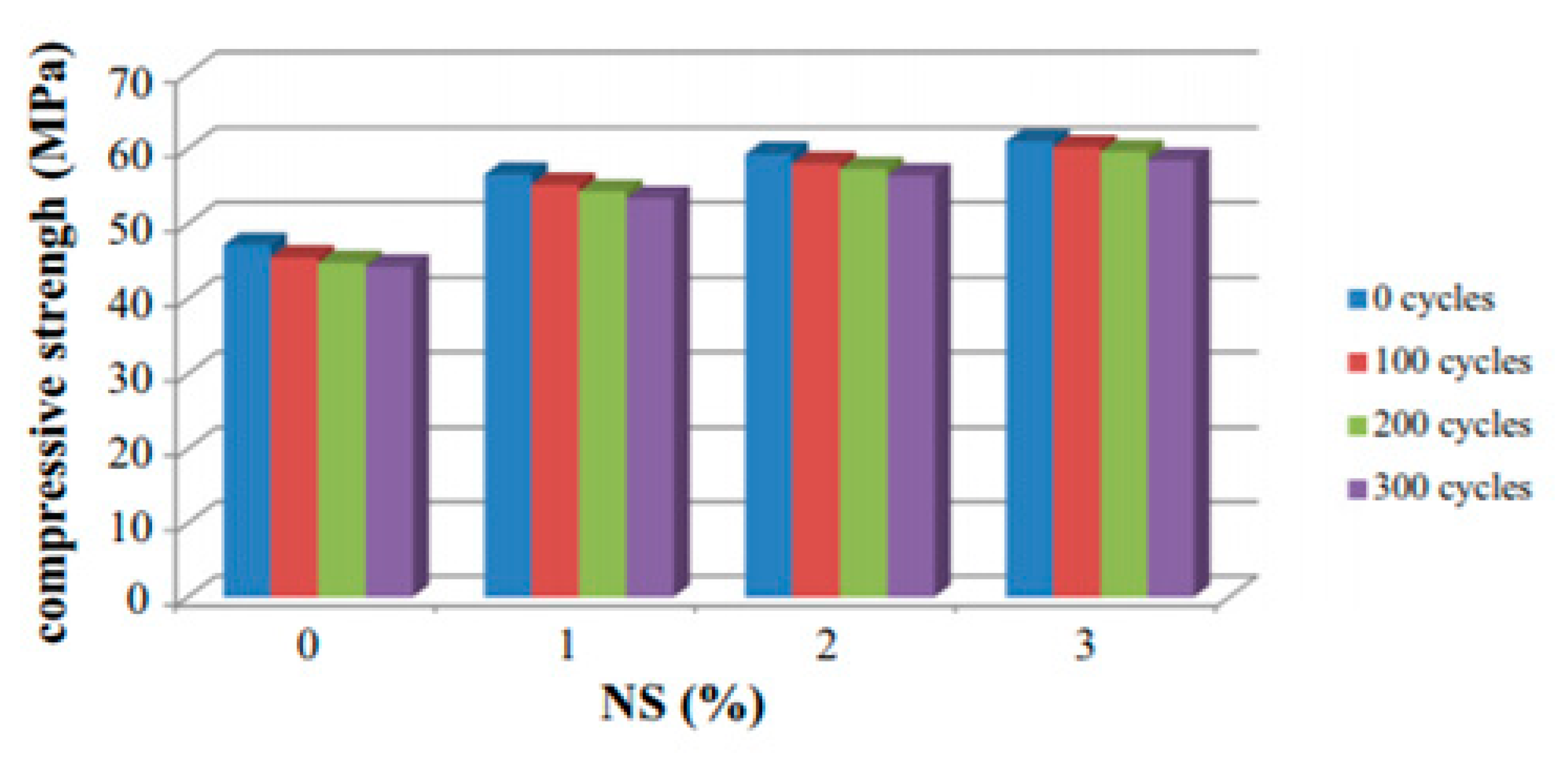

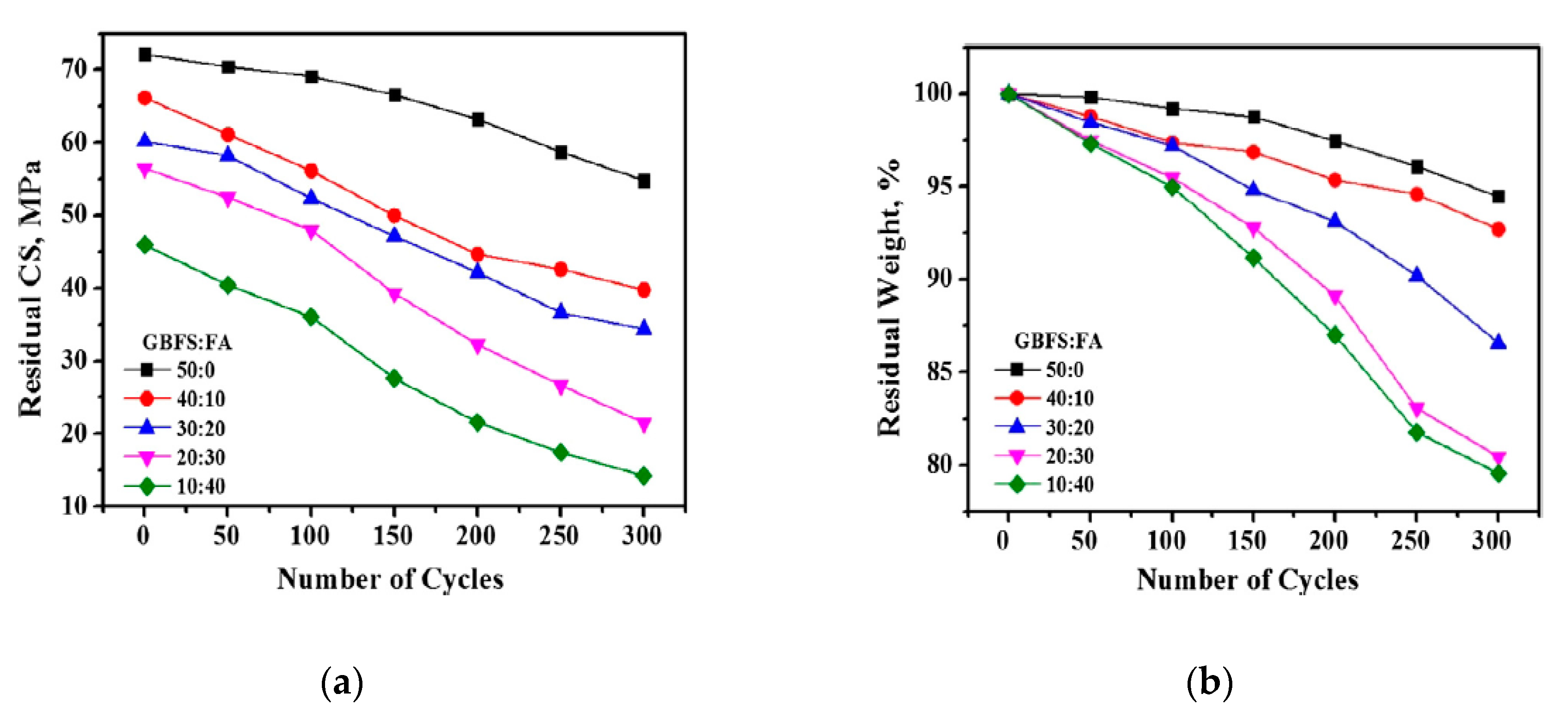



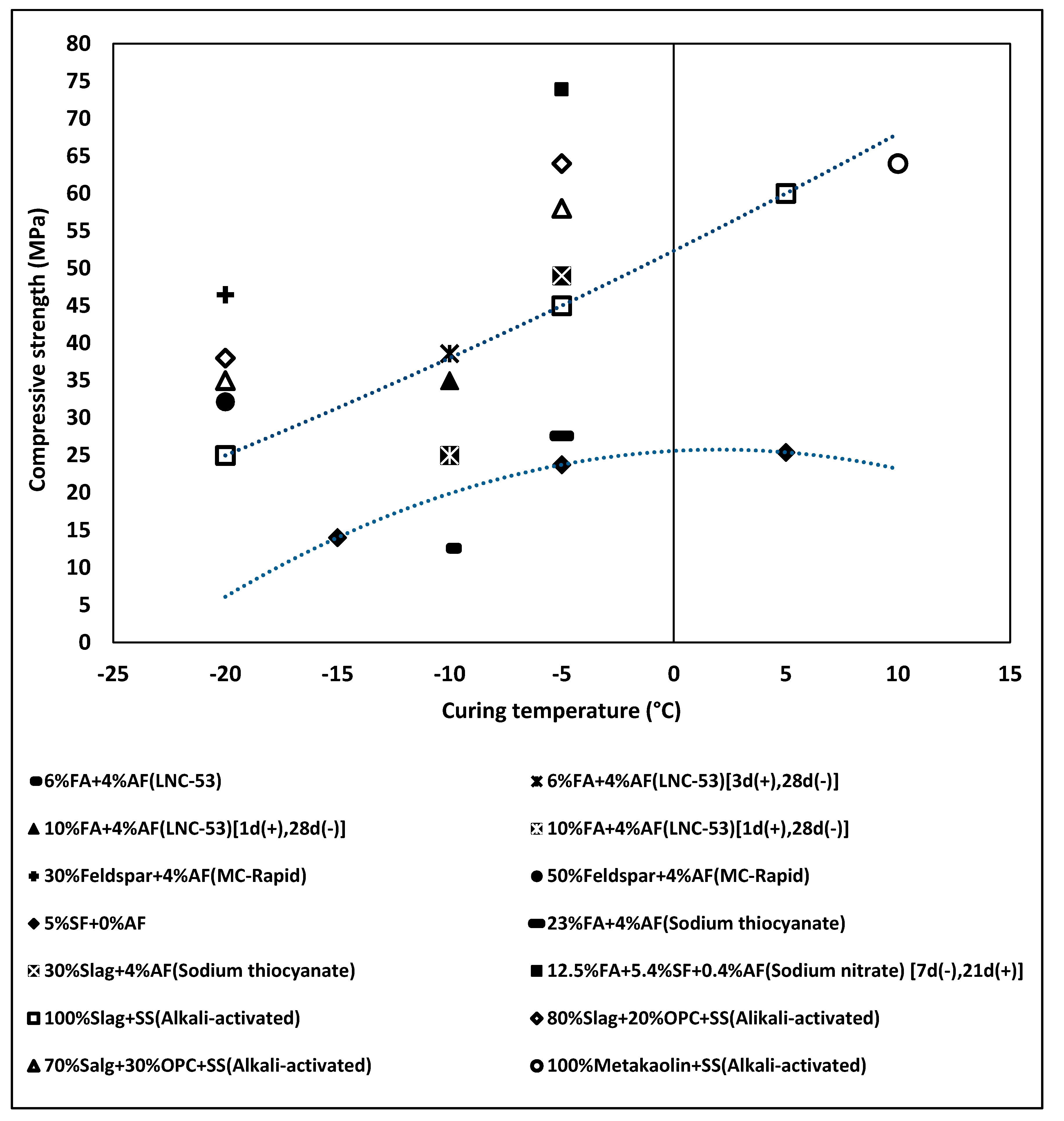
| Admixture | Years | Reference |
|---|---|---|
| Calcium Chloride | 1951, 1952, 1958, 1970, 1976, 1990, 1995, 1998, 2007, 2008, 2013 | [1,7,20,24,27,28,29,30,31,32,33] |
| Sodium Chloride | 1976, 1990, 1998 | [1,7,28] |
| Calcium Nitrate | 1991, 1999, 2003, 2013, 2015, 2016, 2018 | [2,20,25,34,35,36,37,38] |
| Calcium Nitrite | 1989, 1991, 1995, 1996, 1998, 2007, 2013 | [7,16,20,22,27,33,39,40] |
| Magnesium chloride | 2008 | [32] |
| Sodium Nitrate | 1997 | [41] |
| Sodium Nitrite | 1991, 1996, 2012 | [16,39] |
| Sodium Sulfate | 1985, 1990, 1997 | [1,41,42] |
| Potassium carbonate | 1983, 1991, 2019 | [16,43,44] |
| Urea | 2014, 2015, 2016 | [2,14,36] |
| Calcium thiocyanate | 1995, 2007 | [27,45] |
| Polyhydroxy amine | 2011 | [46] |
| Sodium thiocyanate | 1988, 1998, 1999, 2003 | [7,12,34,47] |
| Polyglycolester-based | 1991, 1999 | [34,35] |
| Hydroxyethylamine | 2011, 2014 | [46,48] |
| Polyethylhydrosiloxane | 2007 | [26] |
| Admixture | Section 1 | Section 2 | Section 3 | Section 4 | Section 5 |
|---|---|---|---|---|---|
| Glenium®3000 (fl oz/cwt) | 8 | 6 | 7 | - | 4.9 |
| Pozzutec®20+ (fl oz/cwt) | 68 | 34 | 45 | 34.1 | 22 |
