Role of Magnesium and the Effect of Surface Roughness on the Hydroxyapatite-Forming Ability of Zirconia Induced by Biomimetic Aqueous Solution Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outline of the Experimental Procedure
2.1.1. Fabrication of the Roughened 3Y-TZP Samples
2.1.2. Ca-P Solution Treatments
2.1.3. HAp-Forming Ability
2.1.4. Analysis
2.2. Evaluation of Materials Properties
2.2.1. Surface Roughness Measurement
2.2.2. Evaluation of Ca/P Atomic Ratio and Mg Release
2.2.3. Adhesive Strength Test
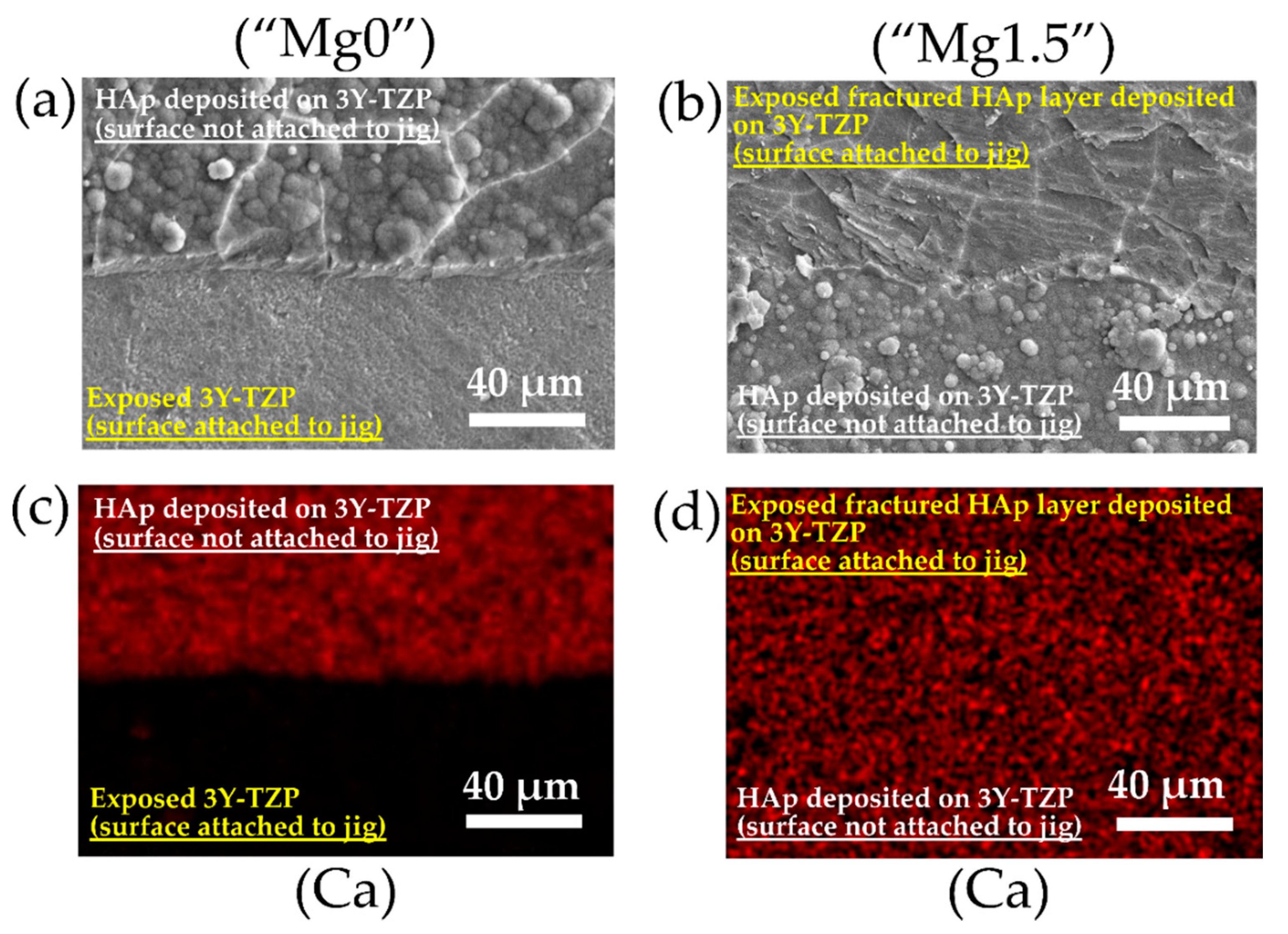
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Burger, W.; Richter, H.G.; Piconi, C.; Vatteroni, R.; Cittadini, A.; Boccalari, M. New Y-TZP powders for medical grade zirconia. J. Mater. Sci. Mater. Med. 1997, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hannink, R.H.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Sennerby, L.; Dasmah, A.; Larsson, B.; Iverhed, M. Bone tissue responses to surface-modified zirconia implants: A histomorphometric and removal torque study in the rabbit. Clin. Implant Dent. R. 2005, 7, 13–20. [Google Scholar] [CrossRef]
- Langhoff, J.D.; Voelter, K.; Scharnweber, D.; Schnabelrauch, M.; Schlottig, F.; Hefti, T.; Kalchofner, K.; Nuss, K.; von Rechenberg, B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: A study in sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Wanpeng, C.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar]
