Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams
Abstract
:1. Introduction
2. Experimental
2.1. Materials
- Kosmos 33 (potassium acetate) and Kosmos 75 (potassium octanoate) purchased from Evonik Industries AG (Essen, Germany) were selected as catalysts;
- TEGOSTAB B8513 purchased from Evonik Industries AG was used as a silicone surfactant;
- Cyclopentane (purity > 98%) and Pentane (purity ≥ 99%) provided by Merck KGaA (Darmstadt, Germany) were used as a blowing agent;
- Polyethylene glycol PEG-400 (purity 95%), sodium hydroxide (anhydrous), phthalic anhydride (purity ≥ 99%), glycerol (purity > 99.5%), imidazole (purity ≥ 99%), sulfuric acid (purity 95–98%), and pyridine (purity ≥ 99.8%) were purchased from Sigma–Aldrich Corporation(St. Louis, MO, USA); and,
- Walnut shells (WS) were obtained from a local company from Poland.
2.2. Methods
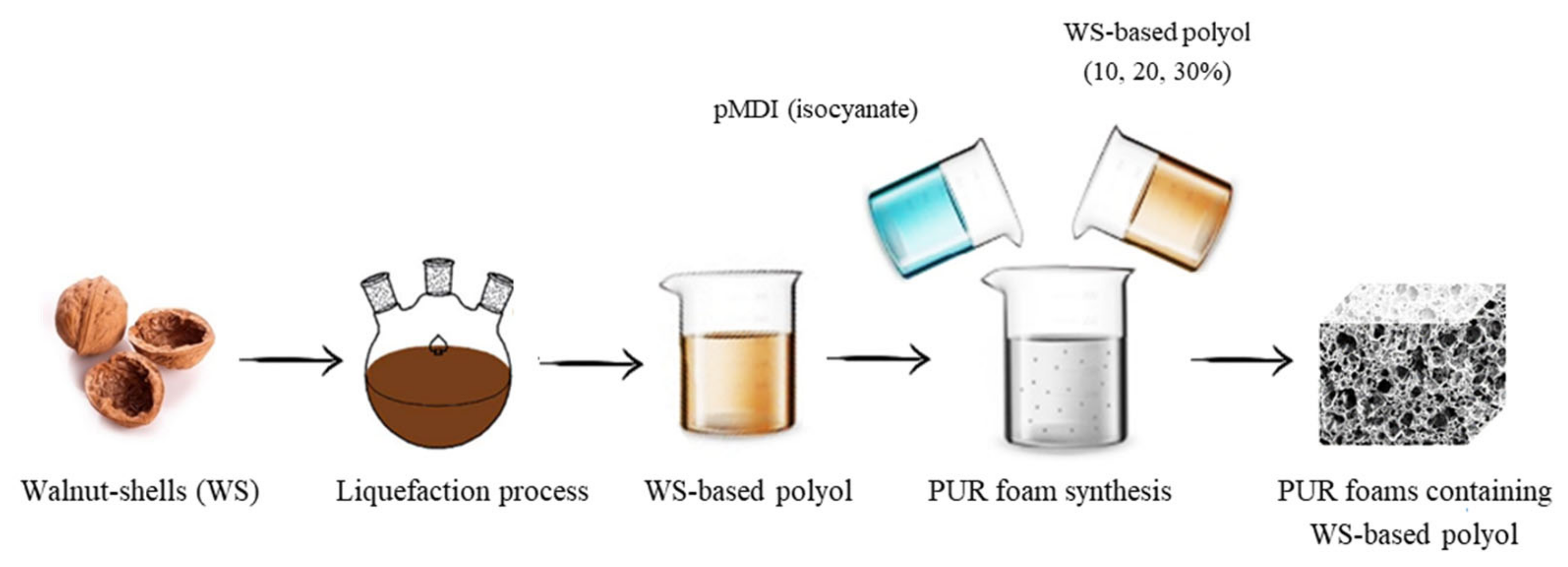
2.2.1. Liquefaction of WS
2.2.2. PUR Foams Preparation
2.3. Methods
3. Results and Discussion
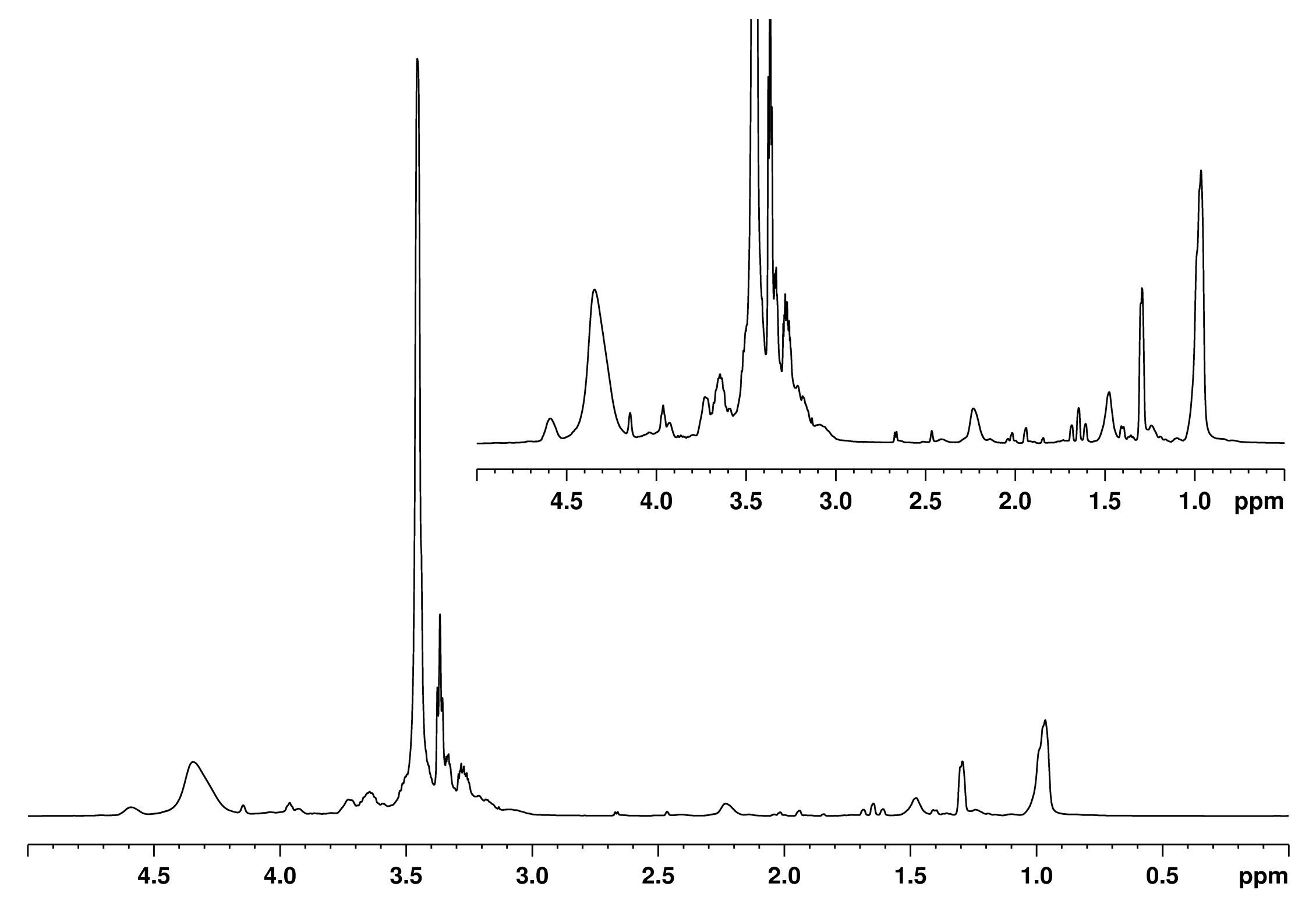
3.1. Characterization of WS-Based Polyol
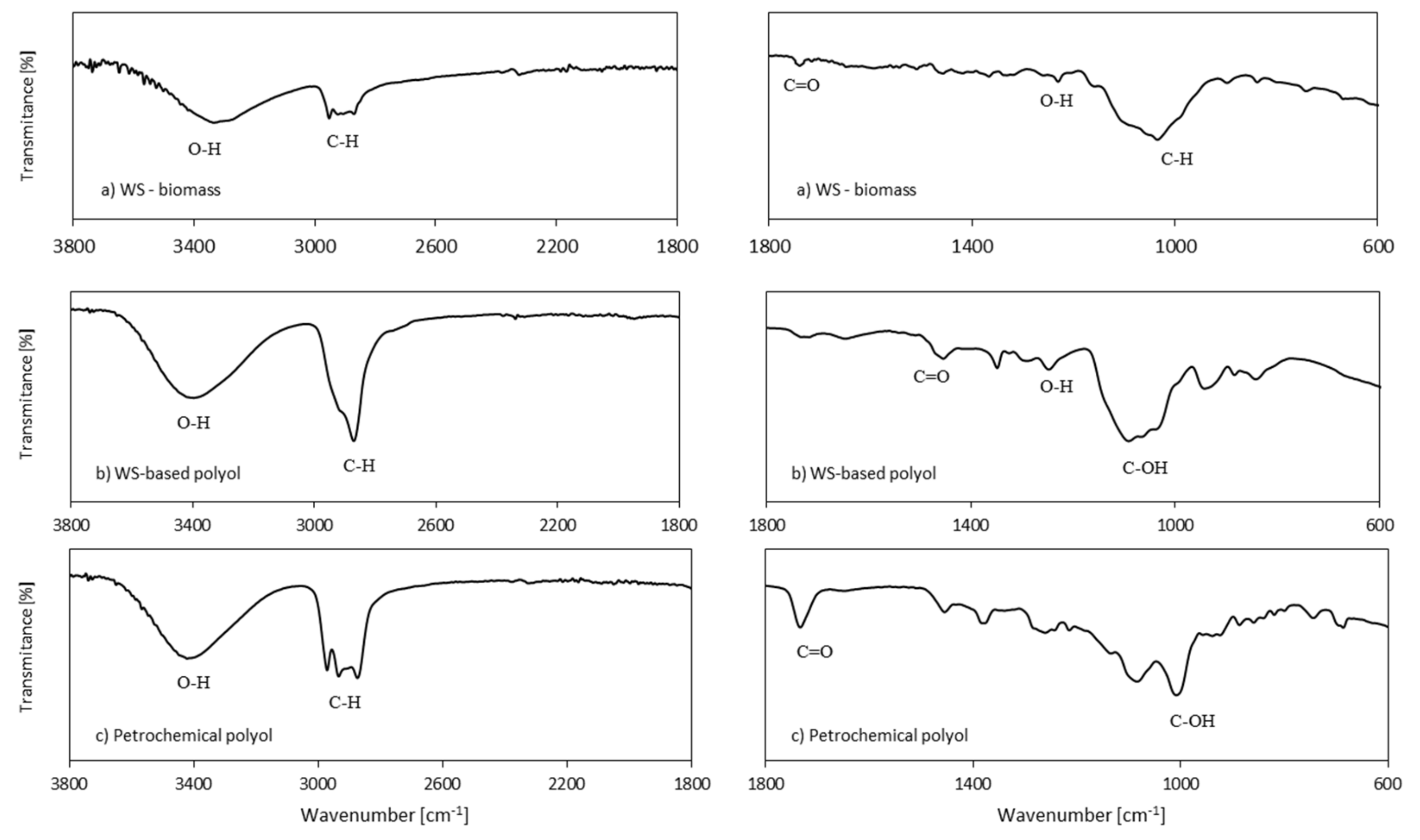
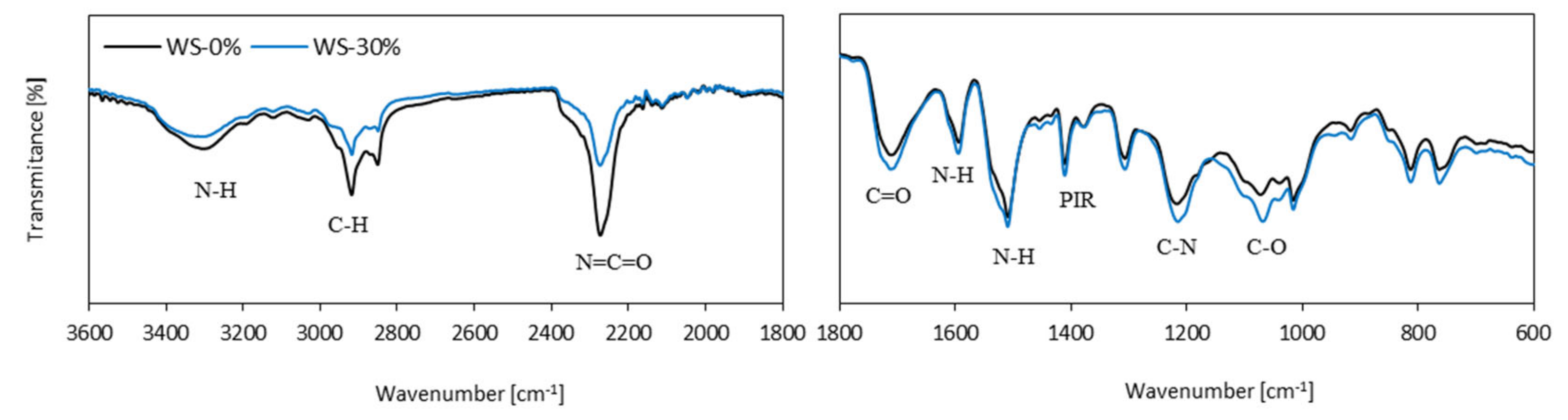
3.2. FTIR Analysis of PUR Foams
3.3. Foaming Kinetic of PUR Foams
3.4. Apparent Density of Pur Foams
3.5. Cellular Morphology of PUR Foams
3.6. Thermal Conductivity of Pur Foams
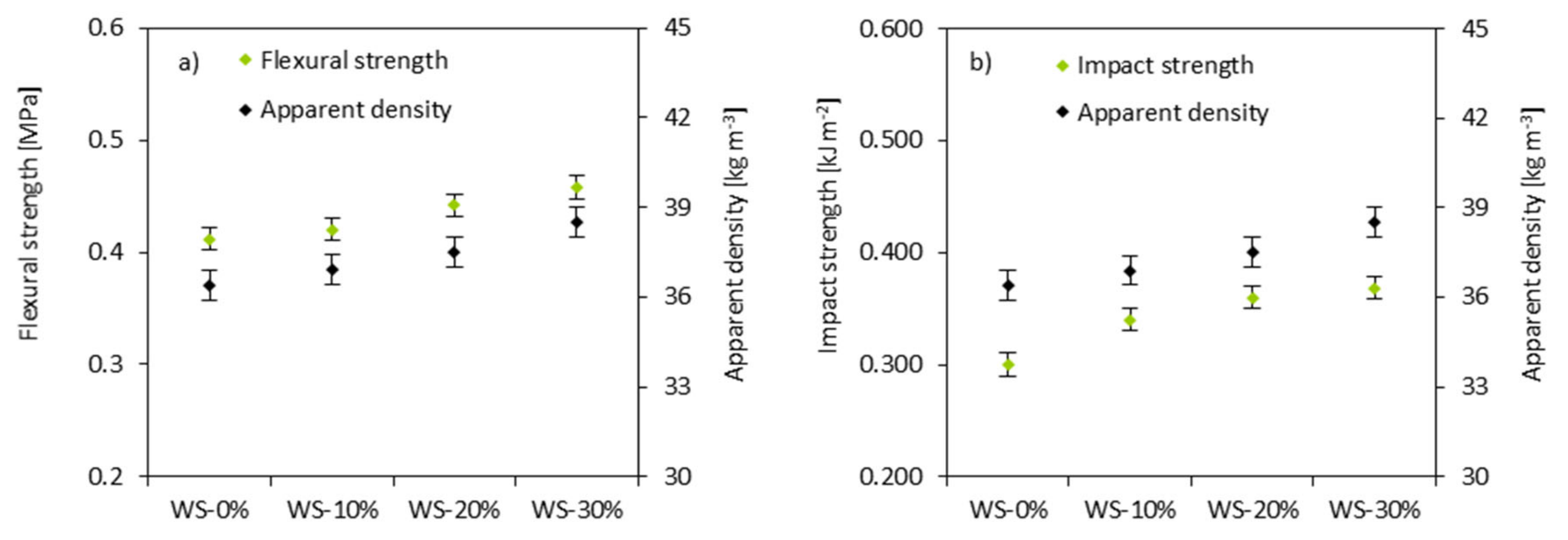
3.7. Mechanical Properties
3.8. Dynamic Mechanical Analysis (DMA) of PUR Foams
3.9. Thermogravimetric Analysis (TGA) of PUR Foams
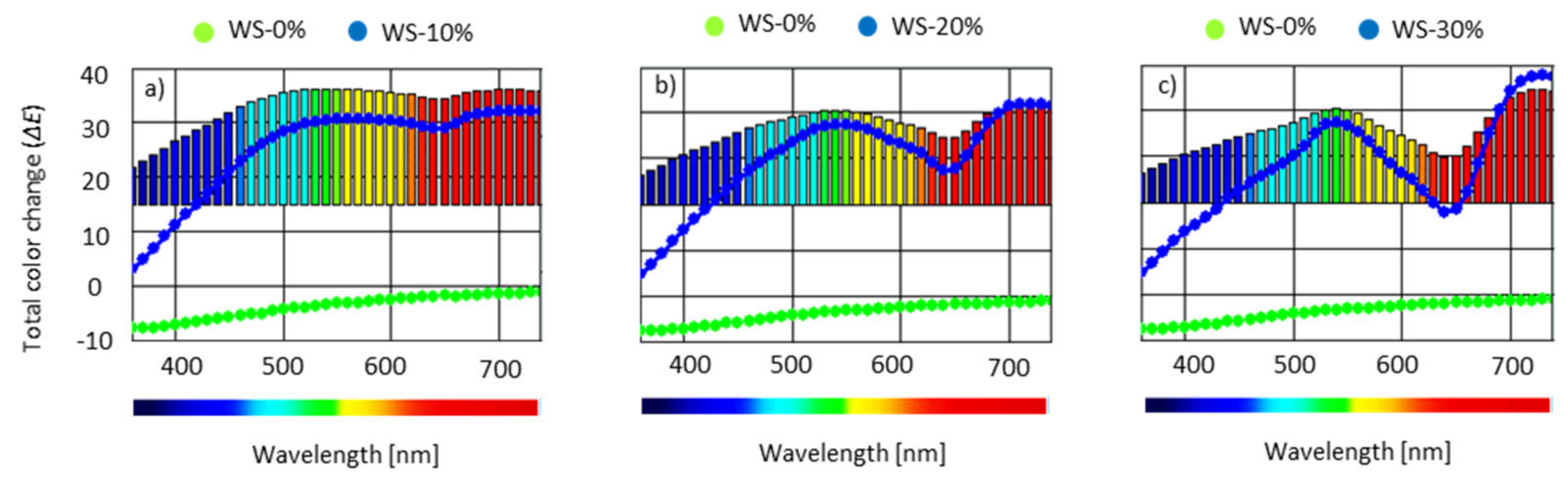
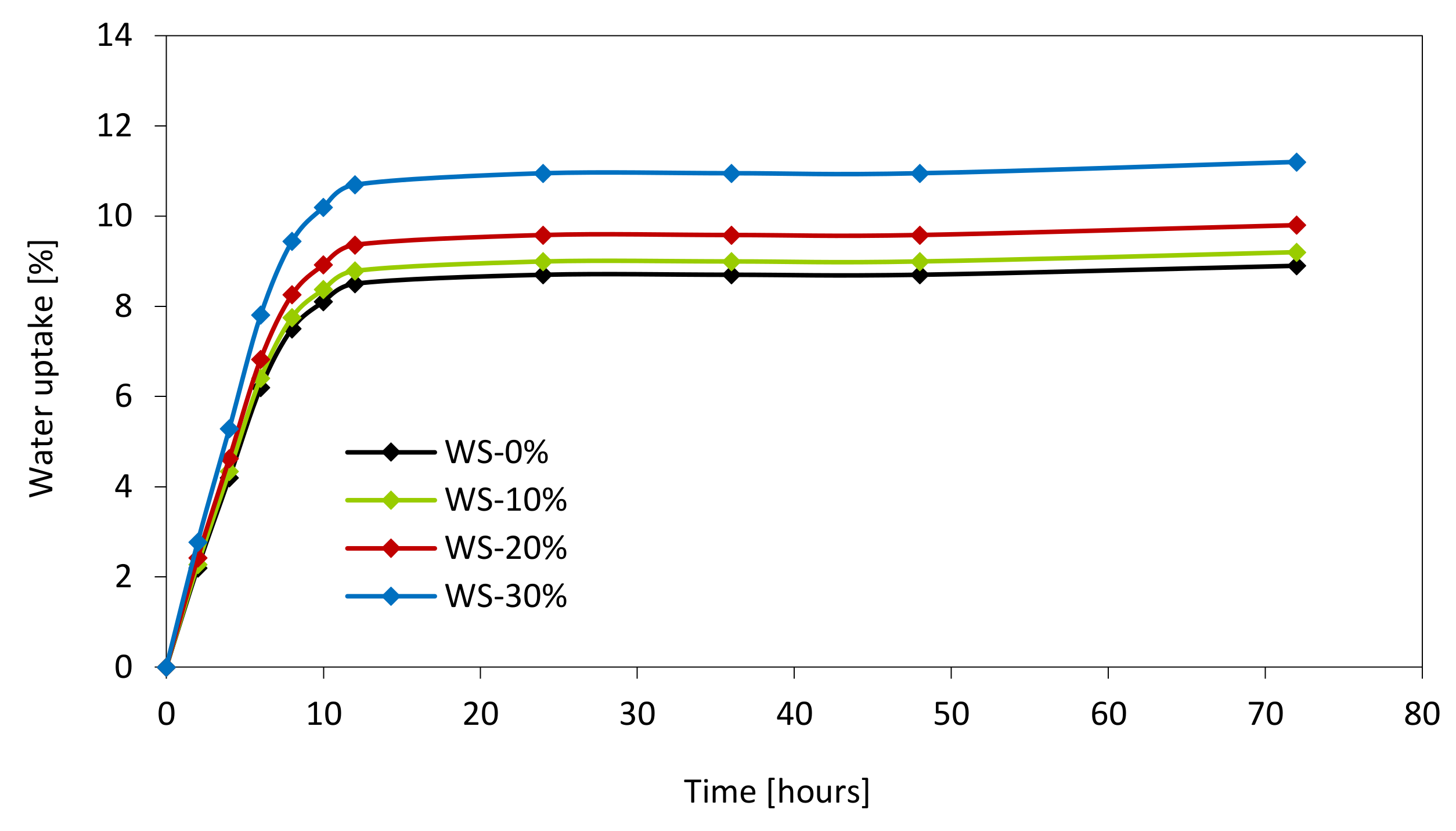
3.10. Color Analysis, Water Uptake and Dimensional Stability of PUR Foams
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czupryński, B.; Apiecionek, Ł. The use of waste from the production of rapeseed oil for obtaining of new polyurethane composites. Polymers 2019, 11, 1431. [Google Scholar] [CrossRef] [Green Version]
- Haponiuk, J.T.; Hejna, A.; Piszczyk, Ł.; Datta, J.; Formela, K. Renewable Resources for Polyurethanes and Polyurethane Composites: A Review. In Chemical Engineering of Polymers; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 407–432. