Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
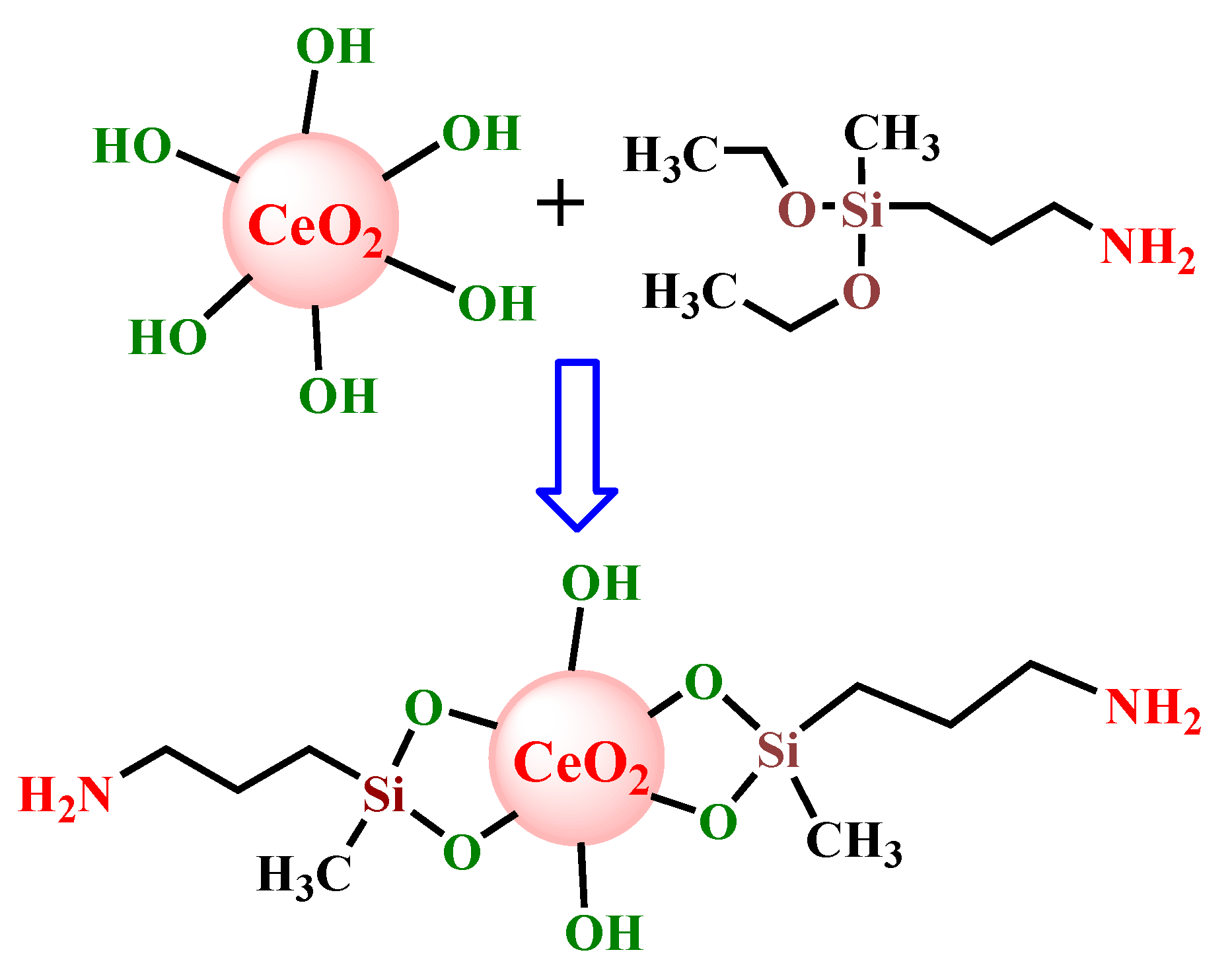
2.2. Preparation of CeO2-Functionalized Nanoparticles (CeO2–APDMS)
2.3. Synthesis of CeO2–APDMS/Cellulose Acetate Nanocomposite Films
2.4. Characterization
3. Results and Discussion
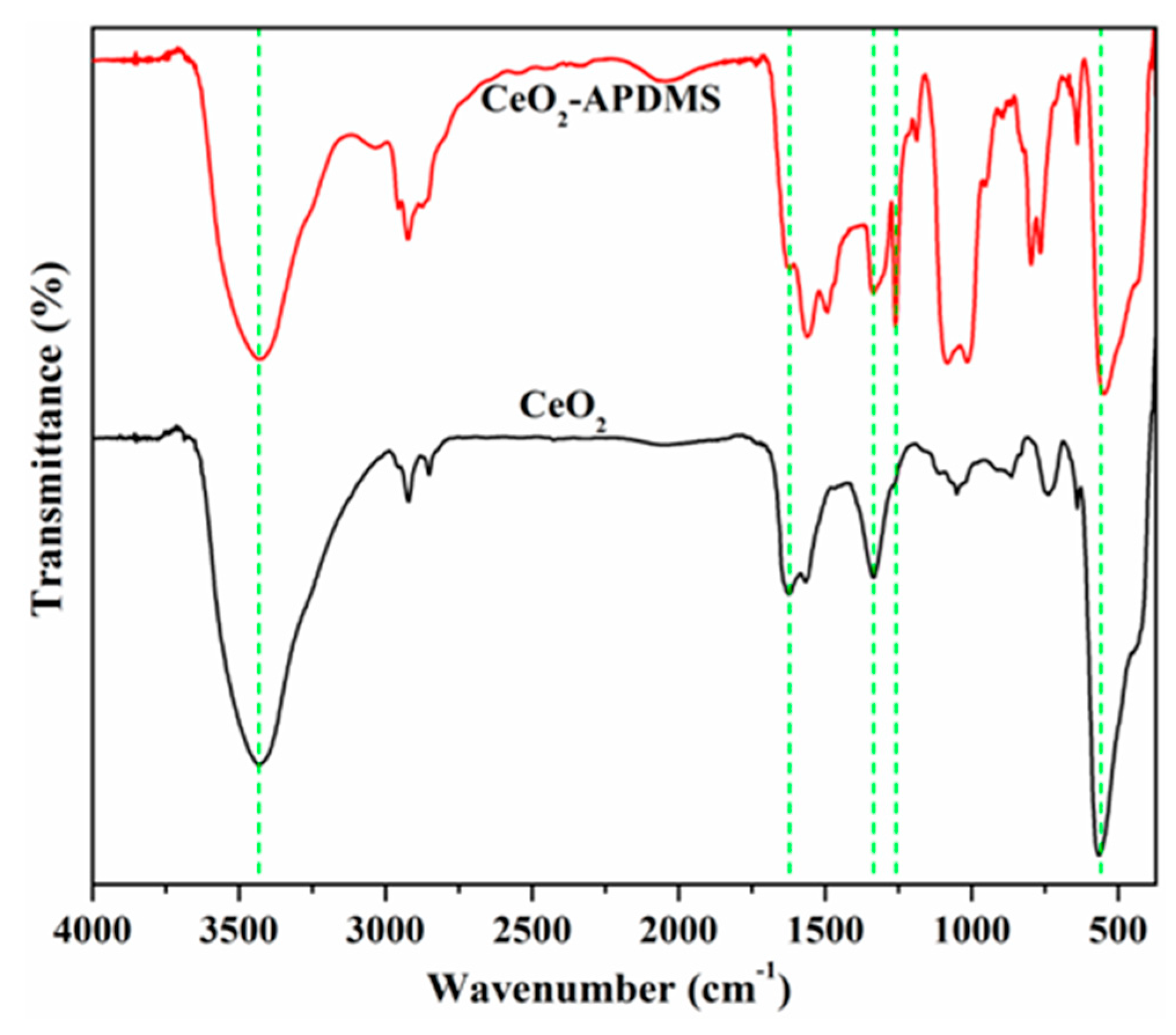
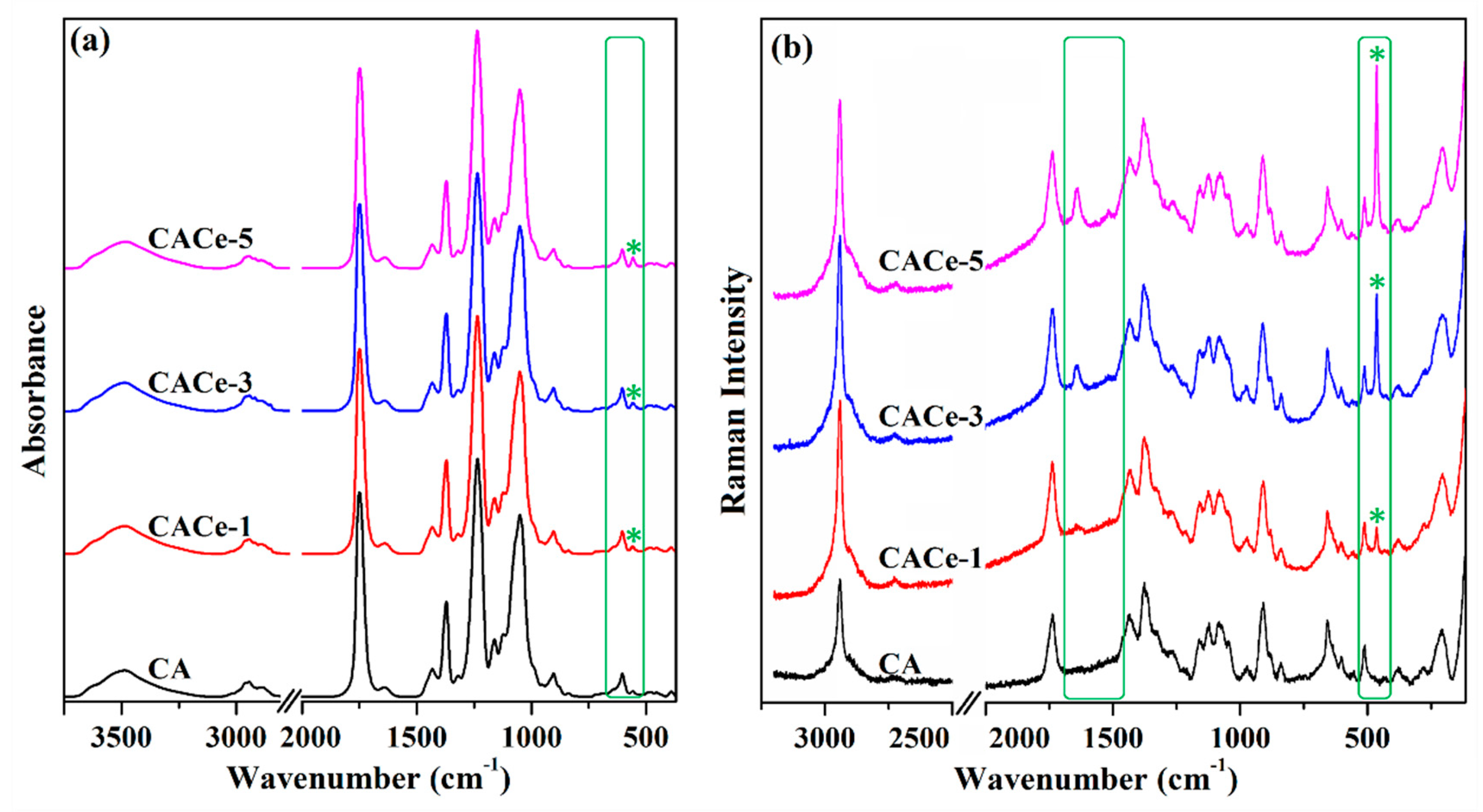
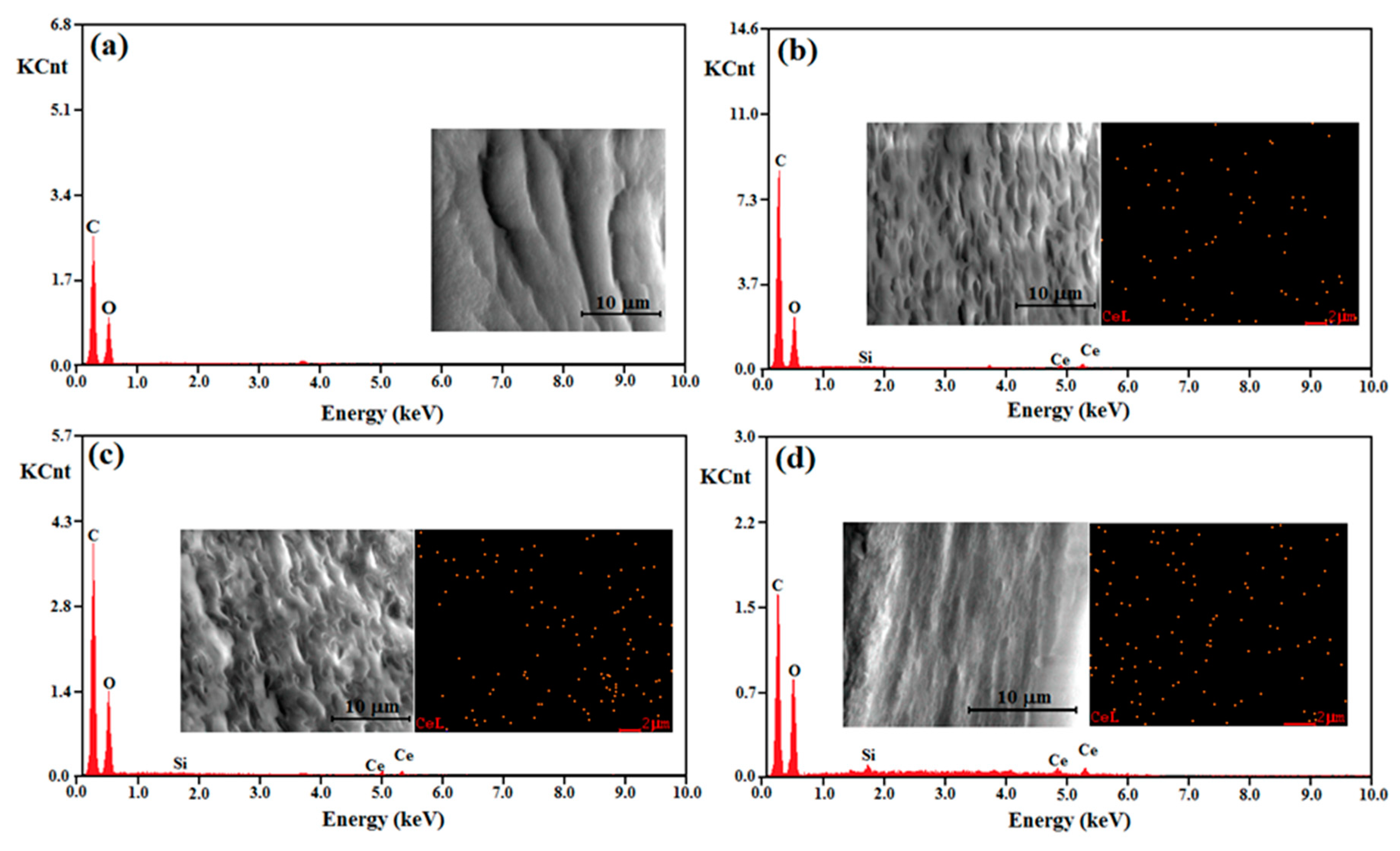
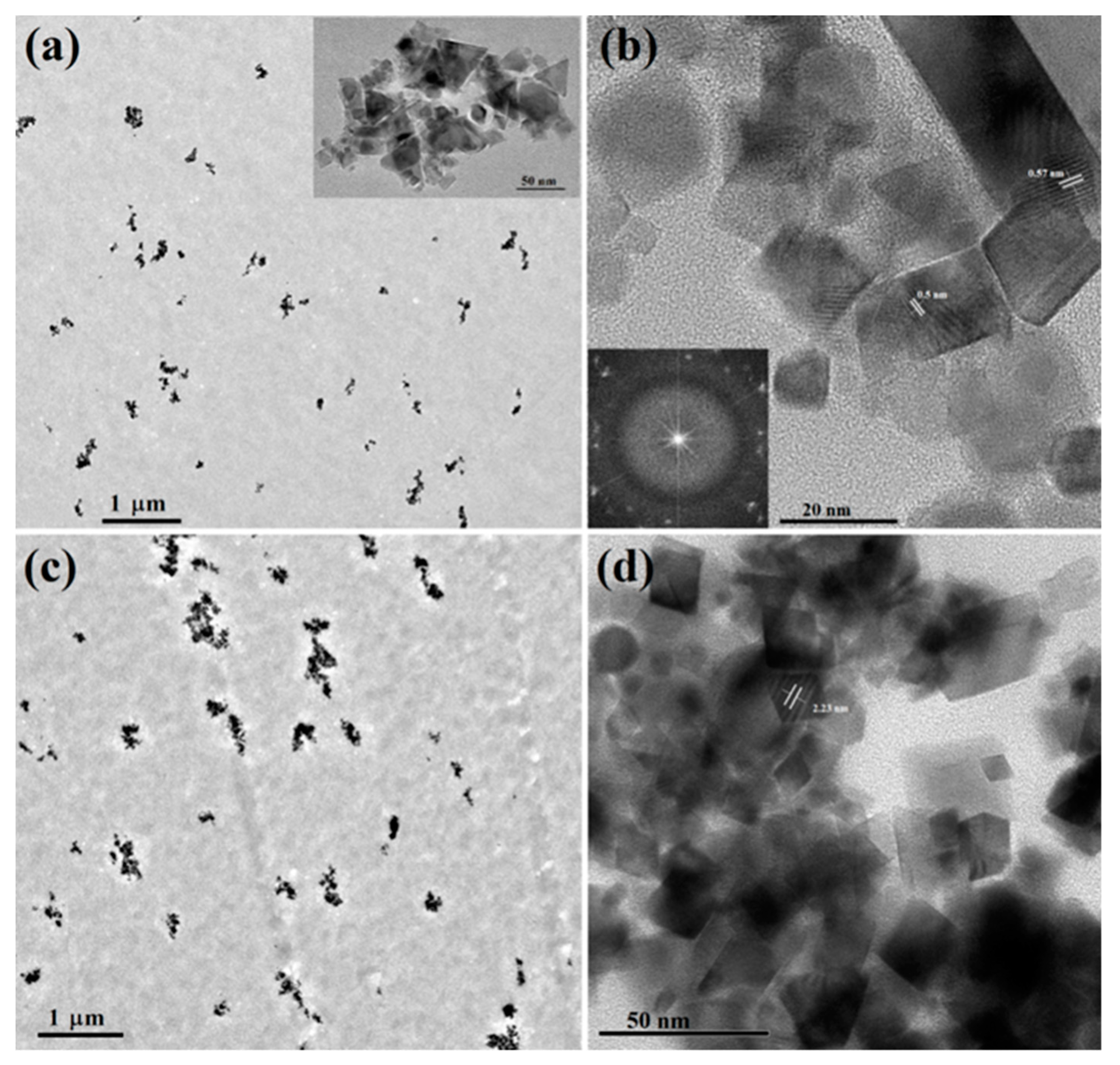
3.1. Structural and Morphological Characterization of the Materials
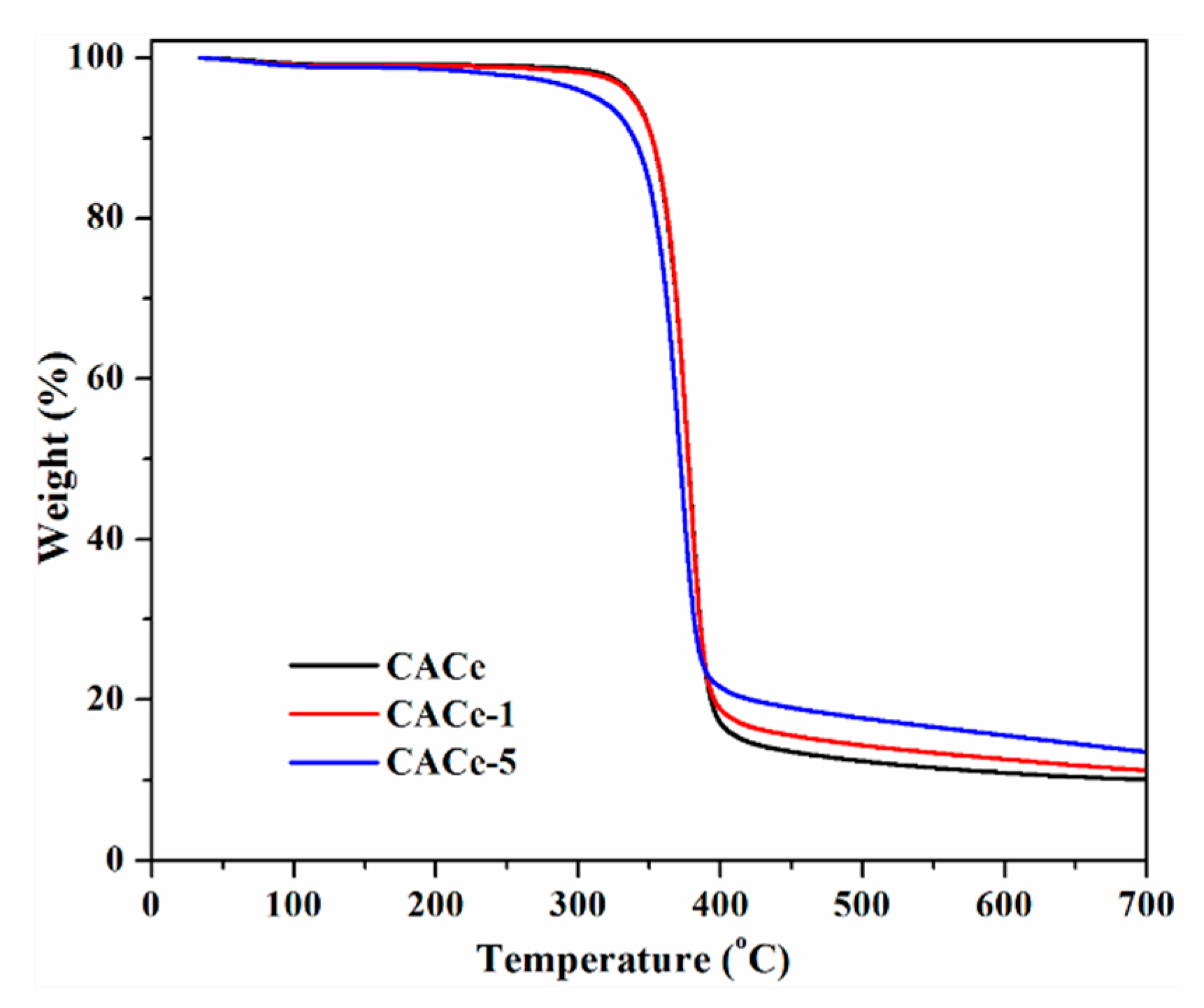
3.2. Mechanical Properties and Thermogravimetric Analysis (TGA)
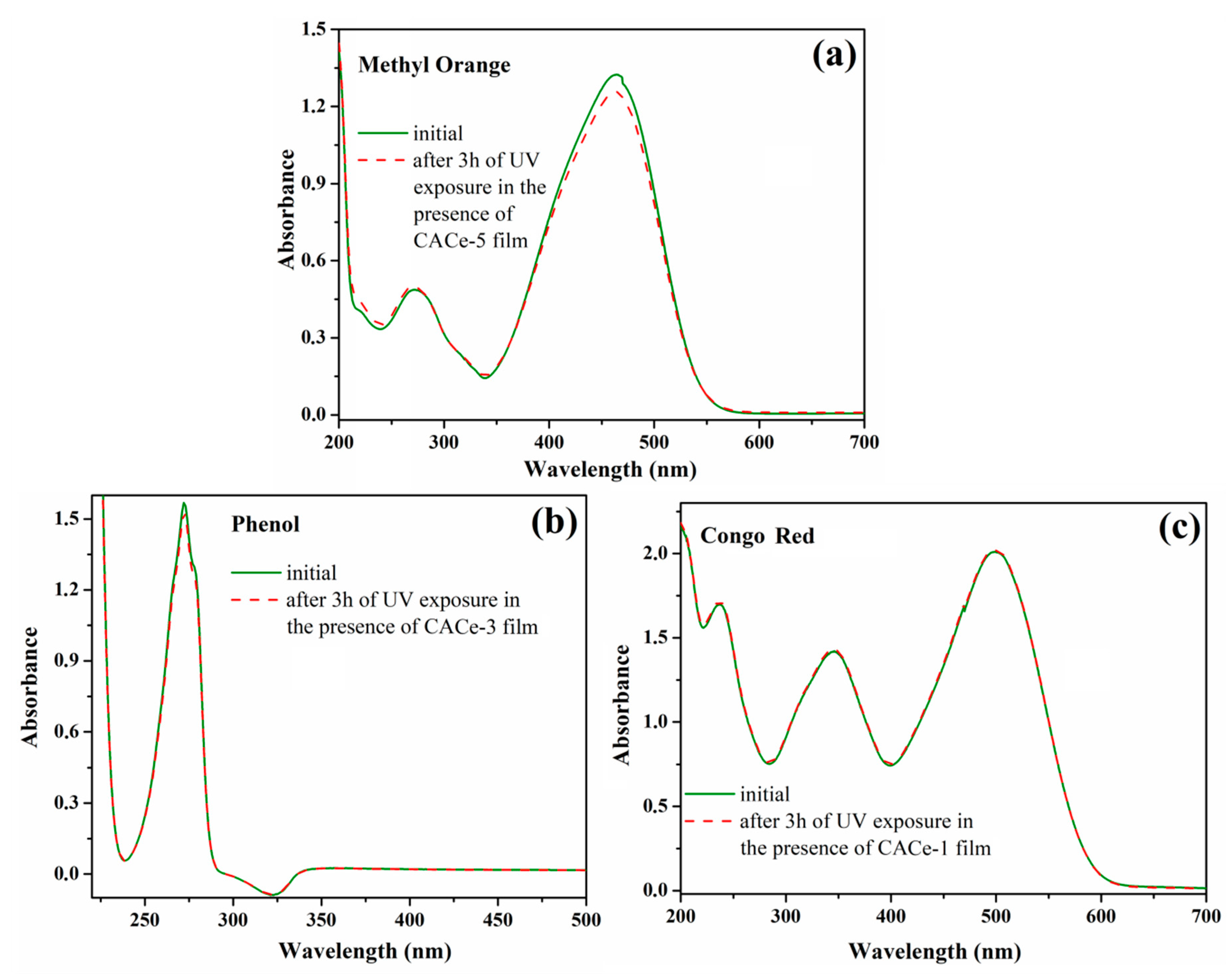
3.3. UV Shielding Ability of CACe Nanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman analysis of mode softening in nanoparticle CeO2-δ and Au-CeO2-δ during CO oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef]
- Xu, C.; Lin, Y.; Wang, J.; Wu, L.; Wei, W.; Ren, J.; Qu, X. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host-guestinteractions and switchable enzymatic activity and cellular effects of CeO2. Adv. Healthc. Mater. 2013, 2, 1591–1599. [Google Scholar] [CrossRef]
- Mandoli, C.; Pagliari, F.; Pagliari, S.; Forte, G.; Nardo, P.; Di Licoccia, S.; Traversa, E. Stem cell aligned growth induced by CeO2 nanoparticles in PLGA scaffolds with improved bioactivity for regenerative medicine. Adv. Funct. Mater. 2010, 20, 1617–1624. [Google Scholar] [CrossRef]
- Kim, C.K.; Kim, T.; Choi, I.Y.; Soh, M.; Kim, D.; Kim, Y.J.; Jang, H.; Yang, H.S.; Kim, J.Y.; Park, H.K.; et al. Ceria nanoparticles that can protect against ischemic stroke. Angew. Chem. Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef]