- Neo, M.; Nakamura, T.; Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Apatite formation on three kinds of bioactive materials at an early stage in vivo: A comparative study by transmission electron microscopy. J. Biomed. Mater. Res. 1993, 27, 999–1006. [Google Scholar] [CrossRef]
- Neo, M.; Kotani, S.; Fujita, Y.; Nakamura, T.; Yamamuro, T.; Bando, Y.; Ohtsuki, C.; Kokubo, T. Differences in ceramics-bone interface between surface-active ceramics and resorbable ceramics: A study by scanning and transmission electron microscopy. J. Biomed. Mater. Res. 1992, 26, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Chem. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H. Medical Applications of Hydroxyapatite.; Ishiyaku EuroAmerica: St. Louis, MO, USA, 1994; pp. 13–74. [Google Scholar]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process-an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tonsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim. 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Ducheyne, P.; Radin, S.; King, L. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. I. Dissolution. J. Biomed. Mater. Res. 1993, 27, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Takadama, H.; Kokubo, T. In vitro evaluation of bone bioactivity. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 165–182. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting In Vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barrere, F.; van Bliterswijk, C.A.; de Groot, K.; Layrolle, P. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N. Apatite formation induced by silica gel in a simulated body fluid. J. Am. Ceram. Soc. 1992, 75, 2094–2097. [Google Scholar] [CrossRef]