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Oenothera biennis seed oil as an alternative raw material for production of bio-polyol for rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower oil based biodegradable hyperbranched polyurethane as a thin film material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Kairytė, A.; Vėjelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crops Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Fourati, Y.; Hassen, R.B.; Bayramoğlu, G.; Boufi, S. A one step route synthesis of polyurethane newtwork from epoxidized rapeseed oil. Prog. Org. Coatings 2017, 105, 48–55. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Spent coffee grounds as a renewable source for ecopolyols production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Huang, G.; Wang, P. Effects of preparation conditions on properties of rigid polyurethane foam composites based on liquefied bagasse and jute fibre. Polym. Test. 2017, 60, 266–273. [Google Scholar] [CrossRef]
- Kang, J.; Chen, W.; Yao, Y.; Jin, Y.; Cheng, X.; Lü, Q. Optimisation of Bio-polyol Production from Cassava Residue Using Ethylene Glycol as the Liquefaction Reagent. J. Wuhan Univ. Technol. Sci. Ed. 2019, 34, 945–949. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.-K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jiang, J.; Hse, C.Y.; Shupe, T.F. Preparation of polyurethane foams using fractionated products in liquefied wood. J. Appl. Polym. Sci. 2014, 131, 1–7. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.T.; Piszczyk, Ł. Biopolyols obtained via crude glycerol-based liquefaction of cellulose: their structural, rheological and thermal characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Zhang, J.; Zhao, J.; Tu, S. Liquefaction of bamboo shoot shell for the production of polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crops Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Sun, R.-C. Lignin-Based Rigid Polyurethane Foam Reinforced with Pulp Fiber: Synthesis and Characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Ertaş, M.; Fidan, M.S.; Alma, M.H. Preparation and characterization of biodegradable rigid polyurethane foams from the liquefied eucalyptus and pine woods. Wood Res. 2014, 59, 97–108. [Google Scholar]
