A Connection Method between Ultrahard PtW8 Wire and a Au Thick Film Based on Parallel-Gap Resistance Microwelding
Abstract
:1. Introduction
2. Process Design and Experiment
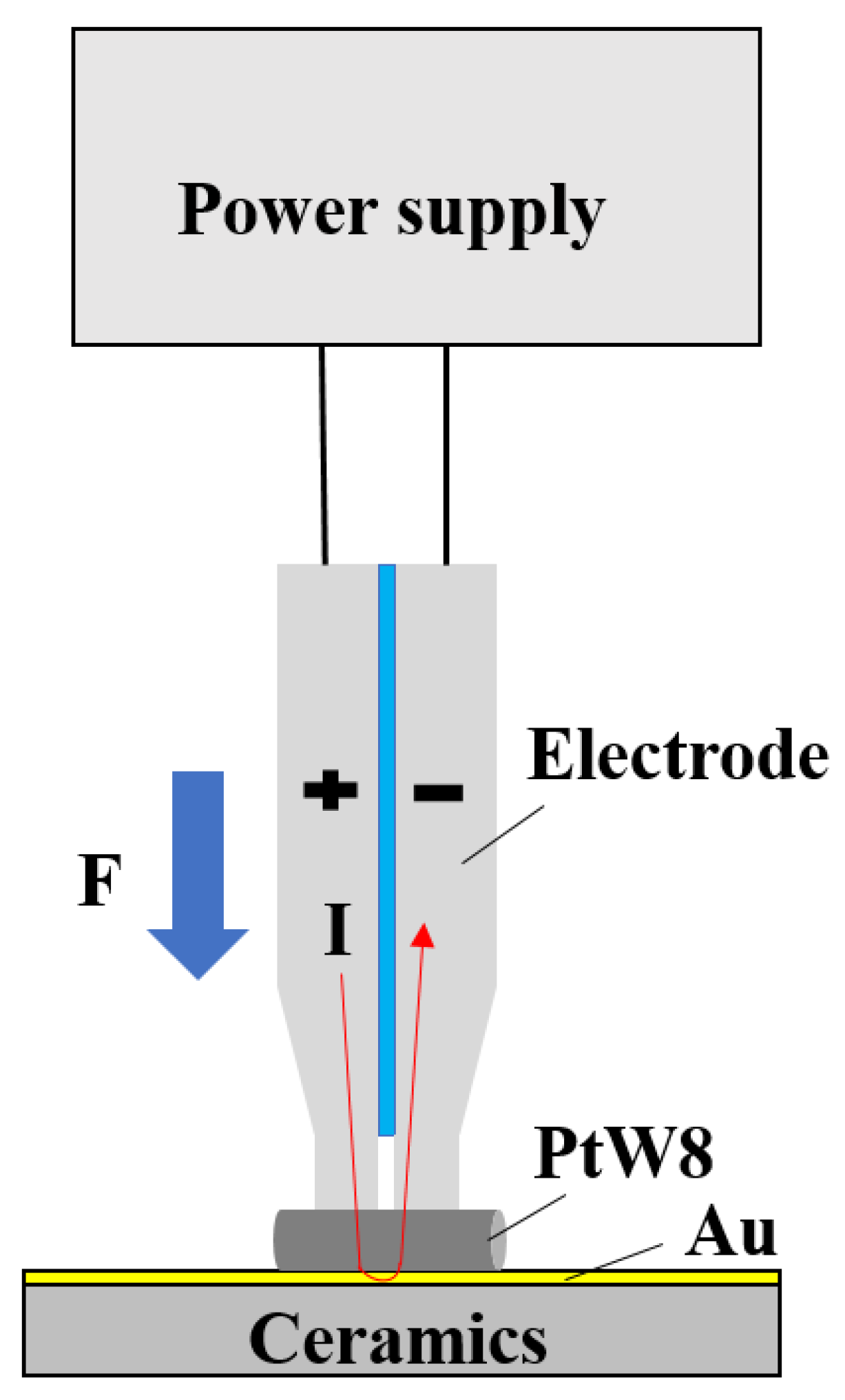
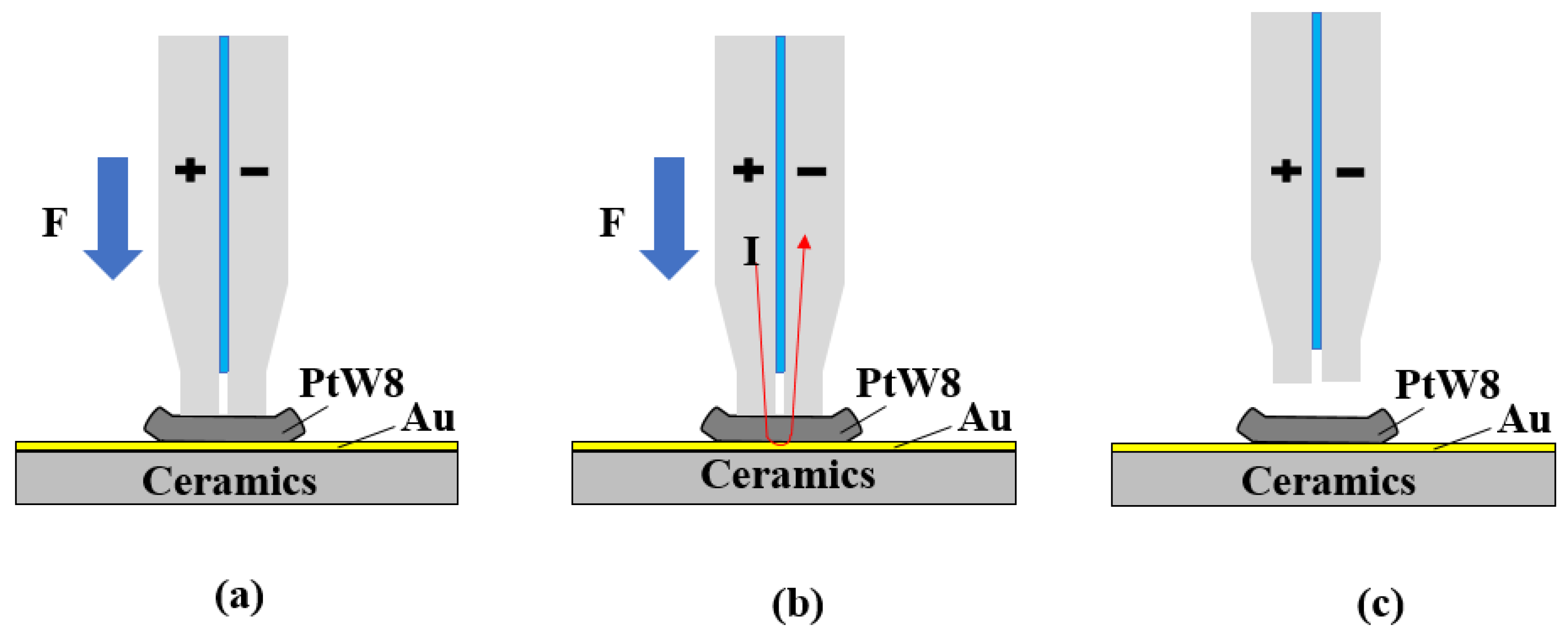
2.1. Process Design of Parallel-Gap Resistance Microwelding
2.2. Experiment
3. Results and Discussion
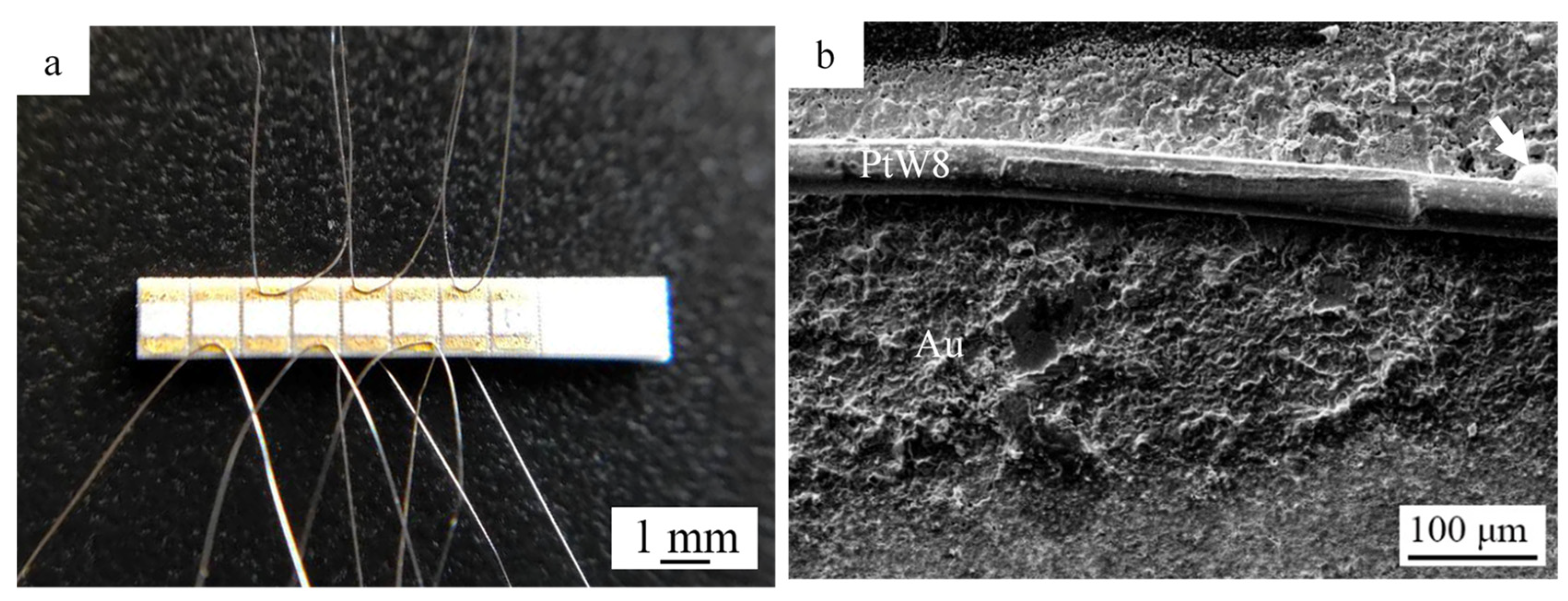
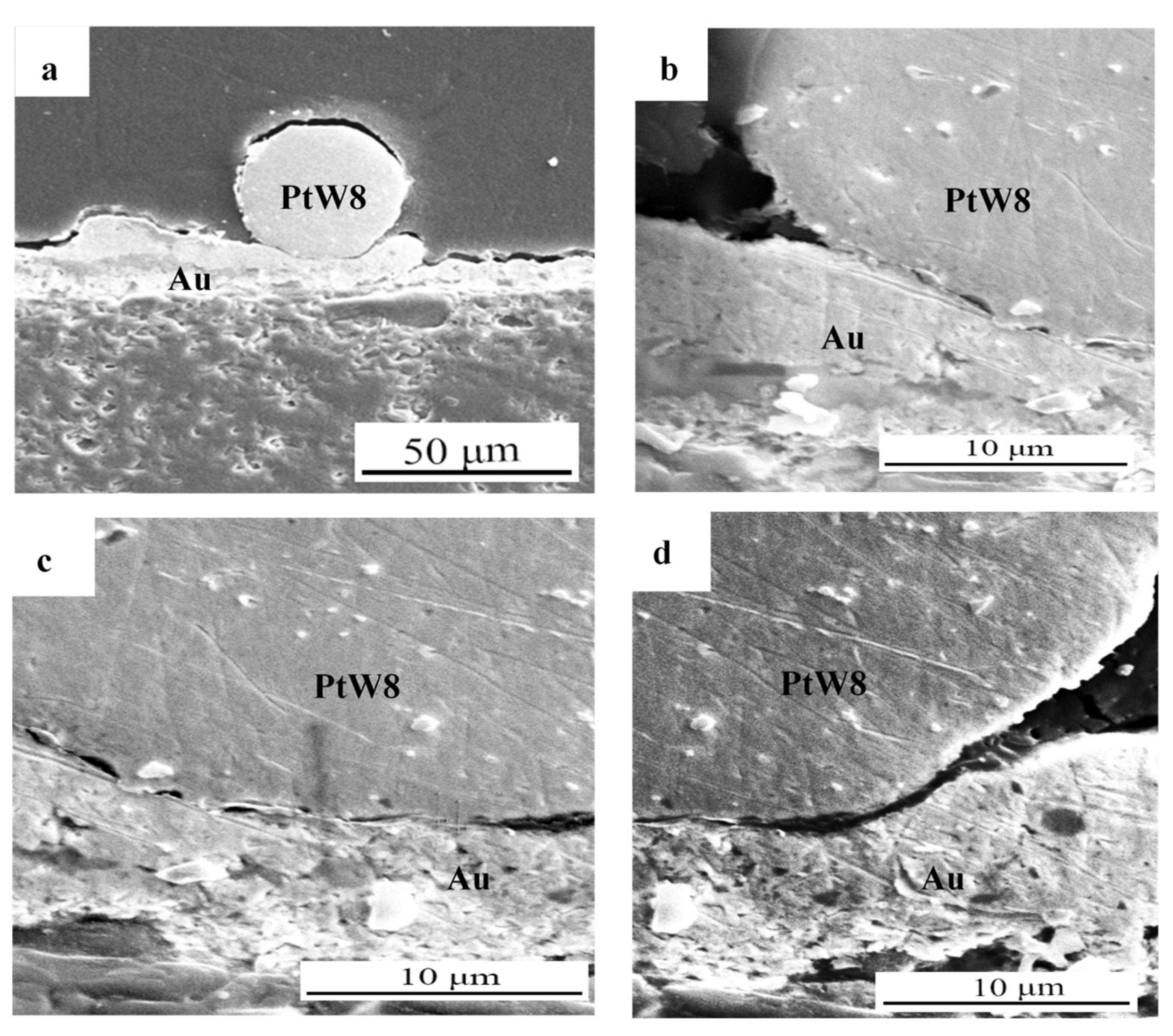
3.1. Welded Joint and Microstructure
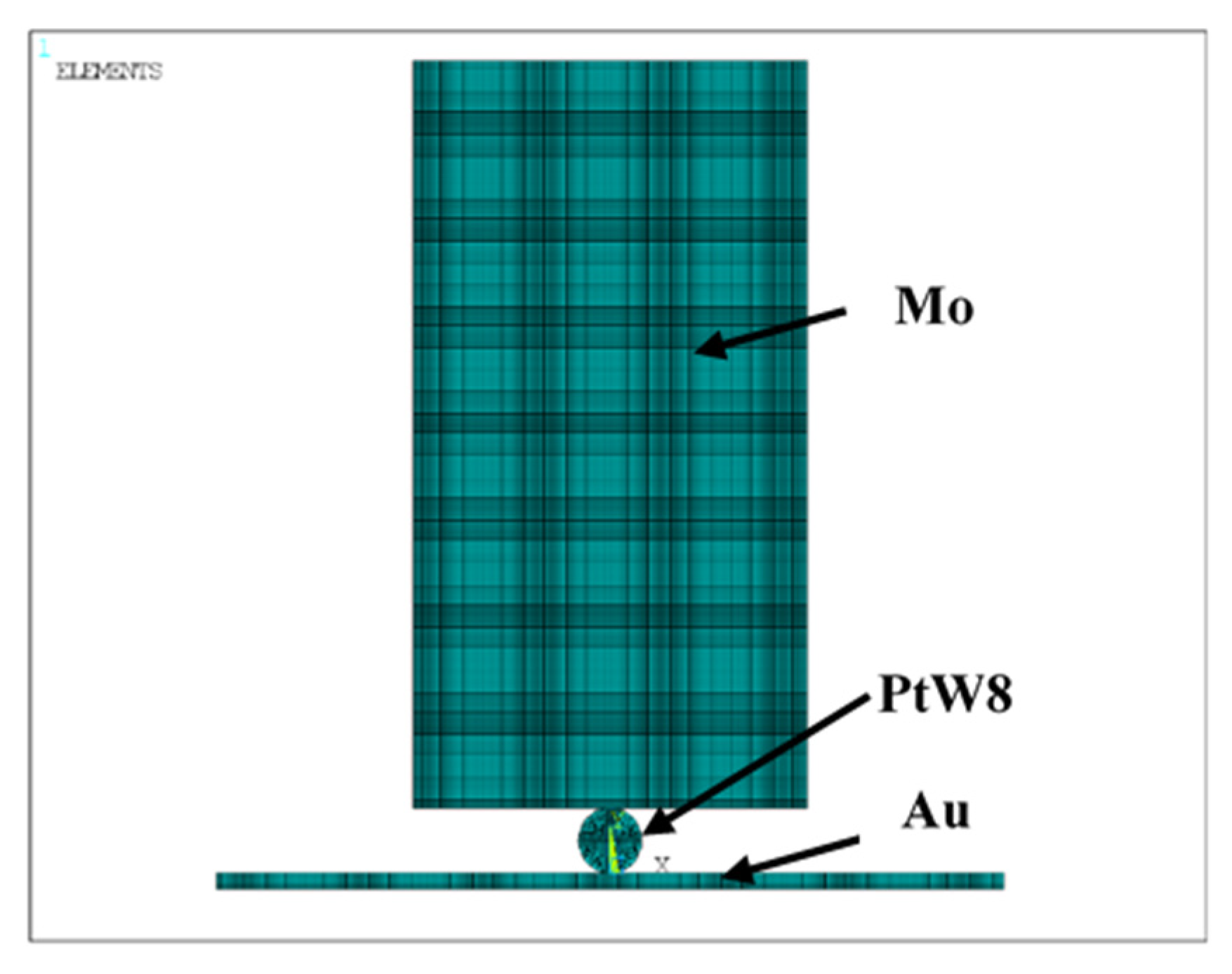