- Kim, H.Y.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Kaynak, G.; Gümüşderelioğlu, M. Bone-like hydroxyapatite precipitated from 10×SBF-like solution by microwave irradiation. Mater. Sci. Eng. C 2015, 49, 713–719. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Nawa, M.; Asano, T.; Tanaka, K.; Nakamura, T. Apatite-forming ability of a zirconia/alumina nano-composite induced by chemical treatment. J. Biomed. Mater. Res. 2002, 60, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Parduna, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Destri, G.L.; Marletta, G.; Rezwana, K. Mixed zirconia calcium phosphate coatings for dental implants: Tailoring coating stability and bioactivity potential. Mater. Sci. Eng. C 2015, 48, 337–346. [Google Scholar] [CrossRef]
- Ponnilavan, V.; Vasanthavel, S.; Khan, M.I.K.; Dhayalan, A.K.; Kannan, S. Structural and bio-mineralization features of alumina zirconia composite influenced by the combined Ca2+ and PO43− additions. Mater. Sci. Eng. C 2019, 98, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shi, T.; Jiao, C.; Liang, H.; Chen, R.; Tian, Z.; Zou, A.; Yang, Y.; Wei, Z.; Wang, C.; et al. Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing. Ceram. Int. 2020, 46, 2300–2308. [Google Scholar] [CrossRef]