- Domingos, I.J.; Fernandes, A.P.; Ferreira, J.; Cruz-Lopes, L.; Esteves, B.M. Polyurethane foams from liquefied Eucalyptus globulus branches. BioResources 2019, 14, 31–43. [Google Scholar]
- Kosmela, P.; Hejna, A.; Suchorzewski, J.; Piszczyk, Ł.; Haponiuk, J.T. Study on the Structure-Property Dependences of Rigid PUR-PIR Foams Obtained from Marine Biomass-Based Biopolyol. Materials 2020, 13, 1257. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S. Polyols and Rigid Polyurethane Foams from Cashew Nut Shell Liquid. J. Polym. Environ. 2012, 20, 647–658. [Google Scholar] [CrossRef]
- Gandhi, T.S.; Patel, M.R.; Dholakiya, B.Z. Mechanical, thermal and fire properties of sustainable rigid polyurethane foam derived from cashew nut shell liquid. Int. J. Plast. Technol. 2015, 19, 30–46. [Google Scholar] [CrossRef]
- De Luca Bossa, F.; Santillo, C.; Verdolotti, L.; Campaner, P.; Minigher, A.; Boggioni, L.; Losio, S.; Coccia, F.; Iannace, S.; Lama, G.C. Greener Nanocomposite Polyurethane Foam Based on Sustainable Polyol and Natural Fillers: Investigation of Chemico-Physical and Mechanical Properties. Materials 2020, 13, 211. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Kosugi, R.; Hatakeyama, T. Thermal properties of lignin-and molasses-based polyurethane foams. J. Therm. Anal. Calorim. 2008, 92, 419–424. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Improved lignin polyurethane properties with lewis acid treatment. ACS Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef] [Green Version]
- Passoni, V.; Scarica, C.; Levi, M.; Turri, S.; Griffini, G. Fractionation of Industrial Softwood Kraft Lignin: Solvent Selection as a Tool for Tailored Material Properties. ACS Sustain. Chem. Eng. 2016, 4, 2232–2242. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- MacLeod, I.T.; Scully, A.D.; Ghiggino, K.P.; Ritchie, P.J.A.; Paravagna, O.M.; Leary, B. Photodegradation at the wood-clearcoat interface. Wood Sci. Technol. 1995, 29, 183–189. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood—The state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Potter, D.; Gao, F.; Baggett, S.; McKenna, J.R.; McGranahan, G.H. Defining the sources of Paradox: DNA sequence markers for North American walnut (Juglans L.) species and hybrids. Sci. Hortic. 2002, 94, 157–170. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut ( Juglans regia L.) shell pyroligneous acid: chemical constituents and functional applications. RSC Adv. 2018, 8, 22376–22391. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar]
