Determining the Degree of Admixing Rate of the Base Material and the Melting Efficiency in Single-Bead Surface Welds Using Different Methods, Including New Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Materials and Samples
2.2. Research Methods
- -
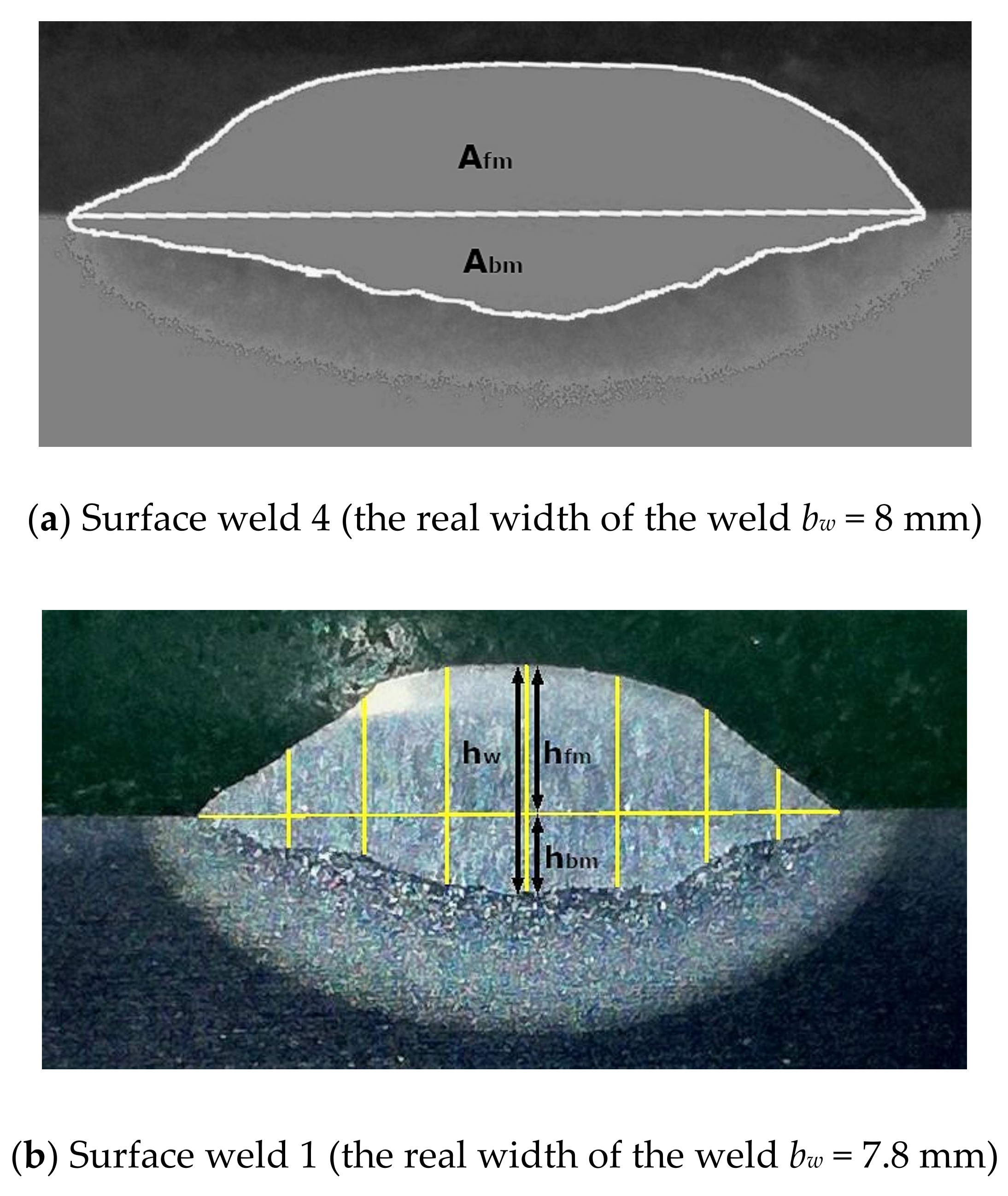
- From the ratio of the areas in the metallographic cross-section, which in a single-bead surface weld belong to the base material and to the filler material; the areas were measured with the “imageJ” computer program.
- -
- From the ratio of the heights on the metallographic cross-section, which in a single-bead surface weld belong to the base material and to the filler material (a new approach); the heights were measured with the “imageJ” computer program.