| Rheocrete®CNI (gal/yd3) | 4 | 2.3 | 3 | 2.3 | 1.5 |
| Rheomac®VMA (fl oz/cwt) | - | - | - | 4 | - |
| Serial No. | Antifreeze Admixtures | % Dosage by Cement Weight |
|---|---|---|
| 1 | Conventional concrete | - |
| 2 | NaNO2 | 15 |
| 3 | Ca(NO3)2 + CO(NH2)2 | 15 |
| 4 | Ca(NO3)2/(NO2)2 + CaCl2 + CO(NH2)2 | 15 |
| 5 | NaNO2 + CaCl2 | 15 |
| 6 | K2CO3 | 15 |
| 7 | K2CO3 | 25 |
| Antifreeze Admixture | OPC | Composite | Comments | References |
|---|---|---|---|---|
| Calcium Chloride | × | - | Expansive oxychloride Susceptible to reinforcement corrosion | [1,7,24,28,29,30,31] |
| Sodium Chloride | × | - | ||
| Calcium Nitrate | ×××× | - | Accelerates hydration reaction Efficient up to −10 °C Needs additional standard curing | [2,16,20,22,39] |
| Calcium Nitrite | ×××× | - | ||
| Polyhydroxy amine | ××× | - | Post-curing boosts strength and microstructure | [46] |
| Polyglycolester-based | ××× | - | Depends on the dosage of admixture | [34,35] |
| Urea | ××××× | - | Efficient with calcium nitrate Breaks hydrogen bond and enhances workability Accelerates and nucleates at the same time | [36] |
| Calcium thiocyanate | ×× | - | Accelerator | [27,45] |
| Sodium thiocyanate | × | × (OPC + FA) ×× (OPC + Slag) | Risk of alkali–aggregate reaction | [7,12,34,47] |