- Sarsari, N.A.; Pourmousa, S.; Tajdini, A. Physical and mechanical properties of walnut shell flour-filled thermoplastic starch composites. BioResources 2016, 11, 6968–6983. [Google Scholar]
- Ayrilmis, N.; Kaymakci, A.; Ozdemir, F. Physical, mechanical, and thermal properties of polypropylene composites filled with walnut shell flour. J. Ind. Eng. Chem. 2013, 19, 908–914. [Google Scholar] [CrossRef]
- Yu, F.; Saha, P.; Suh, P.W.; Kim, J.K. Green polyurethane from dimer acid based polyether polyols: Synthesis and characterization. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [Green Version]
- Cichosz, S.; Masek, A. Drying of the Natural Fibers as A Solvent-Free Way to Improve the Cellulose-Filled Polymer Composite Performance. Polymers 2020, 12, 484. [Google Scholar] [CrossRef] [Green Version]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Superiority of Cellulose Non-Solvent Chemical Modification over Solvent-Involving Treatment: Solution for Green Chemistry (Part I). Materials 2020, 13, 2552. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of different heat treatment temperatures on the chemical composition and structure of chinese fir wood. BioResources 2016, 11, 4006–4016. [Google Scholar] [CrossRef] [Green Version]
- Hejna, A.; Barczewski, M.; Andrzejewski, J.; Kosmela, P.; Piasecki, A.; Szostak, M.; Kuang, T. Rotational Molding of Linear Low-Density Polyethylene Composites Filled with Wheat Bran. Polymers 2020, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Proc. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Production of polyols via direct hydrolysis of kraft lignin: Effect of process parameters. Bioresour. Technol. 2013, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Faris, A.H.; Ibrahim, M.N.M.; Rahim, A.A.; Hussin, M.H.; Brosse, N. Preparation and characterization of lignin polyols from the residues of oil palm empty fruit bunch. BioResources 2015, 10, 7339–7352. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, J.; Yan, N. Producing bark-based polyols through liquefaction: Effect of liquefaction temperature. ACS Sustain. Chem. Eng. 2013, 1, 534–540. [Google Scholar] [CrossRef]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of lignin by polyethyleneglycol and glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef]