- Faga, M.G.; Vallée, A.; Bellosi, A.; Mazzocchi, M.; Thinh, N.N.; Martra, G.; Coluccia, S. Chemical treatment on alumina–zirconia composites inducing apatite formation with maintained mechanical properties. J. Eur. Ceram. 2012, 32, 2113–2120. [Google Scholar] [CrossRef]
- Baino, F.; Canela, J.M.; Korkusuz, F.; Korkusuz, P.; Kankılıç, B.; Montealegre, M.A.; López, M.A.D.S.; Brovarone, C.V. In Vitro assessment of bioactive glass coatings on alumina/zirconia composite implants for potential use in prosthetic applications. Int. J. Mol. Sci. 2019, 20, 722. [Google Scholar] [CrossRef] [Green Version]
- Dehestani, M.; Zemlyanov, D.; Adolfsson, E.; Stanciu, L.A. Improving bioactivity of inert bioceramics by a novel Mg-incorporated solution treatment. Appl. Surf. Sci. 2017, 425, 564–575. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, S.; Khor, K.A.; Lye, S.W.; Zeng, X.; Weng, W.; Liu, C.; Venkatraman, S.S.; Ma, L.L. Osteoblastic cell response on magnesium-incorporated apatite coatings. Appl. Surf. Sci. 2008, 225, 304–307. [Google Scholar] [CrossRef]
- Ren, L.; Yang, K. Bio-functional design for metal implants, a new concept for development of metallic biomaterials. J. Mater. Sci. Technol. 2013, 29, 1005–1010. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Zhang, W.; Xu, L.; Pan, H.; Wen, J.; Wu, Q.; She, W.; Jiao, T.; Liu, X.; et al. Magnesium ion implantation on a micro/nanostructured titanium surface promotes its bioactivity and osteogenic differentiation function. Int. J. Nanomed. 2014, 9, 2387–2398. [Google Scholar]