- Poonia, E.; Mishra, P.K.; Kiran, V.; Sangwan, J.; Kumar, R.; Rai, P.K.; Malik, R.; Tomer, V.K.; Ahuja, R.; Mishra, Y.K. Aero-gel based CeO2 nanoparticles: Synthesis, structural properties and detailed humidity sensing response. J. Mater. Chem. C 2019, 7, 5477–5487. [Google Scholar] [CrossRef]
- Aguirre, M.; Johansson Salazar-Sandoval, E.; Johansson, M.; Ahniyaz, A.; Paulis, M.; Leiza, J.R. Hybrid acrylic/CeO2 nanocomposites using hydrophilic, spherical and high aspect ratio CeO2 nanoparticles. J. Mater. Chem. A 2014, 2, 20280–20287. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Sandoval, E.J.; Aguirre, M.; Paulis, M.; Leiza, J.R.; Johansson, M.; Ahniyaz, A. Radical initiator modified cerium oxide nanoparticles for polymer encapsulation via grafting from the surface. RSC Adv. 2014, 4, 61863–61868. [Google Scholar] [CrossRef]
- Martín-Fabiani, I.; Koh, M.L.; Dalmas, F.; Elidottir, K.L.; Hinder, S.J.; Jurewicz, I.; Lansalot, M.; Bourgeat-Lami, E.; Keddie, J.L. Design of waterborne nanoceria/polymer nanocomposite UV-absorbing coatings: Pickering versus blended particles. ACS Appl. Nano Mater. 2018, 1, 3956–3968. [Google Scholar] [CrossRef]
- Saadat-Monfared, A.; Mohseni, M.; Tabatabaei, M.H. Polyurethane nanocomposite films containing nano-cerium oxide as UV absorber. Part 1. Static and dynamic light scattering, small angle neutron scattering and optical studies. Colloids Surf. A Physicochem. Eng. Asp. 2012, 408, 64–70. [Google Scholar] [CrossRef]