- Wong, C.S.; Badri, K.H. Chemical Analyses of Palm Kernel Oil-Based Polyurethane Prepolymer. Mater. Sci. Appl. 2012, 03, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Kim, Y.; Elsayed, I.; Taylor, M.; Eberhardt, T.L.; Hassan, E.B.; Shmulsky, R. Rigid polyurethane foams containing lignin oxyalkylated with ethylene carbonate and polyethylene glycol. Ind. Crops Prod. 2019, 141. [Google Scholar] [CrossRef]
- Stirna, U.; Beverte, I.; Yakushin, V.; Cabulis, U. Mechanical properties of rigid polyurethane foams at room and cryogenic temperatures. J. Cell. Plast. 2011, 47, 337–355. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Ionomers for proton exchange membrane fuel cells with sulfonic acid groups on the end-groups: Novel branched poly(ether-ketone)s. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 2008, 49, 511–512. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Kodippili, G.; Kinyanjui, J.M.; Benincasa, F.; Sapochak, L. FTIR analysis of thermally processed PU foam. Polym. Degrad. Stab. 2005, 87, 555–561. [Google Scholar] [CrossRef]
- Ma, S.R.; Shi, L.Y.; Feng, X.; Yu, W.J.; Lu, B. Graft modification of ZnO nanoparticles with silane coupling agent KH570 in mixed solvent. J. Shanghai Univ. 2008, 12, 278–282. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open cell semi-rigid polyurethane foams synthesized using palm oil-based bio-polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Hayati, A.N.; Evans, D.A.C.; Laycock, B.; Martin, D.J.; Annamalai, P.K. A simple methodology for improving the performance and sustainability of rigid polyurethane foam by incorporating industrial lignin. Ind. Crops Prod. 2018, 117, 149–158. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Xu, M.; Li, B. Synthesis of a novel phosphorus and nitrogen-containing flame retardant and its application in rigid polyurethane foam with expandable graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Zhu, M. Flame retardant, mechanical and thermal insulating properties of rigid polyurethane foam modified by nano zirconium amino-tris-(methylenephosphonate) and expandable graphite. Polym. Degrad. Stab. 2019, 170, 108997. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, M.C.; Kim, H.D.; Park, H.C.; Jeong, H.M.; Yoon, K.S.; Kim, B.K. Nanoclay reinforced rigid polyurethane foams. J. Appl. Polym. Sci. 2010, 117, 1992–1997. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguścik, M. Innovative porous polyurethane-polyisocyanurate foams based on rapeseed oil and modified with expandable graphite. Ind. Crops Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Wang, J.; Zhang, B. Synergistic flame-retardant effect of expandable graphite and phosphorus-containing compounds for epoxy resin: Strong bonding of different carbon residues. Polym. Degrad. Stab. 2016, 128, 89–98. [Google Scholar] [CrossRef]
- Kurańska, M.; Barczewski, M.; Uram, K.; Lewandowski, K.; Prociak, A.; Michałowski, S. Basalt waste management in the production of highly effective porous polyurethane composites for thermal insulating applications. Polym. Test. 2019, 76, 90–100. [Google Scholar] [CrossRef]
- Barczewski, M.; Kurańska, M.; Sałasińska, K.; Michałowski, S.; Prociak, A.; Uram, K.; Lewandowski, K. Rigid polyurethane foams modified with thermoset polyester-glass fiber composite waste. Polym. Test. 2020, 81, 106190. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Przybysz, M.; Ryl, J.; Saeb, M.R.; Piszczyk, Ł. Structural, thermal and physico-mechanical properties of polyurethane/brewers’ spent grain composite foams modified with ground tire rubber. Ind. Crops Prod. 2017, 108, 844–852. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg filler as a natural compound for the production of polyurethane composite foams with antibacterial and anti-aging properties. Polym. Test. 2020, 106479. [Google Scholar] [CrossRef]
- Federation of European Rigid Polyurethane Foam Associations. Thermal Insulation Materials Made of Rigid Polyurethane Foam (PUR/PIR) Properties-Manufacture; Federation of European Rigid Polyurethane Foam Associations: Brussels, Belgium, 2006. [Google Scholar]