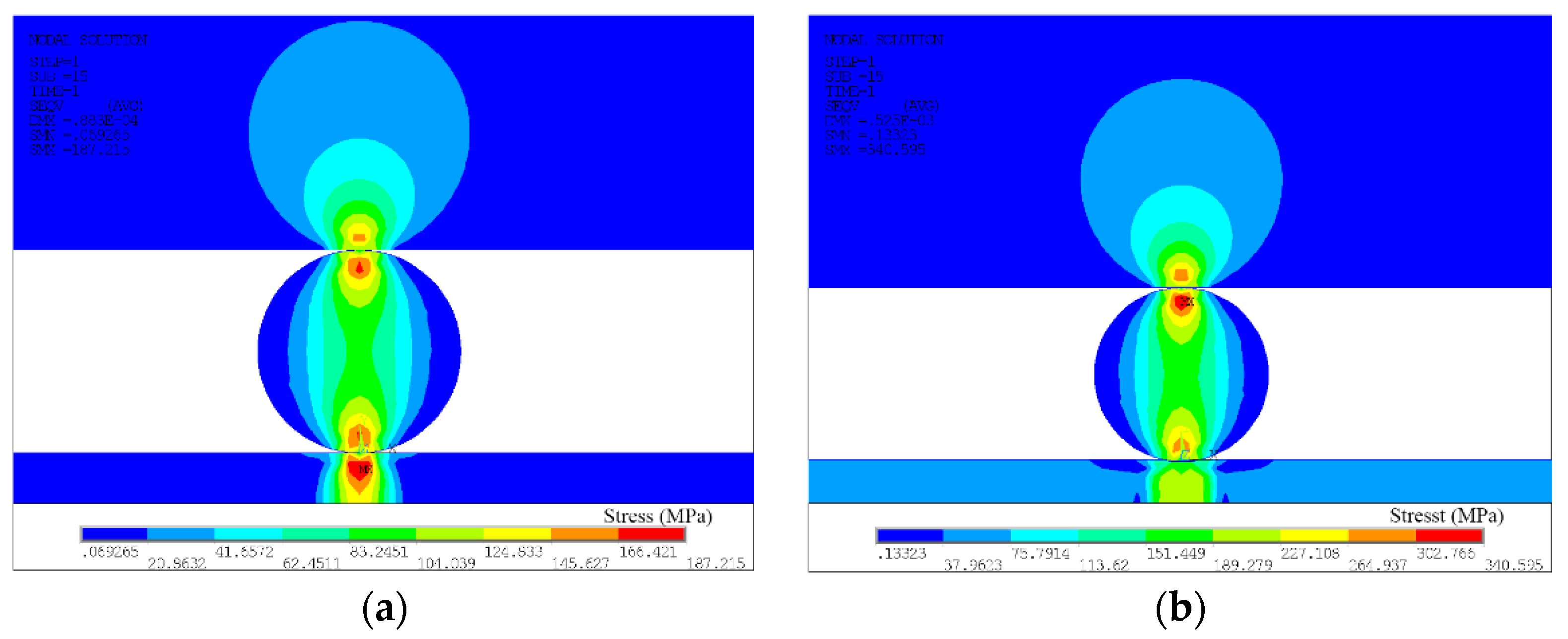
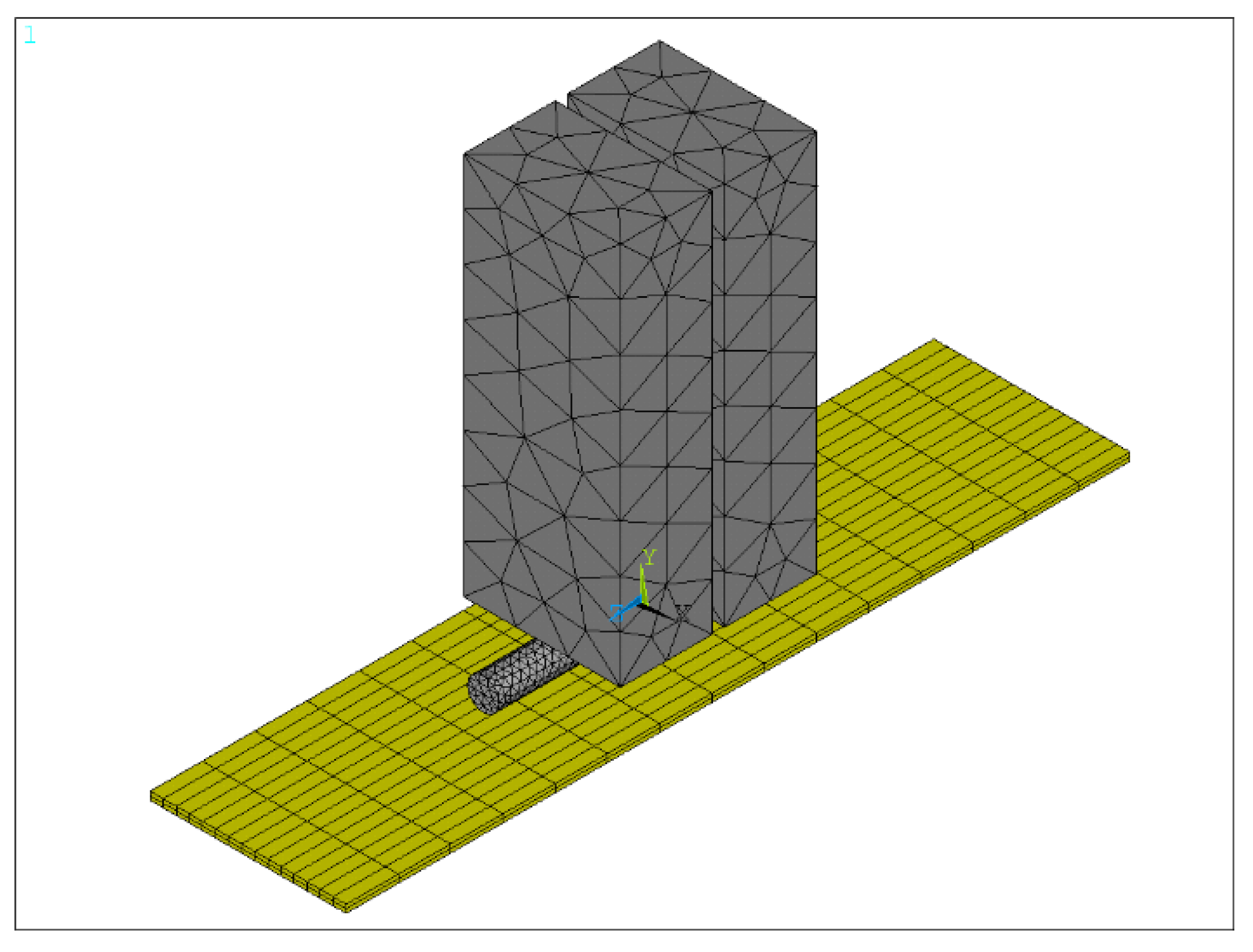
3.2. Preloading Analysis of Parallel-Gap Resistance Microwelding
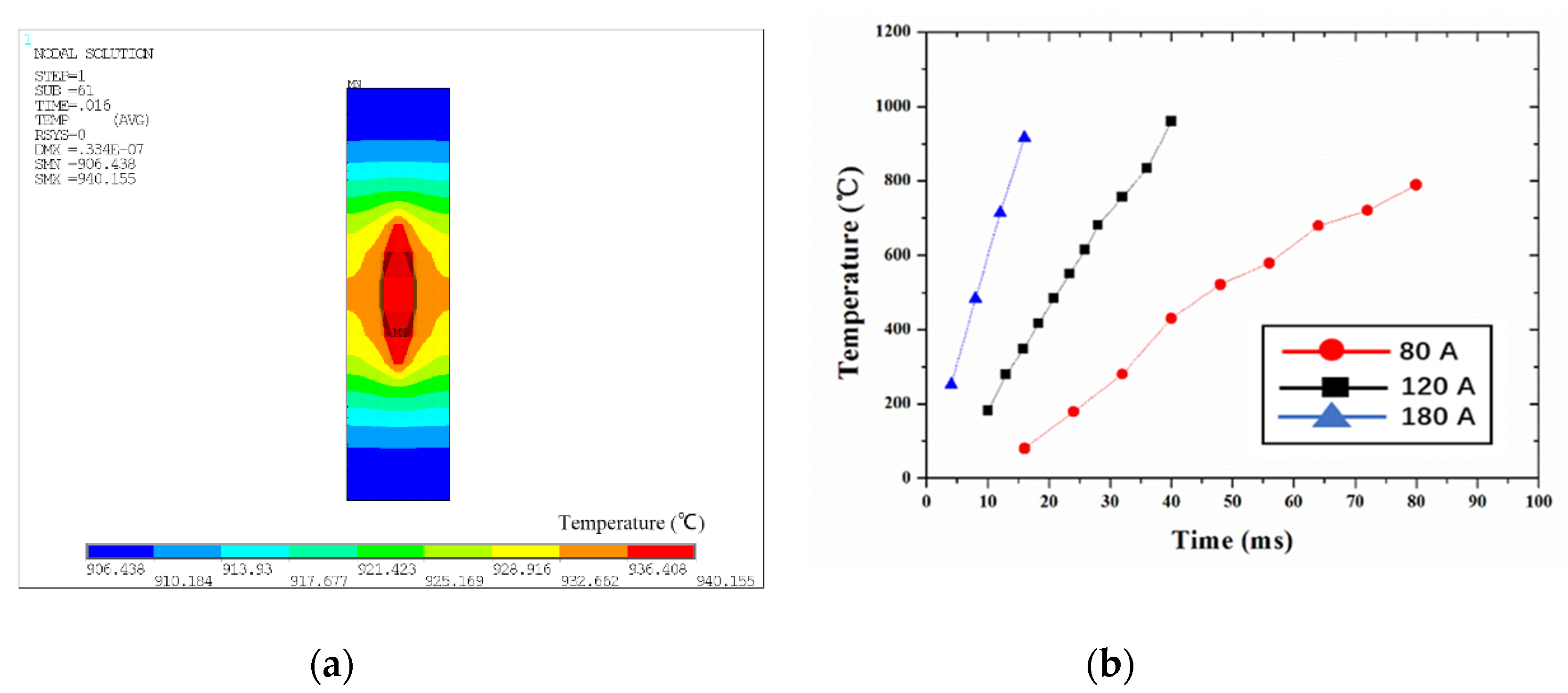
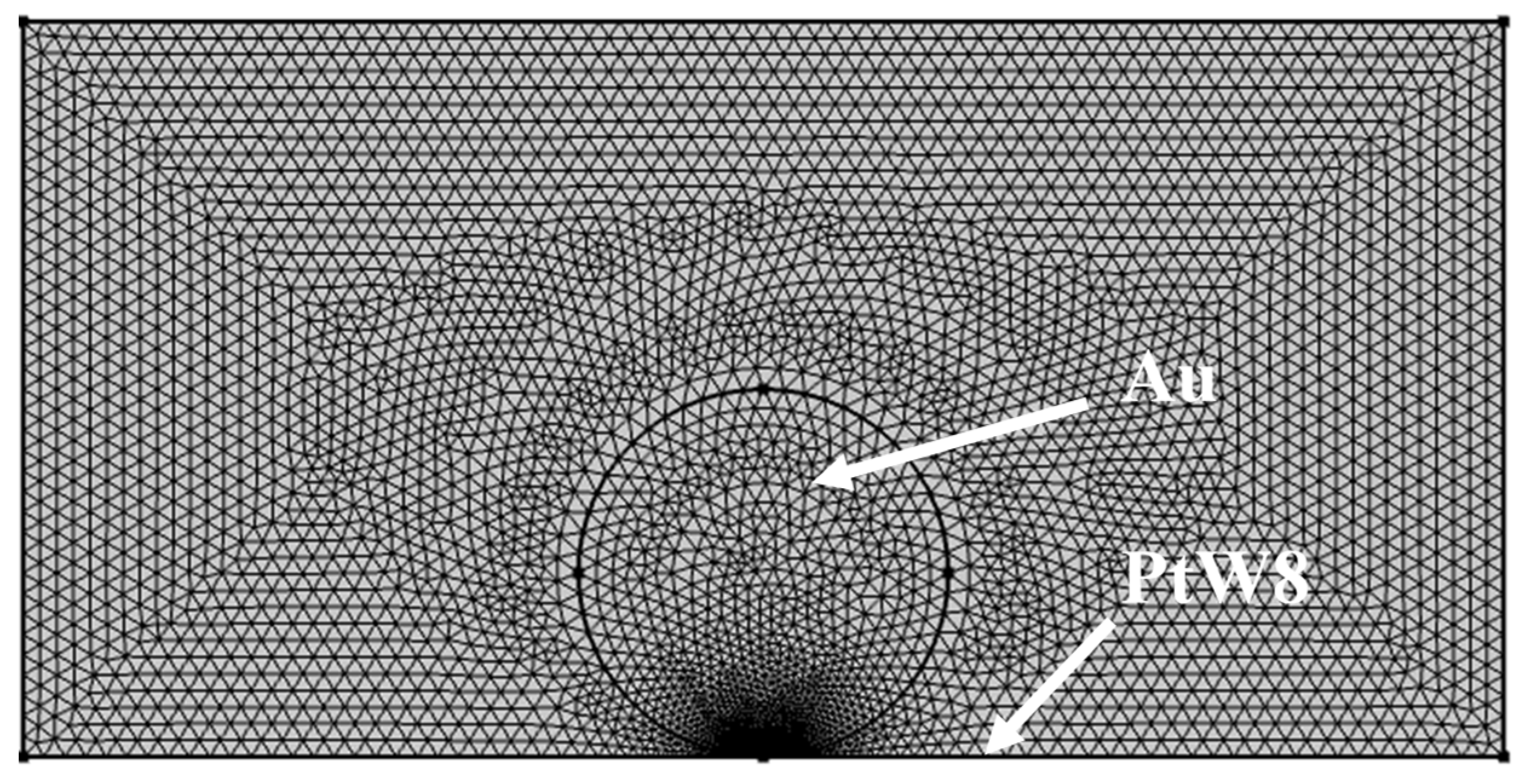
3.3. The Temperature Field of the Parallel-Gap Resistance Microwelding
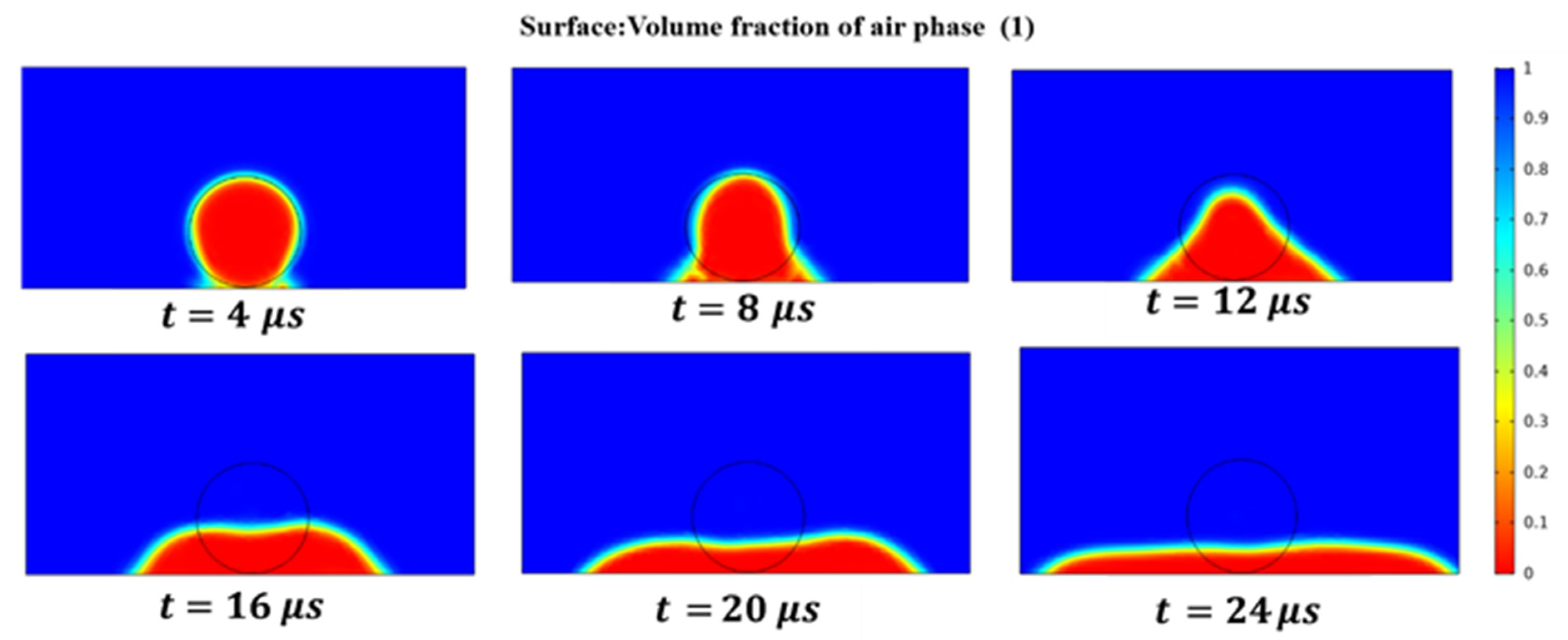
3.4. Wetting Behavior of Liquid Au on the Surface of the Platinum-Tungsten Alloy Wire
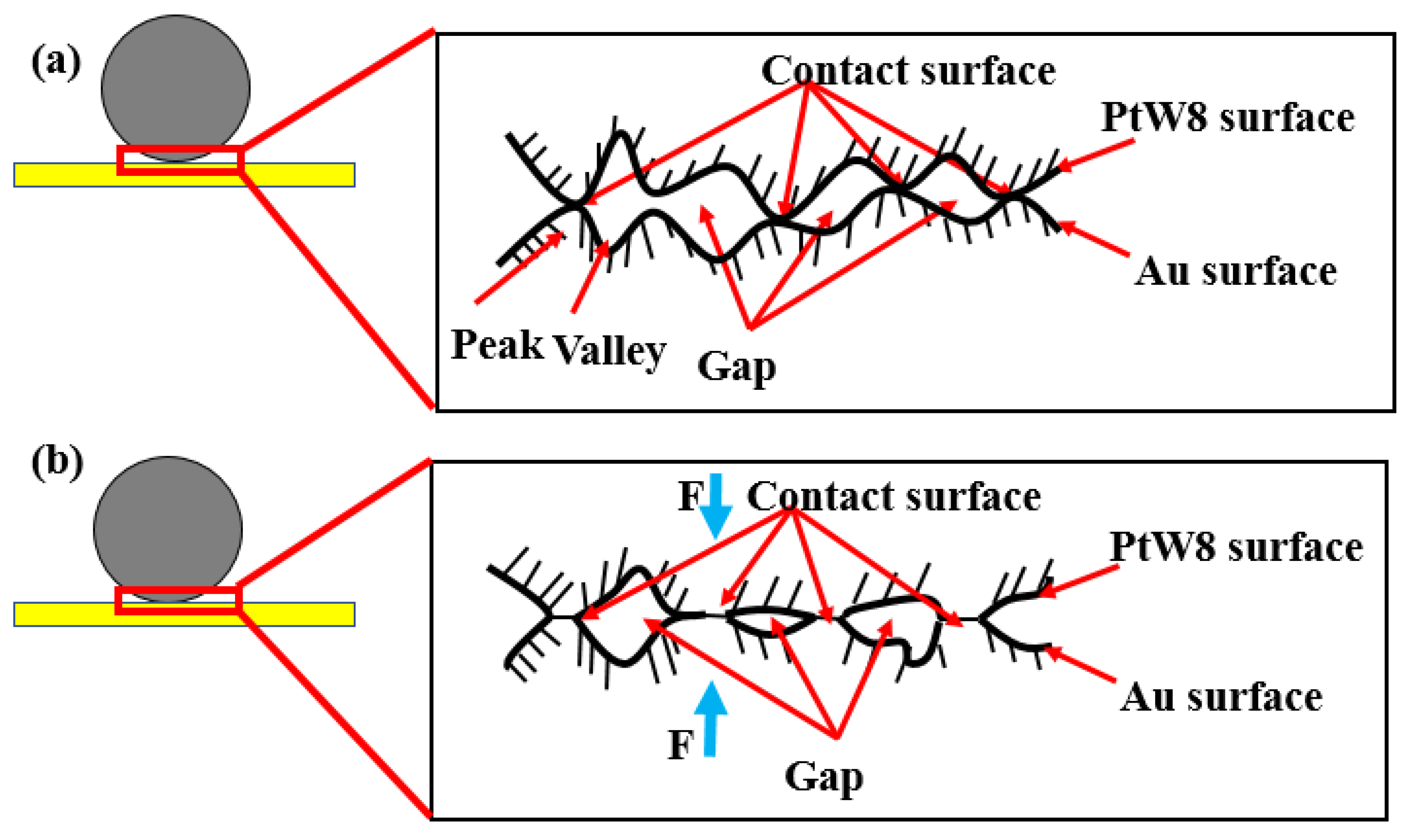
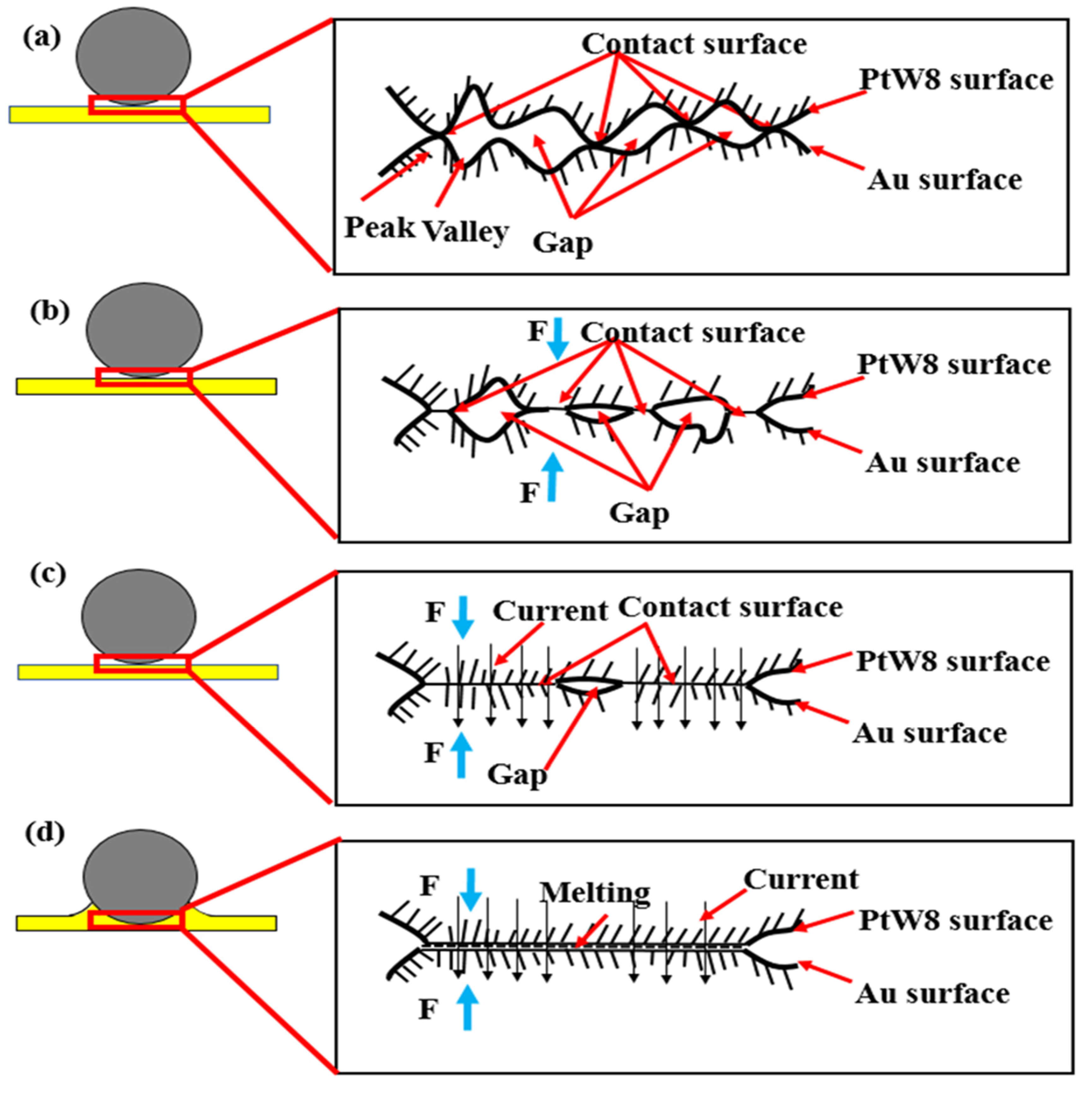