- Jia, R.P.; Wang, C.F.; Zheng, K.S.; He, X.Y.; Huang, M.S. Preparation, characterization, and properties of CeO2/thermoplastic polyurethane nanocomposites. J. Reinf. Plast. Compos. 2015, 34, 1090–1098. [Google Scholar] [CrossRef]
- Gofman, I.; Nikolaeva, A.; Yakimansky, A.; Ivanova, O.; Baranchikov, A.; Ivanov, V. Unexpected selective enhancement of the thermal stability of aromatic polyimide materials by cerium dioxide nanoparticles. Polym. Adv. Technol. 2019, 30, 1518–1524. [Google Scholar] [CrossRef]
- Latha, P.; Dhanabackialakshmi, R.; Kumar, P.S.; Karuthapandian, S. Synergistic effects of trouble free and 100% recoverable CeO2/Nylon nanocomposite thin film for the photocatalytic degradation of organic contaminants. Sep. Purif. Technol. 2016, 168, 124–133. [Google Scholar] [CrossRef]
- An, K.; Long, C.; Sui, Y.; Qing, Y.; Zhao, G.; An, Z.; Wang, L.; Liu, C. Large-scale preparation of superhydrophobic cerium dioxide nanocomposite coating with UV resistance, mechanical robustness, and anti-corrosion properties. Surf. Coat. Technol. 2020, 384, 125312. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Jiang, S.; Bai, H.; Zhang, S. Use of CeO2 nanoparticles to enhance UV-shielding of transparent regenerated cellulose films. Polymers 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Shu, S.; Chen, R.; Chen, B.; Dong, W. Functionalization of electrospun nanofibers of natural cotton cellulose by cerium dioxide nanoparticles for ultraviolet protection. J. Appl. Polym. Sci. 2013, 130, 1524–1529. [Google Scholar] [CrossRef]