- Wu, L.; Gemert, J.V.; Camargo, R.E. Rheology Study in Polyurethane Rigid Foams; Huntsman Corporation: Auburn Hills, MI, USA, 2008. [Google Scholar]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Producing lignin-based polyols through microwave-assisted liquefaction for rigid polyurethane foam production. Materials 2015, 8, 586–599. [Google Scholar] [CrossRef] [Green Version]
- Jasiūnas, L.; McKenna, S.T.; Bridžiuvienė, D.; Miknius, L. Mechanical, Thermal Properties and Stability of Rigid Polyurethane Foams Produced with Crude-Glycerol Derived Biomass Biopolyols. J. Polym. Environ. 2020. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Calvo-Correas, T.; Peña-Rodríguez, C.; Corcuera, M.A.; Eceiza, A. Properties of flexible polyurethane foams containing isocyanate functionalized kraft lignin. Ind. Crops Prod. 2017, 100, 51–64. [Google Scholar] [CrossRef]
- Dominic C.D., M.; Joseph, R.; Begum, P.M.S.; Joseph, M.; Padmanabhan, D.; Morris, L.A.; Kumar, A.S.; Formela, K. Cellulose Nanofibers Isolated from the Cuscuta Reflexa Plant as a Green Reinforcement of Natural Rubber. Polymers 2020, 12, 814. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Li, Y. Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, Y.; Wu, Q.; Zeng, J. Development of high-performance biodegradable rigid polyurethane foams using all bioresource-based polyols: Lignin and soy oil-derived polyols. Int. J. Biol. Macromol. 2018, 115, 786–791. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Preparation of bio-based rigid polyurethane foam using hydrolytically depolymerized Kraft lignin via direct replacement or oxypropylation. Eur. Polym. J. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Członka, S.; Sienkiewicz, N.; Strąkowska, A.; Strzelec, K. Keratin feathers as a filler for rigid polyurethane foams on the basis of soybean oil polyol. Polym. Test. 2018, 72, 32–45. [Google Scholar] [CrossRef]
- Kairytė, A.; Kirpluks, M.; Ivdre, A.; Cabulis, U.; Vėjelis, S.; Balčiūnas, G. Paper waste sludge enhanced eco-efficient polyurethane foam composites: Physical–mechanical properties and microstructure. Polym. Compos. 2018, 39, 1852–1860. [Google Scholar] [CrossRef]
- Structural Insulated Panels (SIPS): BASF Polyurethanes. Available online: http://www.polyurethanes.basf.de/pu/Great_Britain/insulation/SIPS (accessed on 11 June 2020).
