Investigation of Fresh Gastric Normal and Cancer Tissues Using Terahertz Time-Domain Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Details
2.3. Data Analysis
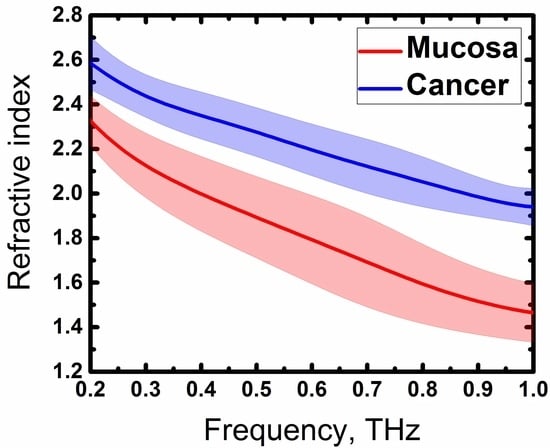
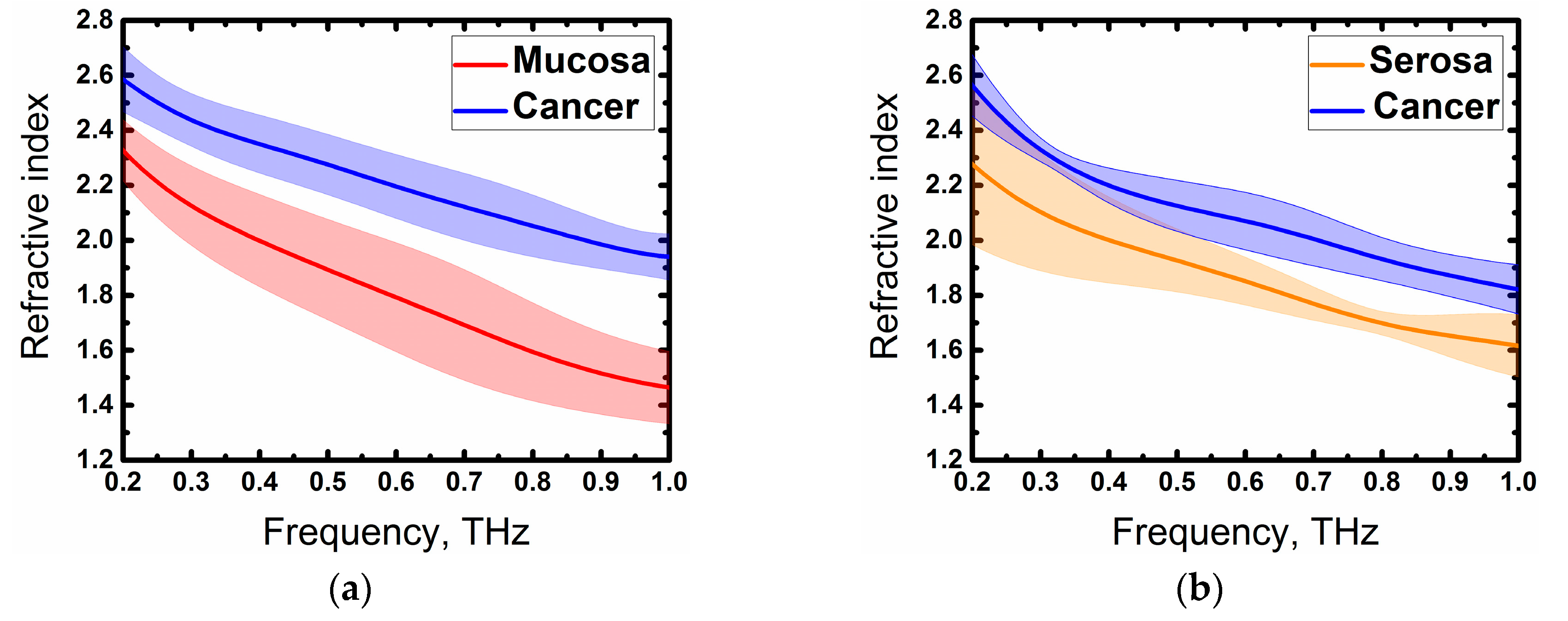
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotoda, T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-C.; Xu, J. Introduction to THz wave photonics; Springer: Berlin, Germany, 2010. [Google Scholar]
- Son, J.-H. Terahertz Biomedical Science and Technology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Smolyanskaya, O.A.; Chernomyrdin, N.V.; Konovko, A.A.; Zaytsev, K.I.; Ozheredov, I.A.; Cherkasova, O.P.; Nazarov, M.M.; Guillet, J.-P.; Kozlov, S.A.; Kistenev, Y.V. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog. Quantum Electron. 2018, 62, 1–77. [Google Scholar] [CrossRef]
- Danciu, M.; Alexa-Stratulat, T.; Stefanescu, C.; Dodi, G.; Tamba, B.I.; Mihai, C.T.; Stanciu, G.D.; Luca, A.; Spiridon, I.A.; Ungureanu, L.B. Terahertz Spectroscopy and Imaging: A Cutting-Edge Method for Diagnosing Digestive Cancers. Materials 2019, 12, 1519. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.B.; Park, C.H.; Kim, H.; Kim, S.-H.; Lee, G.M.; Noh, S.K.; Jeon, T.-I.; Son, J.-H.; Huh, Y.-M.; Haam, S. Feasibility of terahertz reflectometry for discrimination of human early gastric cancers. Biomed. Opt. Express 2015, 6, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Wahaia, F.; Kasalynas, I.; Seliuta, D.; Molis, G.; Urbanowicz, A.; Silva, C.D.C.; Carneiro, F.; Valusis, G.; Granja, P.L. Terahertz spectroscopy for the study of paraffin-embedded gastric cancer samples. J. Mol. Struct. 2015, 1079, 391–395. [Google Scholar] [CrossRef]
- Hou, D.; Li, X.; Cai, J.; Ma, Y.; Kang, X.; Huang, P.; Zhang, G. Terahertz spectroscopic investigation of human gastric normal and tumor tissues. Phys. Med. Biol. 2014, 59, 5423. [Google Scholar] [CrossRef]
- Goryachuk, A.; Simonova, A.; Khodzitsky, M.; Borovkova, M.; Khamid, A. Gastrointestinal cancer diagnostics by terahertz time domain spectroscopy. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017. [Google Scholar]
- Cao, Y.; Huang, P.; Li, X.; Ge, W.; Hou, D.; Zhang, G. Terahertz spectral unmixing based method for identifying gastric cancer. Phys. Med. Biol. 2018, 63, 035016. [Google Scholar] [CrossRef]
- Ji, Y.B.; Lee, E.S.; Kim, S.-H.; Son, J.-H.; Jeon, T.-I. A miniaturized fiber-coupled terahertz endoscope system. Opt. Express 2009, 17, 17082–17087. [Google Scholar] [CrossRef]