- Biliuta, G.; Coseri, S. Cellulose: A ubiquitous platform for ecofriendly metal nanoparticles preparation. Coord. Chem. Rev. 2019, 383, 155–173. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized cellulose—Survey of the most recent achievements. Carbohydr. Polym. 2013, 93, 207–215. [Google Scholar] [CrossRef]
- Biliuta, G.; Suteu, D.; Malutan, T.; Chirculescu, A.I.; Nica, I.; Coseri, S. Valorization of tempo-oxidized cellulosic fractions for efficient dye removal from wastewaters. Cellul. Chem. Technol. 2018, 52, 609–618. [Google Scholar]
- Baron, R.I.; Bercea, M.; Avadanei, M.; Lisa, G.; Biliuta, G.; Coseri, S. Green route for the fabrication of self-healable hydrogels based on tricarboxy cellulose and poly(vinyl alcohol). Int. J. Biol. Macromol. 2019, 123, 744–751. [Google Scholar] [CrossRef]
- Culica, M.E.; Biliuta, G.; Rotaru, R.; Lisa, G.; Baron, R.I.; Coseri, S. New electromagnetic shielding materials based on viscose-carbon nanotubes composites. Polym. Eng. Sci. 2019, 59, 1499–1506. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Liu, L.; Yang, S.; He, G.; Jin, H. Preparation, characteristics, and application of bifunctional TiO2 sheets. Materials 2020, 13, 1615. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Berglund, L.; Abou-Zeid, R.; Hassan, E.; Abou-Elseoud, W.; Oksman, K. Nanocomposite film based on cellulose acetate and lignin-rich rice straw nanofibers. Materials 2019, 12, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, C.; Liu, M.; Huang, Y.; Yu, X.; Ding, E. Synthesis and characterization of asymmetric polymer/inorganic nanocomposites with pH/temperature sensitivity. Appl. Surf. Sci. 2013, 264, 636–643. [Google Scholar] [CrossRef]