3.5. Bonding Mechanism
3.6. Factors Affecting Welded Quality
4. Conclusions
- The ultrahard PtW8 wire was successfully bonded with the Au thick film using parallel-gap resistance microwelding under appropriate welding parameters. The joint strength could be up to 5 cN.
- It was found that the parallel-gap resistance microwelding of PtW8 wire and Au thick film included the following stages: (1) deformation after pressing, (2) partial melting, (3) molten metal wetting and spreading, and (4) solid-state bonding.
- Sufficient local heat generation was the key to high-quality welds because first, it generated sufficient molten metal, and second, it created plastic deformation, and third, it facilitated the wetting and spreading of the molten metal to expand the bonded area.
- Optimizing only the welding voltage, time, and force was insufficient to achieve acceptable joint strength.
Author Contributions
Funding
Conflicts of Interest
References
- Zaza, F.; Pallozzi, V.; Serra, E. Optimization of Working Conditions for Perovskite-Based Gas Sensor Devices by Multiregression Analysis. J. Nanotechnol. 2019, 2019, 4628765. [Google Scholar] [CrossRef] [Green Version]
- Il’in, O.; Rudyk, N.; Il’ina, M.; Fedotov, A.; Guryanov, A. Design of the gas sensor prototype with CNTs-based sensitive element and application of the FFT technique for gas identification. In Proceedings of the 2020 Moscow Workshop on Electronic and Networking Technologies (MWENT), Moscow, Russia, 11–13 March 2020; pp. 1–4. [Google Scholar]
- Zhang, K.; Qin, S.; Tang, P.; Feng, Y.; Li, D. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef]
- Bin, S.; Shengwu, C.; Peiqing, Y.; Xinlei, L. Study on thermal field coupling simulation of methane sensor based on ceramic micro-hot plate. In Proceedings of the 2nd International Symposium on Application of Materials Science and Energy Materials, Shanghai, China, 17–18 December 2018; p. 022007. [Google Scholar]
- Yamazoe, N.; Shimanoe, K. New perspectives of gas sensor technology. Sens. Actuators B Chem. 2009, 138, 100–107. [Google Scholar] [CrossRef]
- Simon, I.; Barsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B Chem. 2001, 73, 1–26. [Google Scholar] [CrossRef]
- Guo, L.; Kou, X.; Ding, M.; Chong, W.; Dong, L.; Hong, Z.; Feng, C.; Sun, Y.; Yuan, G.; Peng, S. Reduced graphene oxide/α-Fe_2O_3 composite nanofibers for application in gas sensors. Sens. Actuators B Chem. 2017, b244, 233–242. [Google Scholar] [CrossRef]
- Enhanced sensitive and selective xylene sensors using W-doped NiO nanotubes. Sens. Actuators B Chem. 2015, B221, 1475–1482.