- -
- By calculating from the chemical composition of the filler metal and the chemical composition of the areas, which in a single-bead surface weld belong to the filler material (four measurements) and to the base material (two measurements).
- -
- By calculating from the welding parameters and the material constants.
3. Results and Discussion
3.1. Admixing Rate from the Ratio of the Areas
3.2. Admixing Rate from the Ratio of the Heights
3.3. Admixing Rate from the Chemical Composition of the Surface Weld
3.4. Summary of the Experimental Determination of the Admixing
3.5. Theoretical Determination of the Admixing Rate from the Welding Parameters
3.5.1. Determination of the Melting Efficiency from the Welding Parameters
3.5.2. Determining the Melting Efficiency from the Heat Balance of the Welding Process
4. Conclusions
- -
- The weld pool is very intensely and well mixed during arc surfacing with coated electrodes. This is proven by the very small deviation in the chemical compositions of the different regions in the single-bead surface welds.
- -
- The admixing rate of the base material can be determined in various ways: from the ratio of the areas on the cross-section of the surface weld; from the ratio of the heights on the cross-section of the surface weld; with EDS analyses of different areas of the surface weld; and theoretically from the welding parameters, the correctly determined heat and melting efficiency, the materials constants and the dimensions of the weld.
- -
- The admixing rate determined from the ratio of the heights is accurate only if there are many measurements made in the direction from the middle towards both edges of the surface weld in each individual cross-section, as the ratio of the heights changes in the same direction. Similarly, in the case of the EDS chemical analyses, it is necessary to analyse the chemical composition of the surface weld cross-section at various points in the region that belongs to the base material and in the region that belongs to the filler material. For each cross-section, it is useful to calculate the admixing rate of all the measured values and of the two lowest values separately, and then finally calculate the average admixing rate of all these values. The results show that this method leads to a very accurately determined admixing rate.
- -
- The average admixing rate determined from eight cross-sections and with different experimental methods is the same and equals Dm ≈ 30%, regardless of the deviations in individual surface welds. This means that for an accurate determination of the degree of admixing of the base material in an individual surface weld, regardless of the method used, it is necessary to analyse more metallographic cross-sections, taken from the entire length of the weld at various distances from the start to the end of the weld.
- -
- The admixing rate in each surface weld estimated from the ratio of areas was checked and additionally confirmed by comparing the calculated and measured values of chromium in the surface welds. These values match with a 90% to 99% accuracy.
- -
- The average melting efficiency of arc surface welding with a coated electrode is ηm = 0.30, and this matches well with the value reported in [26]. The average degree of admixing calculated using Equation (6) with the average melting efficiency ηm = 0.3 and the experimentally used welding parameters and material constants is Dm = 32%, which is 94% accurate with respect to the experimentally measured value.
- -
- The experimentally determined admixing rate Dm = 30% shows, from Equation (6), that the melting efficiency in our case of surface welding was ηm = 0.29. Irrespective of the value (ηm = 0.29 or ηm = 0.30), the result shows the credibility of Equation (6) as well as a correct determination of the values of all the material and process quantities used in the calculations.
- -
- The melting efficiency can be estimated using various equations on the basis of the welding parameters, the geometry of the weld, the material quantities and the admixing rate. The melting efficiency can also be estimated from a good knowledge of the heat balance of the welding process. It is the sum of the melting efficiency of the base material ηmbm and the melting efficiency of all the metallic components in the electrode ηme:
- -
- Rykalin’s method only gives a melting efficiency of 40% for the heat that is used to melt the base material. Therefore, the values of the melting efficiency are low and do not apply to the surface welding with the filler material. If this low value of the melting efficiency ηRykalin is added to the melting efficiency for all the metallic components in the electrode ηme, calculated from the known heat balance, and if we also take into account the deposition efficiency of the filler metal ηdep during the melting of the coated electrode, we obtain a correct value for the melting efficiency of the surface arc welding of thick and medium-thick plates with the newly developed equation:
- -
- A new equation for the estimation of the melting efficiency of arc welding with a coated electrode from the welding parameters and the material constants was also developed:
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Castro, R.; de Cadenet, J.J. Welding Metallurgy of Stainless and Heat-Resisting Steels; Cambridge University Press: Cambridge, UK, 1975; pp. 92–93, 159–160. [Google Scholar]
- Hrivñák, I. Theory of Weldability of Metals and Alloys; Elsevier: London, UK, 1992; p. 82. [Google Scholar]
- Kiser, S.D.; Case, C.W.; Devletian, J.H.; King, J.F.; Lippold, J.C.; Olson, D.L.; Schmidt, C.L. Clad and Dissimilar Metals. In Welding Handbook: Materials and Applications—Part 2, 8th ed.; Oates, R.W., Saitta, A.M., Eds.; AWS: Miami, FL, USA, 1998; Volume 4, Chapter 6; pp. 335–338. [Google Scholar]
- Lancaster, J.F. Metallurgy of Welding, 6th ed.; Abington Publishing: Cambridge, UK, 1999; pp. 192–193. [Google Scholar]
- Kou, S. Welding Metallurgy, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 243–259. [Google Scholar]
- Du Pont, J.N.; Marder, A.R. Dilution in Single Pass Arc Welds. Metall. Mater. Trans. B 1996, 27B, 481–489. [Google Scholar] [CrossRef]
- Du Pont, J.N. Dilution in Fusion Welding. In ASM Handbook: Welding Fundamentals and Processes; Lienert, T., Babu, S., Siewert, T., Acoff, V., Eds.; ASM: Materials Park, OH, USA, 2011; Volume 6A, pp. 115–121. [Google Scholar]
- Probst, H. Kompendium der Schweißtechnik-Band 2: Schweißmetallurgie; DVS-Verlag GmbH: Düsseldorf, Germany, 1997; pp. 11–12, 229–232. [Google Scholar]
- Pohle, C. Schweiβen von Werkstoffkombinationen; DVS-Verlag GmbH: Düsseldorf, Germany, 1999; pp. 143–145, 193–194. [Google Scholar]
- Szymura, M.; Czuprynski, A.; Rožanski, M. Research on the properties of high chromium cast iron overlay welds deposited by tubular electrodes. Weld. Technol. Rev. 2018, 90, 26–30. [Google Scholar] [CrossRef]
- Riabcew, L.A.; Babinec, A.A.; Lentjugow, L.P. The effect of the parameters of submerged arc surfacing with oscillating electrode on a padding weld forming, depth of fusion penetration and the content of parent metal in a weld. Weld. Technol. Rev. 2018, 90, 26–30. [Google Scholar]
- Killing, R. Angewandte Schweißmetallurgie; DVS-Verlag GmbH: Düsseldorf, Germany, 1996; pp. 49, 115–118. [Google Scholar]
- Mas, F.; Tassin, C.; Roch, F.; Yesaas, M.; Todeschim, P.; Bréchet, Y. Growth Morphologies and Primary Solidification Modes in a Dissimilar Weld between a Low-Alloy Steel and Austenitic Stainless Steel. Metals 2018, 8, 1–21. [Google Scholar] [CrossRef]
- Ornath, F.; Soudry, J.; Weiss, B.Z.; Minkoff, I. Weld Pool Segregation during the Welding of Low Alloy Steels with Austenitic Electrodes. Weld. J. 1981, 60, 227s–230s. [Google Scholar]
- Baeslack, W.A., III; Lippold, J.C.; Savage, W.F. Unmixed Zone Formation in Austenitic Stainless Steel Weldments. Weld. J. 1979, 58, 168s–176s. [Google Scholar]
- Kou, S.; Yang, Y.K. Mechanisms of Macrosegregation Formation near Fusion Boundary in Welds Made with Dissimilar Filler Metals. In Proceedings of the Conference Processing and product Manufacturing: Joining of Advanced and Specialty Materials IX 2007, Detroit, MI, USA, 16–20 September 2007; ASM International: Materials Park, OH, USA, 2007; pp. 329–340. [Google Scholar]
- EN 1011-1: Recommendations for Welding of Metallic Materials, General Guidance for ARC Welding; CEN: Brussels, Belgium, 2009.