- Chibac, A.L.; Buruiana, T.; Melinte, V.; Mangalagiu, I.; Buruiana, E.C. Tuning the size and the photocatalytic performance of gold nanoparticles in situ generated in photopolymerizable glycomonomers. RSC Adv. 2015, 5, 90922–90931. [Google Scholar] [CrossRef]
- Chibac, A.L.; Buruiana, T.; Melinte, V.; Buruiana, E.C. Photocatalysis applications of some hybrid polymeric composites incorporating TiO2 nanoparticles and their combinations with SiO2/Fe2O3. Beilstein J. Nanotechnol. 2017, 8, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Melinte, V.; Stroea, L.; Buruiana, T.; Chibac, A.L. Photocrosslinked hybrid composites with Ag, Au or Au-Ag NPs as visible-light triggered photocatalysts for degradation/reduction of aromatic nitroderivatives. Eur. Polym. J. 2019, 121, 109289. [Google Scholar] [CrossRef]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.A. In situ synthesis of hybrid inorganic-polymer nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yu, L.; Liu, W.; Song, Z. Surface modification of ceria nanoparticles and their chemical mechanical polishing behavior on glass substrate. Appl. Surf. Sci. 2010, 256, 3856–3861. [Google Scholar] [CrossRef]
- Nethi, S.K.; Nanda, H.S.; Steele, T.W.J.; Patra, C.R. Functionalized nanoceria exhibit improved angiogenic properties. J. Mater. Chem. B 2017, 5, 9371–9383. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Akhtar, K.; Asiri, A.M.; Khan, A.; Alamry, K.A. Effect of particle size on the photocatalytic activity and sensing properties of CeO2 nanoparticles. Int. J. Electrochem. Sci. 2013, 8, 7284–7297. [Google Scholar]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Triggs, N.E.; Valentine, J.J. Raman studies of hydrogen bonding in polyamides. Isr. J. Chem. 1994, 34, 89–93. [Google Scholar] [CrossRef]