| Sodium Nitrite | ×××× | ×××× (OPC + FA + SF) | Prolonged pre-curing will develop cracks | [40] |
| Potassium carbonate | ××× | - | Detrimental effect at standard temperature | [44] |
| Sodium Sulfate | ×× | - | Need pre-curing | [43] |
| Hydroxyethylamine | ×× | - | Does not withstand corrosive environment | [46,48] |
| MC Rapid 25/15 | - | ×××× (OPC + Feldspar) | Dissolute silica compound and maintain liquid phases | [9,17] |
| Air-Entraining (AE) Admixture | OPC | Composite | Comments | References |
|---|---|---|---|---|
| Diamidoamine salt | ××× | - | Closed spaced air-void Promote strength development at later age | [62] |
| Saponin based | - | ×××× (OPC + FA) | Stable in acidic and alkaline environment | [76,77,78,79] |
| Resin based | - | × (OPC + SF) | High porosity | [88,89] |
| Polyethylhydrosiloxane (PEHSO) | - | ×××× (OPC + SF + Slag) ×××× (OPC + FA + SF) | High reactive with hydroxyl group | [26,117] |
| System | Activator | AE | Rating | Comments | References |
|---|---|---|---|---|---|
| Slag | SS | √ | ××× | Refined air-voids | [127] |
| Slag | SC | √ | × | Unstable microstructure | [126] |
| Slag | SS | - | ×××× | Low temperature less microcracks | [128] |
| Slag/OPC | SS | - | ××××× | Acceleration and geopolymerization | [6] |
| Slag/SF | SS | - | ××××× | SF complimented to fill pores | [54,130] |