- Doradla, P.; Alavi, K.; Joseph, C.S.; Giles, R.H. Single-channel prototype terahertz endoscopic system. J. Biomed. Opt. 2014, 19, 080501. [Google Scholar] [CrossRef] [Green Version]
- Doradla, P.; Joseph, C.; Giles, R.H. Terahertz endoscopic imaging for colorectal cancer detection: Current status and future perspectives. World J. Gastrointest. Endosc. 2017, 9, 346. [Google Scholar] [CrossRef]
- Hwang, S.I.; Kim, H.O.; Yoo, C.H.; Shin, J.H.; Son, B.H. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg. Endosc. 2009, 23, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Grigorev, R.O.; Khodzitsky, M.K.; Zhang, T.; Demchenko, P.S. The study of optical properties and spectral characteristics of brain glioblastoma and lung adenocarcinoma. In Proceedings of the Optics in Health Care and Biomedical Optics VIII, Beijing, China, 23 October 2018; p. 1082032. [Google Scholar]
- Globus, T.; Theodorescu, D.; Frierson, H.; Khromova, T.; Woolard, D. Terahertz spectroscopic characterization of cancer cells. In Proceedings of the Advanced Biomedical and Clinical Diagnostic Systems III, San Jose, CA, USA, 1 April 2005; pp. 233–240. [Google Scholar]
- Naftaly, M. Terahertz Metrology; Artech House: Norwood, MA, USA, 2015. [Google Scholar]
- Vázquez-Cabo, J.; Chamorro-Posada, P.; Fraile-Peláez, F.J.; Rubiños-López, Ó.; López-Santos, J.M.; Martín-Ramos, P. Windowing of THz time-domain spectroscopy signals: A study based on lactose. Opt. Commun. 2016, 366, 386–396. [Google Scholar] [CrossRef]
- Ro/nne, C.; Thrane, L.; Astrand, P.-O.; Wallqvist, A.; Mikkelsen, K.V.; Keiding, S.R. Investigation of the temperature dependence of dielectric relaxation in liquid water by THz reflection spectroscopy and molecular dynamics simulation. J. Chem. Phys. 1997, 107, 5319–5331. [Google Scholar] [CrossRef] [Green Version]
- Gavdush, A.A.; Chernomyrdin, N.V.; Malakhov, K.M.; Beshplav, S.-I.T.; Dolganova, I.N.; Kosyrkova, A.V.; Nikitin, P.V.; Musina, G.R.; Katyba, G.M.; Reshetov, I.V. Terahertz spectroscopy of gelatin-embedded human brain gliomas of different grades: A road toward intraoperative THz diagnosis. J. Biomed. Opt. 2019, 24, 027001. [Google Scholar] [CrossRef]
- Reid, C.B.; Fitzgerald, A.; Reese, G.; Goldin, R.; Tekkis, P.; O’Kelly, P.S.; Pickwell-MacPherson, E.; Gibson, A.P.; Wallace, V.P. Terahertz pulsed imaging of freshly excised human colonic tissues. Phys. Med. Biol. 2011, 56, 4333. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, P.C.; Pickwell-MacPherson, E.; Provenzano, E.; Pinder, S.E.; Purushotham, A.D.; Pepper, M.; Wallace, V.P. Terahertz pulsed spectroscopy of freshly excised human breast cancer. Opt. Express 2009, 17, 12444–12454. [Google Scholar] [CrossRef]