- Park, J.W.; An, C.H.; Jeong, S.H.; Suh, J.Y. Osseointegration of commercial microstructured titanium implants incorporating magnesium: A histomorphometric study in rabbit cancellous bone. Clin. Oral Implant. Res. 2012, 23, 294–300. [Google Scholar] [CrossRef]
- Barbosa, M.A.; Monteiro, F.J.; Correia, R.; Leon, B. The effect of magnesium ions on bone bonding to hydroxyapatite coating on titanium alloy implants. Key Eng. Mater. 2003, 254, 447–450. [Google Scholar]
- Zhao, S.; Jiang, Q.; Peel, S.; Wang, X.; He, F. Effects of magnesium-substituted nanohydroxyapatite coating on implant osseointegration. Clin. Oral Implant. Res. 2013, 24, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, J.A.; Best, S.M.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T.; Bonfield, W. Bonding strength of the apatite layer formed on glass-ceramic apatite-wollastonite-polyethylene composites. J. Biomed. Mater. Res. 2003, 67, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Lacefield, W.R. Hydroxyapatite coatings. In An Introduction to Bioceramics, 2nd ed.; Hench, L.L., Ed.; Imperial College Press: London, UK, 2013; pp. 331–347. ISBN 9781908977151. [Google Scholar]
- Miyazaki, T.; Kim, H.-M.; Kokubo, T.; Ohtsuki, C.; Kato, K.; Nakamura, T. Enhancement of bonding strength by graded structure at interface between apatite layer and bioactive tantalum metal. J. Mater. Sci. Mater. Med. 2002, 13, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Mechanism