- Salama, A.; Mohamed, A.; Aboamera, N.M.; Osman, T.; Khattab, A. Characterization and mechanical properties of cellulose acetate/carbon nanotube composite nanofibers. Adv. Polym. Technol. 2018, 37, 2446–2451. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.B.; Ferrarezi, M.M.F.; Leite, C.A.P.; Alves, R.M.V.; Gonçalves, M.d.C. Influence of the layered silicate type on the structure, morphology and properties of cellulose acetate nanocomposites. Cellulose 2013, 20, 675–686. [Google Scholar] [CrossRef]
- Gomez-Hermoso-de-Mendoza, J.; Gutierrez, J.; Tercjak, A. Improvement of macroscale properties of TiO2/cellulose acetate hybrid films by solvent vapour annealing. Carbohydr. Polym. 2020, 231, 115683. [Google Scholar] [CrossRef]
- Qu, P.; Zhou, Y.; Zhang, X.; Yao, S.; Zhang, L. Surface modification of cellulose nanofibrils for poly(lactic acid) composite application. J. Appl. Polym. Sci. 2012, 125, 3084–3091. [Google Scholar] [CrossRef]
- De Campos, E.A.; De Campos, S.D.; Roos, A.A.; De Souza, B.V.C.; Schneider, J.M.; Uliana, M.B.; De Oliveira, R.C. Titanium dioxide dispersed on cellulose acetate and its application in methylene blue photodegradation. Polym. Polym. Compos. 2013, 21, 423–430. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Amorphous Semiconductors. In Amorphous and Liquid Semiconductors, 1st ed.; Springer: Boston, MA, USA, 1974. [Google Scholar]
- Liu, K.Q.; Kuang, C.X.; Zhong, M.Q.; Shi, Y.Q.; Chen, F. Synthesis, characterization and UV-shielding property of polystyrene-embedded CeO2 nanoparticles. Opt. Mater. 2013, 35, 2710–2715. [Google Scholar] [CrossRef]