- Zhang, W. Fluid Flow Phenomena during Welding. In ASM Handbook: Welding Fundamentals and Processes; Lienert, T., Babu, S., Siewert, T., Acoff, V., Eds.; ASM: Materials Park, OH, USA, 2011; Volume 6A, pp. 89–95. [Google Scholar]
- Glickstein, S.S.; Ferguson, H.S.; Friedman, E.; Jackson, C.E.; Kou, S. Heat Flow in Welding. In Welding Handbook: Welding Technology, 8th ed.; Conor, L.P., Ed.; AWS: Miami, FL, USA, 1991; Volume 1, Chapter 3; pp. 65–87. [Google Scholar]
- Rykalin, N.N. Berechung der Wärmevorgänge beim Schweissen; VEB Verlag Technik: Berlin, Germany, 1957; pp. 100, 111, 243–249. [Google Scholar]
- Radaj, D. Schweiβprozeβsimulation-Grundlagen und Anwendungen; DVS-Verlag GmbH: Düsseldorf, Germany, 1999; pp. 19–20, 52–55, 155, 158. [Google Scholar]
- Wells, A.A. Heat Flow in Welding. Weld. J. 1952, 31, 263s–267s. [Google Scholar]
- Tadamalle, A.P.; Reddy, Y.P.; Ramjee, E.; Reddy, V.K. Influence of welding speed on the melting efficiency of Nd:YAG laser welding. Adv. Prod. Eng. Manag. 2014, 9, 128–138. [Google Scholar] [CrossRef]
- Okada, A. Application of Melting Efficiency and its Problem. J. Jpn. Weld. Soc. 1977, 46, 53–61. [Google Scholar] [CrossRef]
- Fuerschbach, P.W.; Knorovsky, G.A. A Study of Melting Efficiency in Plasma Arc and Tungsten Arc Welding. Weld. J. 1991, 70, 287s–297s. [Google Scholar]
- Lyttle, K.A.; Anderson, D.G.; Frick, H.D.; McGrew, J.; Meyer, D.W.; Miller, R.A.; Telford, R.T. Physics of Welding. In Welding Handbook: Welding Technology, 8th ed.; Conor, L.P., Ed.; AWS: Miami, FL, USA, 1991; Volume 1, Chapter 2; pp. 31–63. [Google Scholar]
- Tušek, J. Varjenje in sorodne tehnike spajanja materialov v neločljivo zvezo (Welding and related processes for joining of materials into the non-separable joint); Univerza v Ljubljani-Fakulteta za strojništvo: Ljubljana, Slovenia, 2014; pp. 50–52. [Google Scholar]
- Seferian, D. Metalurgija zavarivanja (Welding Metallurgy), Serbian Translation of Metallurgie de la soudure; Radojković, M., Ed.; Građevinska knjiga: Beograd, Serbia, 1969; p. 104. [Google Scholar]
- Arc Welding Processes and Their Application to Low-Carbon Steel, Shielded Metal-Arc Welding. In Metals Handbook: Welding and Brazing, 8th ed.; Taylor, L. (Ed.) ASM: Metals Park, OH, USA, 1971; Volume 6, pp. 1–23. [Google Scholar]
- Lancaster, J.F. The Physics of Welding; Pergamon Press: Oxford, UK, 1984; pp. 36–39. [Google Scholar]
- Eagar, T.W.; Mazzeo, A.D. Welding Process Fundamentals. In ASM Handbook: Welding Fundamentals and Processes; Lienert, T., Babu, S., Siewert, T., Acoff, V., Eds.; ASM: Materials Park, OH, USA, 2011; Volume 6A, pp. 29–34. [Google Scholar]
- SIJ Elektrode Jesenice d.o.o. (producer of welding electrodes) Jesenice, Slovenia.
- Inženjersko tehnički priručnik: Zavarivanje-2 (Engineering Technical Manual: Welding—Vol. 2), Serbian Translation of Cправoчник пo сварке-2; Vlajković, M. (Ed.) RAD: Beograd, Serbia, 1979; p. 16. [Google Scholar]

| Weld | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| (mm2) | 4.403 | 6.424 | 4.131 | 3.738 | 6.755 | 3.094 | 4.448 | 5.983 |
| (mm2) | 13.067 | 17.875 | 18.693 | 12.017 | 17.990 | 14.721 | 11.472 | 26.427 |
| 33.7 | 35.9 | 22.1 | 31.1 | 37.5 | 21.0 | 38.8 | 22.6 |
| Weld | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| (mm) | 4.775 | 5.769 | 3.347 | 4.164 | 5.593 | 2.486 | 4.680 | 4.710 |