of apatite formation on CaO-SiO2-P2O5 glasses in a simulated body fluid. J. Non-Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Bigi, A.; Falini, G.; Foresti, E.; Ripamonti, A.; Gazzano, M.; Roveri, N. Magnesium influence on hydroxyapatite crystallization. J. Inorg. Biochem. 1993, 49, 69–78. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The tetragonal—Monoclinic transformation in zirconia: Lessons learned and future trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Chevalier, J.; Cales, B.; Drouin, J.M. Low-temperature aging of Y-TZP ceramics. J. Am. Ceram. Soc. 1999, 82, 2150–2154. [Google Scholar] [CrossRef]
- Yabutsuka, T.; Karashima, R.; Takai, S.; Yao, T. Effect of doubled sandblasting process and basic simulated body fluid treatment on fabrication of bioactive stainless steels. Materials 2018, 11, 1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salimi, M.H.; Heughebaert, J.C.; Nancollas, G.H. Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1985, 1, 119–122. [Google Scholar] [CrossRef]
- Tomazic, B.; Tomson, M.; Nancollas, G.H. Growth of calcium phosphates on hydroxyapatite crystals: The effect of magnesium. Arch. Oral Biol. 1975, 20, 803–808. [Google Scholar] [CrossRef]
- Termine, J.D.; Peckauskas, R.A.; Posner, A.S. Calcium phosphate formation in vitro. II. Effects of environment on amorphous-crystalline transformation. Arch. Biochem. Biophys. 1970, 140, 318–325. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Nucleation of biomimetic Ca–P coatings on Ti6Al4V from a SBF×5 solution: Influence of magnesium. Biomaterials 2002, 23, 2211–2220. [Google Scholar] [CrossRef]