- Mousavi, S.; Shahraki, F.; Aliabadi, M.; Haji, A.; Deuber, F.; Adlhart, C. Nanofiber immobilized CeO2 /dendrimer nanoparticles: An efficient photocatalyst in the visible and the UV. Appl. Surf. Sci. 2019, 479, 608–618. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of polymer nanocomposites based on [methyl cellulose](1-x):(CuS)x (0.02M ≤ x ≤ 0.08 M) with desired optical band gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. J. Mater. Sci. Mater. Electron. 2015, 26, 8022–8028. [Google Scholar] [CrossRef]
- Prasher, S.; Kumar, M.; Singh, S. Electrical and optical properties of O6+ ion beam-irradiated polymers. Int. J. Polym. Anal. Charact. 2014, 19, 204–211. [Google Scholar] [CrossRef]
- Boubaker, K. A physical explanation to the controversial Urbach tailing universality. Eur. Phys. J. Plus 2011, 126, 1–4. [Google Scholar] [CrossRef]
- Choudhury, B.; Borah, B.; Choudhury, A. Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem. Photobiol. 2012, 88, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Akshay, V.R.; Arun, B.; Mandal, G.; Vasundhara, M. Visible range optical absorption, Urbach energy estimation and paramagnetic response in Cr-doped TiO2 nanocrystals derived by a sol-gel method. Phys. Chem. Chem. Phys. 2019, 21, 12991–13004. [Google Scholar] [CrossRef] [PubMed]
- Lagarón, J.M.; López-Rubio, A.; José Fabra, M. Bio-based packaging. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Morice, C.-H.; Radigue, B.; Winckler, C. Cellulose acetate technology to replace banned PE beads. House Pers. Care Today 2015, 10, 57–60. [Google Scholar]