- Changhao, F.; Xin, L.; Jian, M.; Yanfeng, S.; Chong, W.; Peng, S.; Jie, Z.; Geyu, L. Facile synthesis and gas sensing properties of In2O3-WO3 heterojunction nanofibers. Sens. Actuators B Chem. 2015, 209, 622–629. [Google Scholar] [CrossRef]
- Lee, D.S.; Huh, J.S.; Lee, D.D. Classifying combustible gases using micro-gas sensor array. Sens. Actuators B 2003, B93, 1–6. [Google Scholar] [CrossRef]
- Brachmann, E.; Seifert, M.; Ernst, D.; Menzel, S.B.; Gemming, T. Pt-wire bonding optimization for electroplated Pt films on -Al2O3 for high temperature and harsh environment applications. Sens. Actuators A Phys. 2018, 284, 129–134. [Google Scholar] [CrossRef]
- Zeiser, R.; Wagner, P.; Wilde, J. Investigation of ultrasonic platinum and palladium wire bonding as interconnection technology for high-temperature SiC-MEMS. In Proceedings of the 2012 4th Electronic System-Integration Technology Conference, ESTC 2012, Amsterdam, The Netherlands, 17–20 September 2012. [Google Scholar]
- Ernst, D.; Brachmann, E.; Menzel, S.; Bock, K. Wire Bonding of Surface Acoustic Wave (SAW) Sensors for High Temperature Applications. In Proceedings of the 2018 7th Electronic System-Integration Technology Conference (ESTC), Piscataway, NJ, USA, 18–21 September 2018; p. 5. [Google Scholar]
- Schulz, K.; Ernst, D.; Menzel, S.; Gemming, T.; Eckert, J.; Wolter, K.-J. Thermosonic platinum wire bonding on platinum. In Proceedings of the 2014 37th International Spring Seminar on Electronics Technology, ISSE 2014, Dresden, Germany, 7–11 May 2014; pp. 120–124. [Google Scholar]
- Zeiser, R.; Wagner, P.; Wilde, J. Assembly and packaging technologies for high-temperature SiC sensors. In Proceedings of the 2012 IEEE 62nd Electronic Components and Technology Conference (ECTC), Piscataway, NJ, USA, 29 May–1 June 2012; pp. 338–343. [Google Scholar]
- Gierth, P.; Rebenklau, L. Development and analysis of high temperature stable interconnections on thick films using micro resistance welding for sensors and MEMS. In Proceedings of the 2018 7th Electronic System-Integration Technology Conference (ESTC), Piscataway, NJ, USA, 18–21 September 2018; p. 5. [Google Scholar]
- Huang, Y.; Xiao, L.; Pequegnat, A.; Zhou, Y. Evolution of joint formation in resistance microwelding of crossed Pt-10%Ir and 316 LVM stainless steel wires. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2015, 30, 1286–1290. [Google Scholar] [CrossRef]
- Huang, Y.D.; Pequegnat, A.; Feng, J.C.; Khan, M.I.; Zhou, Y. Resistance microwelding of crossed Pt-10Ir and 316 LVM stainless steel wires. Sci. Technol. Weld. Join. 2011, 16, 648–656. [Google Scholar] [CrossRef]
- Huang, Y.D.; Pequegnat, A.; Zou, G.S.; Feng, J.C.; Khan, M.I.; Zhou, Y. Crossed-Wire Laser Microwelding of Pt-10 Pct Ir to 316 LVM Stainless Steel: Part II. Effect of Orientation on Joining Mechanism. Metall. Mater. Trans. A 2012, 43, 1234–1243. [Google Scholar] [CrossRef]
- Lanlan, G.; Xueying, K.; Mengdi, D.; Chong, W.; Linlin, D.; Hong, Z.; Changhao, F.; Yanfeng, S.; Yuan, G.; Peng, S.; et al. Reduced graphene oxide/-Fe2O3 composite nanofibers for application in gas sensors. Sens. Actuators. B. Chem. 2017, 244, 233–242. [Google Scholar] [CrossRef]
- Cullison, A. Welding: A heavyweight in a miniature world. Weld. J. (MiamiFla) 1996, 75, 29–34. [Google Scholar]
- Kozhushko, Y.; Pavkovic, D.; Zinchenko, D.; Karbivska, T.; Sydorets, V.; Bondarenko, O. Hybrid Energy Storage System of Power Supply for Micro Resistance Welding. In Proceedings of the 2019 IEEE 39th International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 16–18 April 2019; pp. 584–589. [Google Scholar]
- Tuchtfeld, M.; Heilmann, S.; Fussel, U.; Juttner, S. Comparing the effect of electrode geometry on resistance spot welding of aluminum alloys between experimental results and numerical simulation. Weld. World 2019, 63, 527–540. [Google Scholar] [CrossRef]
- Fendrock, J.J.; Hong, L.M. Parallel-gap welding to very-thin metallization for high temperature microelectronic interconnects. IEEE Trans. Compon. Hybrids Manuf. Technol. 1990, 13, 376–382. [Google Scholar] [CrossRef]
- Jing, L. A study on the influence factors of parallel-gap welding heat. In Proceedings of the 2017 18th International Conference on Electronic Packaging Technology (ICEPT), Piscataway, NJ, USA, 16–19 August 2017; pp. 1060–1063. [Google Scholar]
- Zhang, W.W.; Cong, S.; Wen, Z.J.; Liu, Y.; Wang, Y.S.; Tian, Y.H. Experiments and reliability research on bonding process of micron copper wire and nanometer gold layer. Int. J. Adv. Manuf. Technol. 2017, 92, 4073–4080. [Google Scholar] [CrossRef]
- Tan, W.; Zhou, Y.; Kerr, H.W. Effects of Au plating on small-scale resistance spot welding of thin-sheet nickel. Metall. Mater. Trans. A (Phys. Metall. Mater. Sci.) 2002, 33A, 2667–2676. [Google Scholar] [CrossRef]
- Dickinson, D.W.; Franklin, J.E.; Stanya, A. Characterization of spot welding behavior by dynamic electrical parameter monitoring. Weld. J. 1980, 59, 170–176. [Google Scholar]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Zhou, Y. Microjoining and Nanojoining; Woodhead Publishing Ltd.: Cambridge, UK, 2008; pp. 25–51. [Google Scholar]
- Lu, H.M.; Jiang, Q. Surface tension and its temperature coefficient for liquid metals. J. Phys. Chem. B 2005, 109, 15463–15468. [Google Scholar] [CrossRef] [PubMed]




| Material | Melting Point (°C) | Elasticity Modulus (GPa) |
|---|---|---|
| Pt | 1769 | 171 |
| PtW8 | 1870 | 230 |
| Material | Melting Temperature (°C) | Electrical Resistivity (10−8 Ω∙m) | Thermal Conductivity (W∙m−1·K−1) | Elasticity Modulus (GPa) |
|---|---|---|---|---|
| PtW8 | 1870 | 62 | 71 | 230 |
| Au | 1064 | 2.21 | 318 | 79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Tu, M.; Liu, J. A Connection Method between Ultrahard PtW8 Wire and a Au Thick Film Based on Parallel-Gap Resistance Microwelding. Materials 2020, 13, 2911. https://doi.org/10.3390/ma13132911
Pan M, Tu M, Liu J. A Connection Method between Ultrahard PtW8 Wire and a Au Thick Film Based on Parallel-Gap Resistance Microwelding. Materials. 2020; 13(13):2911. https://doi.org/10.3390/ma13132911
Chicago/Turabian StylePan, Mingqiang, Minghui Tu, and Jizhu Liu. 2020. "A Connection Method between Ultrahard PtW8 Wire and a Au Thick Film Based on Parallel-Gap Resistance Microwelding" Materials 13, no. 13: 2911. https://doi.org/10.3390/ma13132911