- Van Exter, M.; Fattinger, C.; Grischkowsky, D. Terahertz time-domain spectroscopy of water vapor. Opt. Lett. 1989, 14, 1128–1130. [Google Scholar] [CrossRef]
- Reynolds, T.Y.; Rockwell, S.; Glazer, P.M. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996, 56, 5754–5757. [Google Scholar]
- Wahaia, F.; Kasalynas, I.; Seliuta, D.; Molis, G.; Urbanowicz, A.; Silva, C.D.C.; Carneiro, F.; Valusis, G.; Granja, P.L. Study of paraffin-embedded colon cancer tissue using terahertz spectroscopy. J. Mol. Struct. 2015, 1079, 448–453. [Google Scholar] [CrossRef]
- Joseph, C.S.; Patel, R.; Neel, V.A.; Giles, R.H.; Yaroslavsky, A.N. Imaging of ex vivo nonmelanoma skin cancers in the optical and terahertz spectral regions optical and terahertz skin cancers imaging. J. Biophotonics 2014, 7, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Eroschenko, V.P. DiFiore’s Atlas of Histology with Functional Correlations; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Fan, S.; Ung, B.; Parrott, E.P.; Pickwell-MacPherson, E. Gelatin embedding: A novel way to preserve biological samples for terahertz imaging and spectroscopy. Phys. Med. Biol. 2015, 60, 2703. [Google Scholar] [CrossRef]





| Case | Age | Gender | Tissues | Pathology (TNM) |
|---|---|---|---|---|
| 1 | 71 | Female | Mucosa and serosa | pT4aN2M0G3 |
| 2 | 74 | Female | Mucosa and serosa | pT3N0M0G2 |
| 3 | 74 | Male | Mucosa only | pT2N0M0G2 |
| 4 | 83 | Female | Serosa only | pT3N3bM0G3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorev, R.; Kuzikova, A.; Demchenko, P.; Senyuk, A.; Svechkova, A.; Khamid, A.; Zakharenko, A.; Khodzitskiy, M. Investigation of Fresh Gastric Normal and Cancer Tissues Using Terahertz Time-Domain Spectroscopy. Materials 2020, 13, 85. https://doi.org/10.3390/ma13010085
Grigorev R, Kuzikova A, Demchenko P, Senyuk A, Svechkova A, Khamid A, Zakharenko A, Khodzitskiy M. Investigation of Fresh Gastric Normal and Cancer Tissues Using Terahertz Time-Domain Spectroscopy. Materials. 2020; 13(1):85. https://doi.org/10.3390/ma13010085
Chicago/Turabian StyleGrigorev, Roman, Anna Kuzikova, Petr Demchenko, Artem Senyuk, Anna Svechkova, Abdo Khamid, Alexander Zakharenko, and Mikhail Khodzitskiy. 2020. "Investigation of Fresh Gastric Normal and Cancer Tissues Using Terahertz Time-Domain Spectroscopy" Materials 13, no. 1: 85. https://doi.org/10.3390/ma13010085