- Cosmetics Info. The Science & Safety Behind Your Favorite Products. Available online: https://cosmeticsinfo.org/ingredient/cellulose-acetate (accessed on 27 April 2020).
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef]
- Parwaiz, S.; Khan, M.M.; Pradhan, D. CeO2-based nanocomposites: An advanced alternative to TiO2 and ZnO in sunscreens. Mater. Express 2019, 9, 185–202. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Hanson, J.C.; Kim, J.Y.; Liu, G.; Iglesias-Juez, A.; Fernández-García, M. Properties of CeO2 and Ce1-xZrxO2 nanoparticles: X-ray absorption near-edge spectroscopy, density functional, and time-resolved X-ray diffraction studies. J. Phys. Chem. B 2003, 107, 3535–3543. [Google Scholar] [CrossRef]



| Sample | Maximum Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (GPa) | Toughness (MJ m−3) |
|---|---|---|---|---|
| CA | 54.9 | 8 | 1.65 | 31.03 |
| CACe-1 | 68.2 | 6.82 | 1.98 | 27.92 |
| CACe-3 | 72.3 | 4.75 | 2.09 | 16.46 |
| CACe-5 | 65.7 | 3.5 | 2.56 | 10.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culica, M.E.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. https://doi.org/10.3390/ma13132955
Culica ME, Chibac-Scutaru AL, Melinte V, Coseri S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials. 2020; 13(13):2955. https://doi.org/10.3390/ma13132955
Chicago/Turabian StyleCulica, Madalina Elena, Andreea L. Chibac-Scutaru, Violeta Melinte, and Sergiu Coseri. 2020. "Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications" Materials 13, no. 13: 2955. https://doi.org/10.3390/ma13132955