- Badri, K.H.; Ahmad, S.H.; Zakaria, S. ProductionofaHigh-FunctionalityRBDPalmKernelOil-BasedPolyesterPolyol. J. Appl. Polym. Sci. 2001, 81, 384–389. [Google Scholar] [CrossRef]







| Component | WS-0% | WS-10% | WS-20% | WS-30% |
|---|---|---|---|---|
| Parts by weight (wt%) | ||||
| STEPANPOL PS-2352 | 100 | 90 | 80 | 70 |
| PUROCYN B | 160 | 160 | 160 | 160 |
| Kosmos 75 | 6 | 6 | 6 | 6 |
| Kosmos 33 | 0.8 | 0.8 | 0.8 | 0.8 |
| Tegostab B8513 | 2.5 | 2.5 | 2.5 | 2.5 |
| Water | 0.5 | 0.5 | 0.5 | 0.5 |
| Pentane/cyclopentane | 11 | 11 | 11 | 11 |
| WS-based polyol | 0 | 10 | 20 | 30 |
| Viscosiy [mPa s] | Hydroxyl Number [mg KOH/g] | Water Content [%] | Molecular Weight (Mw) [Da] | Liquefaction Ratio [%] | |
|---|---|---|---|---|---|
| WS-based polyol | 2550 | 340 | 0.8 | 420 | 87.2 |
| Petrochemical polyol | 2000–4500 | 230–250 | 0.2 | 468 | N/D |
| WS-0% | WS-10% | WS-20% | WS-30% | |
|---|---|---|---|---|
| Start time | 55 ± 3 | 61 ± 1 | 64 ± 2 | 70 ± 3 |
| Growth time | 430 ± 8 | 510 ± 9 | 515 ± 9 | 590 ± 10 |
| Tack-free time | 355 ± 9 | 420 ± 8 | 450 ± 9 | 520 ± 8 |
| Tmax | 150 | 125 | 120 | 105 |
| Height | 205 | 180 | 160 | 145 |
| WS-0% | WS-10% | WS-20% | WS-30% | |
|---|---|---|---|---|
| Cell size [µm] | 345 ± 9 | 320 ± 8 | 310 ± 6 | 280 ± 8 |
| Closed-cell content [%] | 92.1 | 89.3 | 86.4 | 80.2 |
| Apparent density [kg m−3] | 36 ± 1 | 37 ± 1 | 38 ± 2 | 39 ± 1 |
| Thermal conductivity [W m−1 K−1] | 0.024 | 0.026 | 0.029 | 0.032 |
| Sample Code | Colorimetric Parameters | |||
|---|---|---|---|---|
| L* | a* | b* | ΔE* | |
| WS-0% | 60.25 | 22.45 | −5.10 | 0 |
| WS-10% | 45.10 | 35.50 | −2.10 | 29.85 |
| WS-20% | 40.20 | 40.20 | −0.60 | 29.94 |
| WS-30% | 24.25 | 48.50 | 1.40 | 39.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Członka, S.; Strąkowska, A.; Kairytė, A. Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams. Materials 2020, 13, 2687. https://doi.org/10.3390/ma13122687
Członka S, Strąkowska A, Kairytė A. Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams. Materials. 2020; 13(12):2687. https://doi.org/10.3390/ma13122687
Chicago/Turabian StyleCzłonka, Sylwia, Anna Strąkowska, and Agnė Kairytė. 2020. "Application of Walnut Shells-Derived Biopolyol in the Synthesis of Rigid Polyurethane Foams" Materials 13, no. 12: 2687. https://doi.org/10.3390/ma13122687