| Metakaoline | SS | - | ××× | Slow early age strength development | [129] |
| WSP/Slag | SS | - | ××× | Increased C-A-S-H gel formation | [143] |
| WSP/Slag/FA | SS | - | × | FA limited the C-A-S-H gel | [143,144] |
| FA | SS | √ | ×××× | AE refined pore structure | [145,146] |
| FA/Slag | SH | - | ××× | Slag produce more alkali during thawing of F-T | [148] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothari, A.; Habermehl-Cwirzen, K.; Hedlund, H.; Cwirzen, A. A Review of the Mechanical Properties and Durability of Ecological Concretes in a Cold Climate in Comparison to Standard Ordinary Portland Cement-Based Concrete. Materials 2020, 13, 3467. https://doi.org/10.3390/ma13163467
Kothari A, Habermehl-Cwirzen K, Hedlund H, Cwirzen A. A Review of the Mechanical Properties and Durability of Ecological Concretes in a Cold Climate in Comparison to Standard Ordinary Portland Cement-Based Concrete. Materials. 2020; 13(16):3467. https://doi.org/10.3390/ma13163467
Chicago/Turabian StyleKothari, Ankit, Karin Habermehl-Cwirzen, Hans Hedlund, and Andrzej Cwirzen. 2020. "A Review of the Mechanical Properties and Durability of Ecological Concretes in a Cold Climate in Comparison to Standard Ordinary Portland Cement-Based Concrete" Materials 13, no. 16: 3467. https://doi.org/10.3390/ma13163467