| (mm) | 14.452 | 16.421 | 15.559 | 12.806 | 15.208 | 11.756 | 12.344 | 19.582 |
| 33.0 | 35.1 | 21.5 | 32.5 | 36.8 | 21.1 | 37.9 | 24.0 |
| Steel | Cr | Cr | Cr | Cr | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S355 | ||||||||||
| 33.7 | ||||||||||
| 1.03 | ||||||||||
| 0.63 | 0.77 | 0.71 | 0.68 | 0.70 | ||||||
| 0.73 | 0.75 | 0.74 | 0.946 | |||||||
| 0.72 | 0.917 | 30.0 | 0.836 | |||||||
| 0.655 | 1.008 | 36.4 | 1.014 | |||||||
| 0.66 | 35.9 | 33.2 | 0.925 | |||||||
| 4.55 | ||||||||||
| 3.24 | 3.33 | 3.17 | 3.28 | 3.255 | ||||||
| 3.31 | 3.42 | 3.365 | 0.967 | |||||||
| 3.31 | 1.069 | 27.2 | 1.231 | |||||||
| 3.20 | 1.106 | 29.7 | 1.344 | |||||||
| 3.54 | 22.1 | 28.4 | 1.285 | |||||||
| 9.32 | ||||||||||
| 6.56 | 6.44 | 6.52 | 6.52 | 6.51 | ||||||
| 6.63 | 6.47 | 6.55 | 0.994 | |||||||
| 6.53 | 0.955 | 29.9 | 0.903 | |||||||
| 6.455 | 0.967 | 30.7 | 0.927 | |||||||
| 6.24 | 31.1 | 30.3 | 0.974 | |||||||
| 1.43 | ||||||||||
| 0.88 | 0.86 | 0.85 | 0.94 | 0.88 | ||||||
| 0.91 | 0.87 | 0.89 | 0.989 | |||||||
| 0.885 | 1.006 | 38.1 | 1.016 | |||||||
| 0.855 | 1.041 | 40.2 | 1.072 | |||||||
| 0.89 | 37.5 | 39.1 | 1.043 | |||||||
| 7.10 | ||||||||||
| 6.14 | 6.17 | 6.26 | 6.34 | 6.23 | ||||||
| 5.86 | 5.39 | 5.625 | 1.107 | |||||||
| 5.93 | 0.946 | 16.5 | 0.786 | |||||||
| 5.625 | 0.997 | 20.8 | 0.990 | |||||||
| 5.61 | 21.0 | 18.6 | 0.886 | |||||||
| 7.60 | ||||||||||
| 4.48 | 4.85 | 4.28 | 4.49 | 4.525 | ||||||
| 4.42 | 4.39 | 4.405 | 1.027 | |||||||
| 4.465 | 1.041 | 41.2 | 1.062 | |||||||
| 4.335 | 1.073 | 42.9 | 1.106 | |||||||
| 4.65 | 38.8 | 42.0 | 1.082 | |||||||
| 7.20 | ||||||||||
| 5.54 | 5.63 | 5.49 | 5.48 | 5.535 | ||||||
| 6.07 | 5.87 | 5.97 | 0.927 | |||||||
| 5.75 | 0.967 | 20.1 | 0.881 | |||||||
| 5.49 | 1.013 | 23.7 | 1.039 | |||||||
| 5.56 | 22.6 | 21.9 | 0.969 |
| Weld | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 33.7 | 35.9 | 22.1 | 31.1 | 37.5 | 21.0 | 38.8 | 22.6 | 30.3 | |
| 33.0 | 35.1 | 21.5 | 32.5 | 36.8 | 21.1 | 37.9 | 24.0 | 30.2 | |
| / | 33.2 | 28.4 | 30.3 | 39.1 | 18.6 | 42.0 | 21.9 | 30.5 |
| Equation | (8) | (9) | (10) | (11) | (12) | (13a) | (13b) | (13c) | (14) | (15) | (16) | (17) | [26] * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ηm | 0.30 # | 0.01 ## | 0.39 | 0.29 | 0.32 | 0.04 | 0.27 | 0.34 | 0.27 | 0.27 | 0.07 | 0.29 | 0.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorc, M.; Nagode, A.; Kosec, B.; Zorc, B. Determining the Degree of Admixing Rate of the Base Material and the Melting Efficiency in Single-Bead Surface Welds Using Different Methods, Including New Approaches. Materials 2019, 12, 1479. https://doi.org/10.3390/ma12091479
Zorc M, Nagode A, Kosec B, Zorc B. Determining the Degree of Admixing Rate of the Base Material and the Melting Efficiency in Single-Bead Surface Welds Using Different Methods, Including New Approaches. Materials. 2019; 12(9):1479. https://doi.org/10.3390/ma12091479
Chicago/Turabian StyleZorc, Matija, Aleš Nagode, Borut Kosec, and Borut Zorc. 2019. "Determining the Degree of Admixing Rate of the Base Material and the Melting Efficiency in Single-Bead Surface Welds Using Different Methods, Including New Approaches" Materials 12, no. 9: 1479. https://doi.org/10.3390/ma12091479