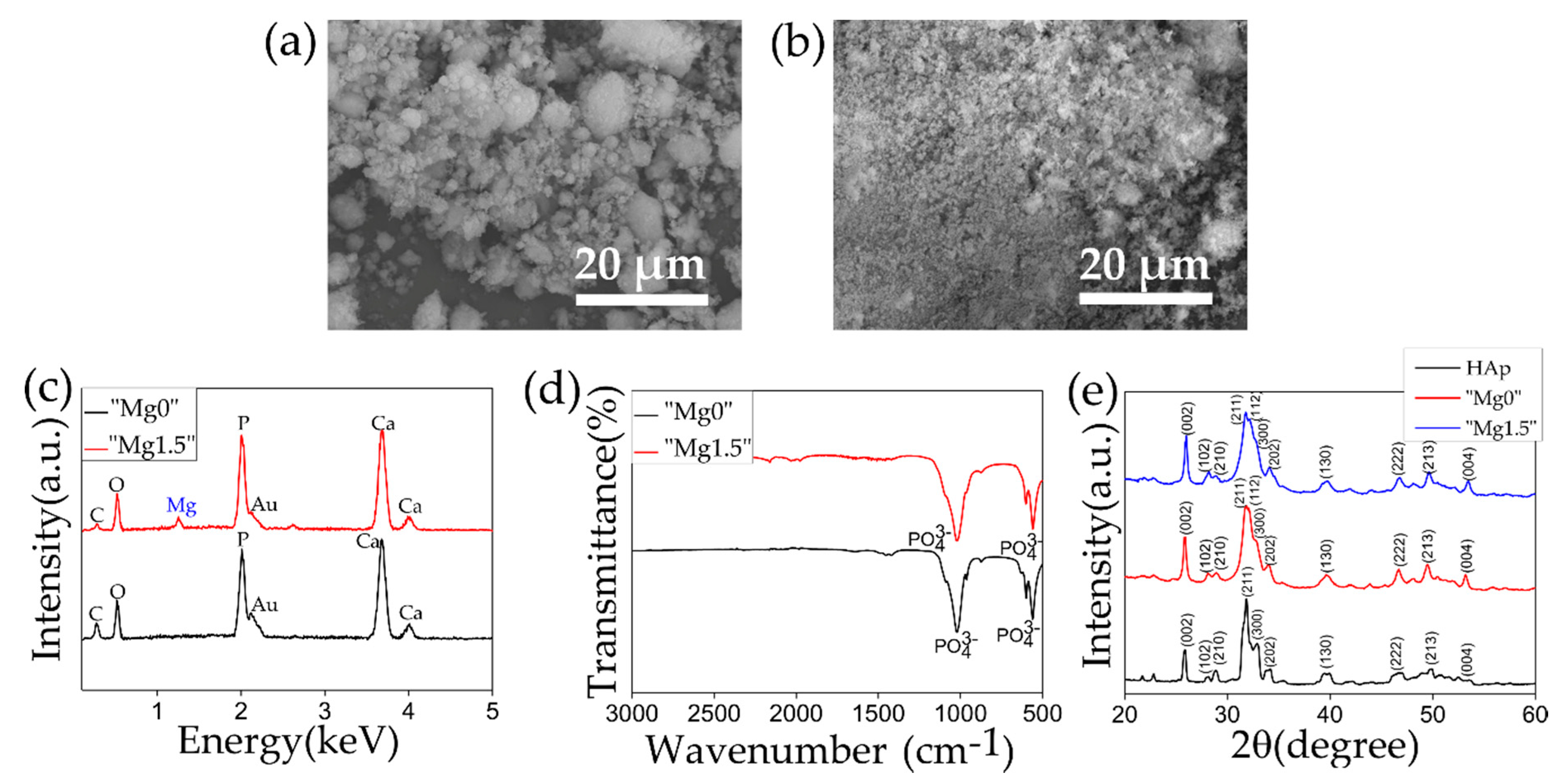
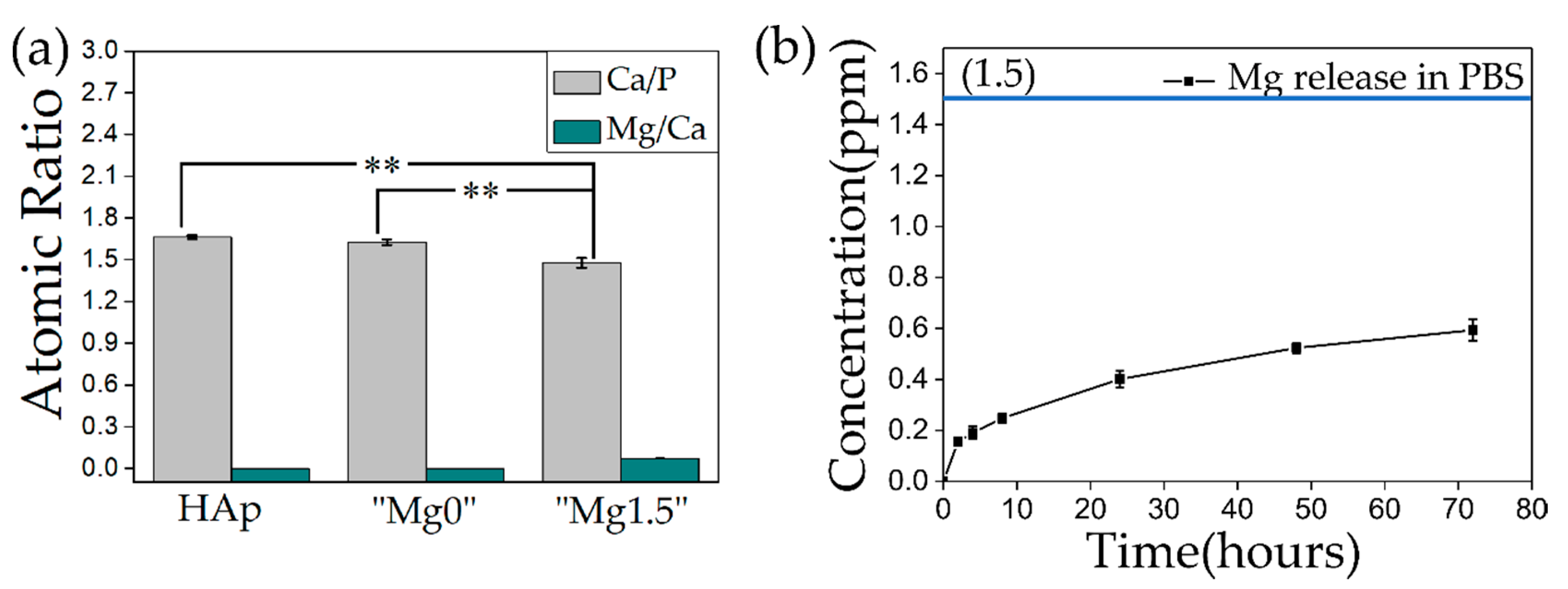
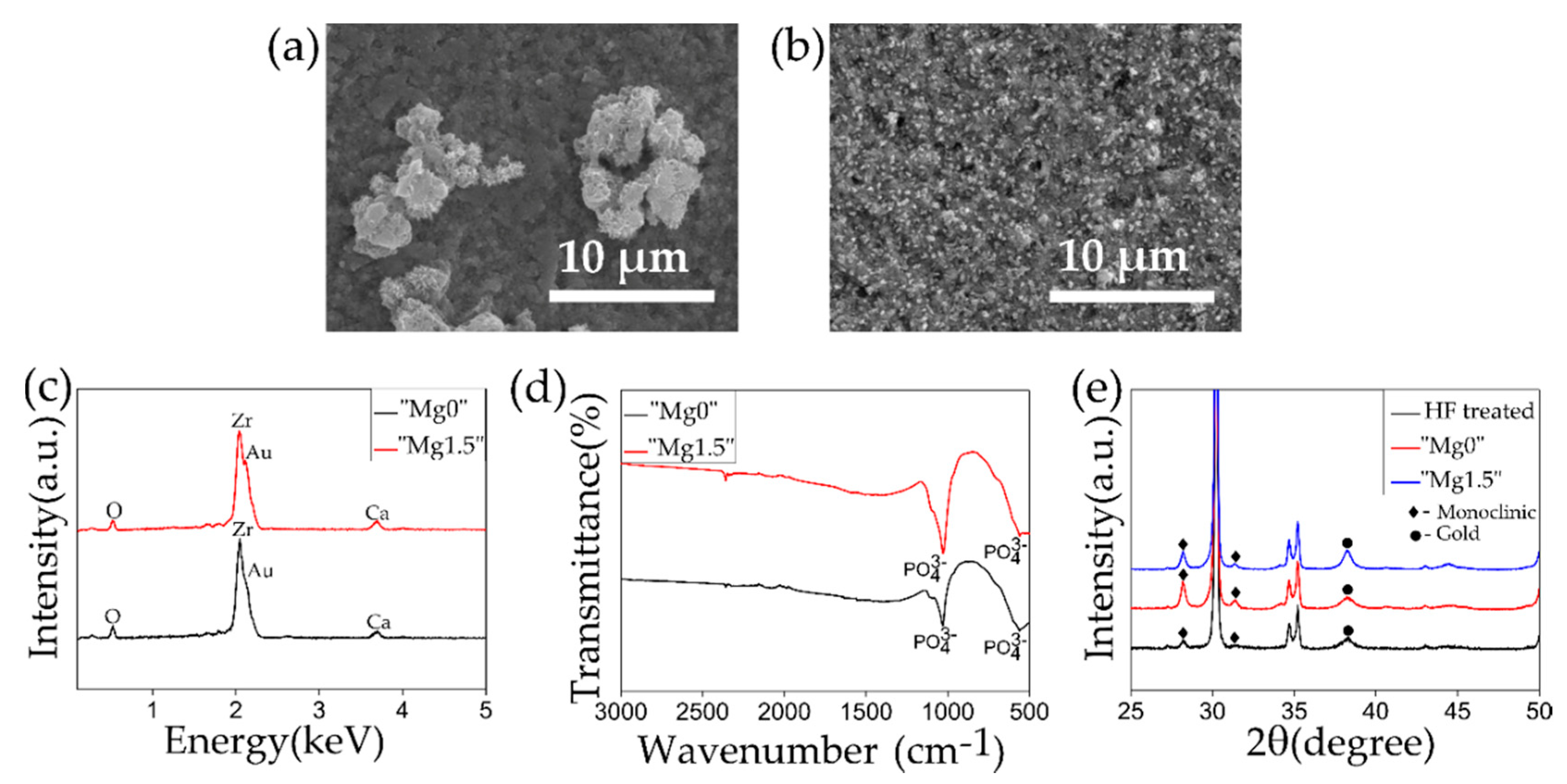

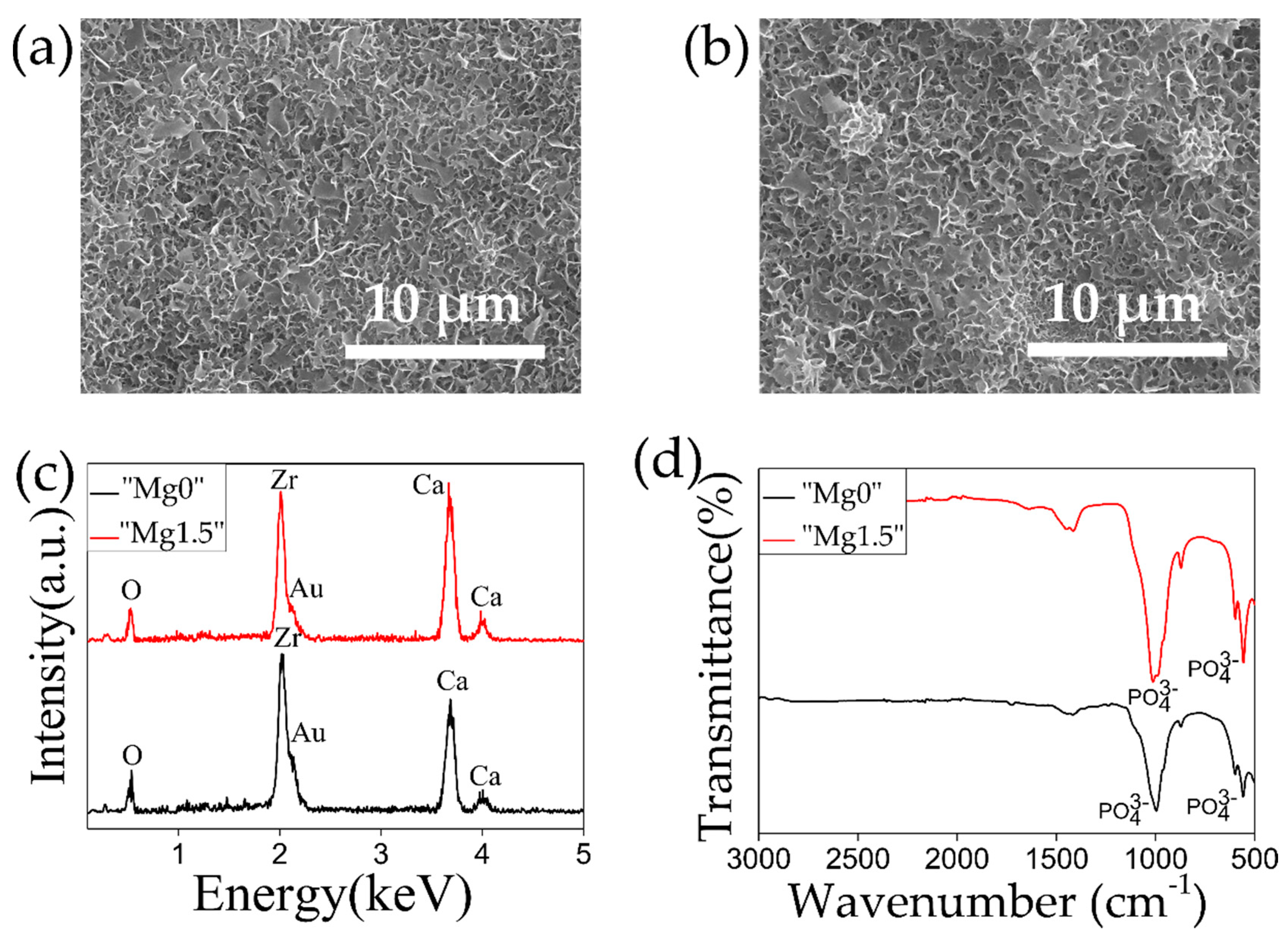


| Name of Ca-P Solution | K2HPO4·3H2O [mM] | MgCl2·6H2O [mM] | CaCl2 [mM] | Name of Precipitated Calcium Phosphate Particles |
|---|---|---|---|---|
| “Mg0” solution | 1.0 | 0 | 2.5 | “Mg0” particles |
| “Mg1.5” solution | 1.0 | 1.5 | 2.5 | “Mg1.5” particles |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamin, H.; Yabutsuka, T.; Takai, S.; Sakaguchi, H. Role of Magnesium and the Effect of Surface Roughness on the Hydroxyapatite-Forming Ability of Zirconia Induced by Biomimetic Aqueous Solution Treatment. Materials 2020, 13, 3045. https://doi.org/10.3390/ma13143045
Zamin H, Yabutsuka T, Takai S, Sakaguchi H. Role of Magnesium and the Effect of Surface Roughness on the Hydroxyapatite-Forming Ability of Zirconia Induced by Biomimetic Aqueous Solution Treatment. Materials. 2020; 13(14):3045. https://doi.org/10.3390/ma13143045
Chicago/Turabian StyleZamin, Hasnat, Takeshi Yabutsuka, Shigeomi Takai, and Hiroshi Sakaguchi. 2020. "Role of Magnesium and the Effect of Surface Roughness on the Hydroxyapatite-Forming Ability of Zirconia Induced by Biomimetic Aqueous Solution Treatment" Materials 13, no. 14: 3045. https://doi.org/10.3390/ma13143045





