The Effect of Specific Energy Density on Microstructure and Corrosion Resistance of CoCrMo Alloy Fabricated by Laser Metal Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Equipment
2.2. Microstructure and Phase Analysis
2.3. Corrosion Resistance Analysis
3. Results & Discussion
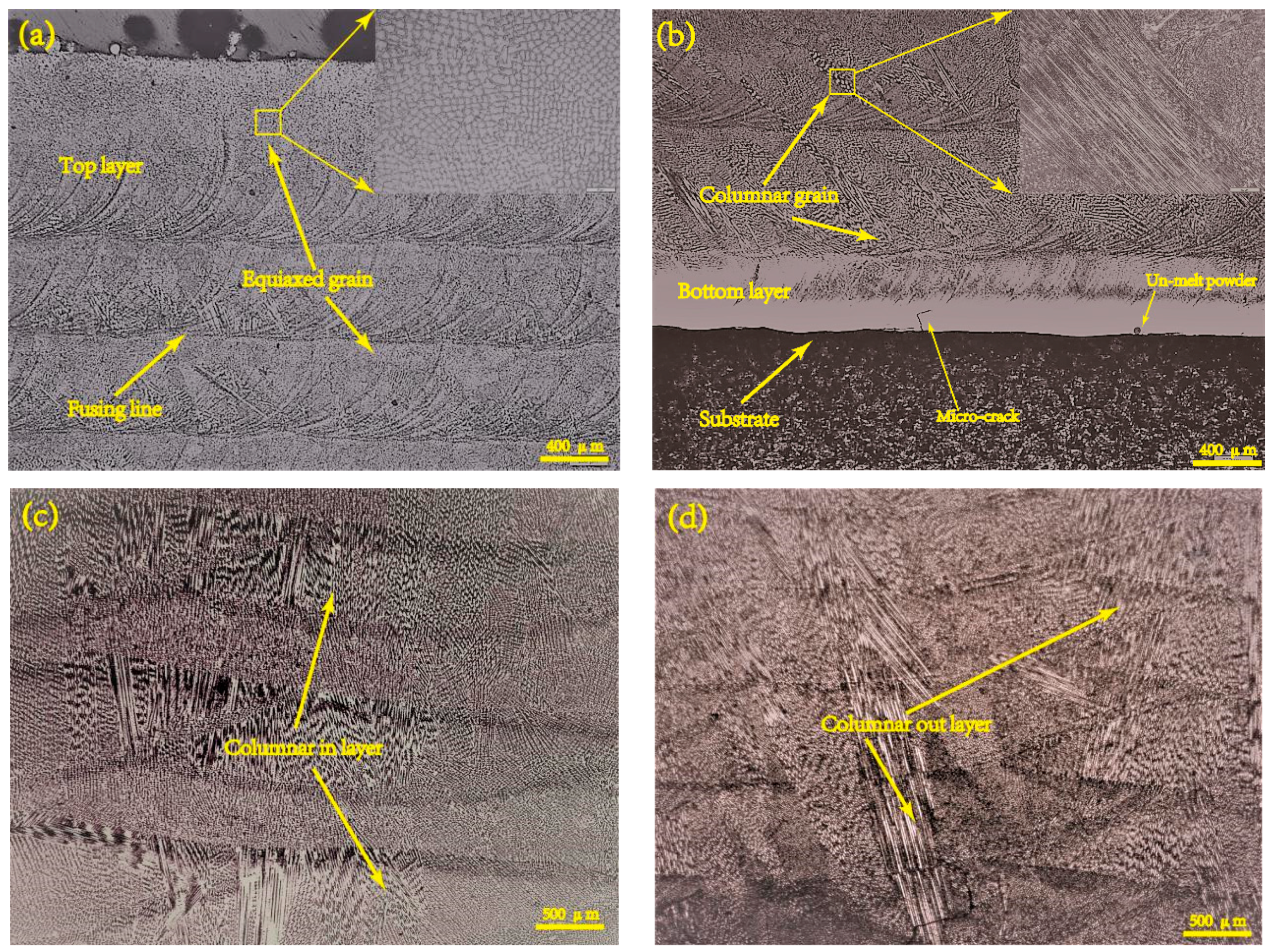
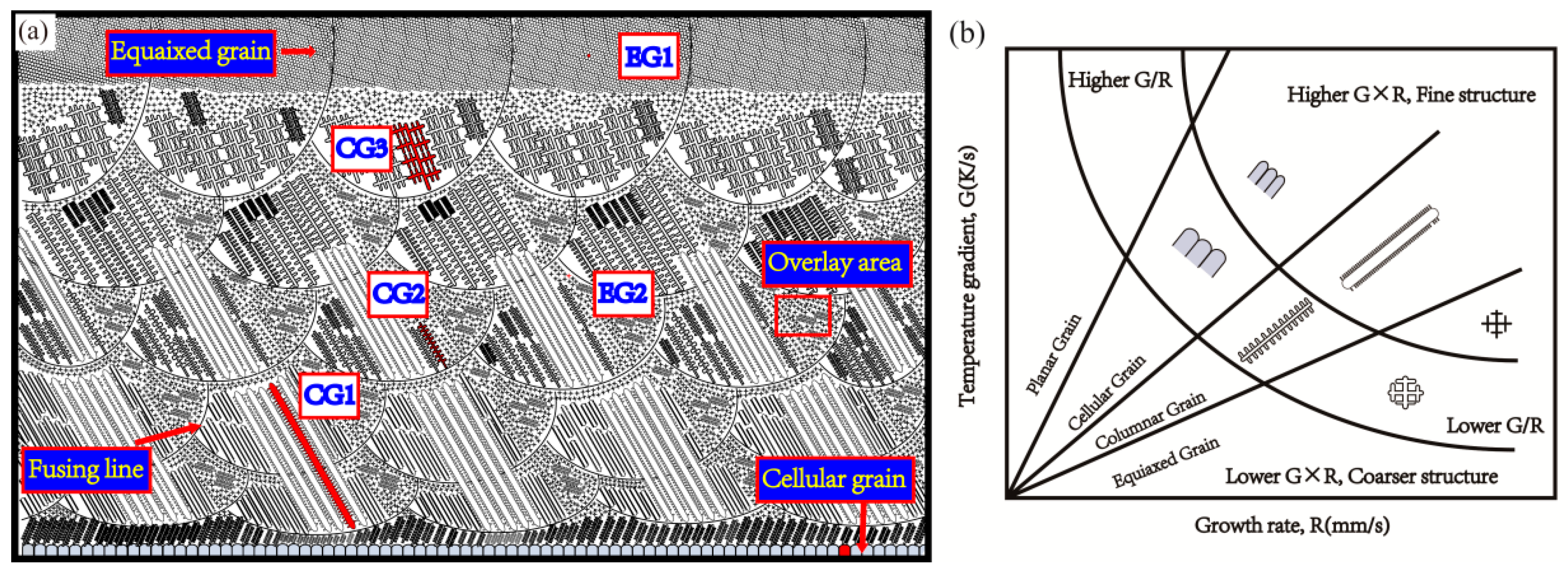
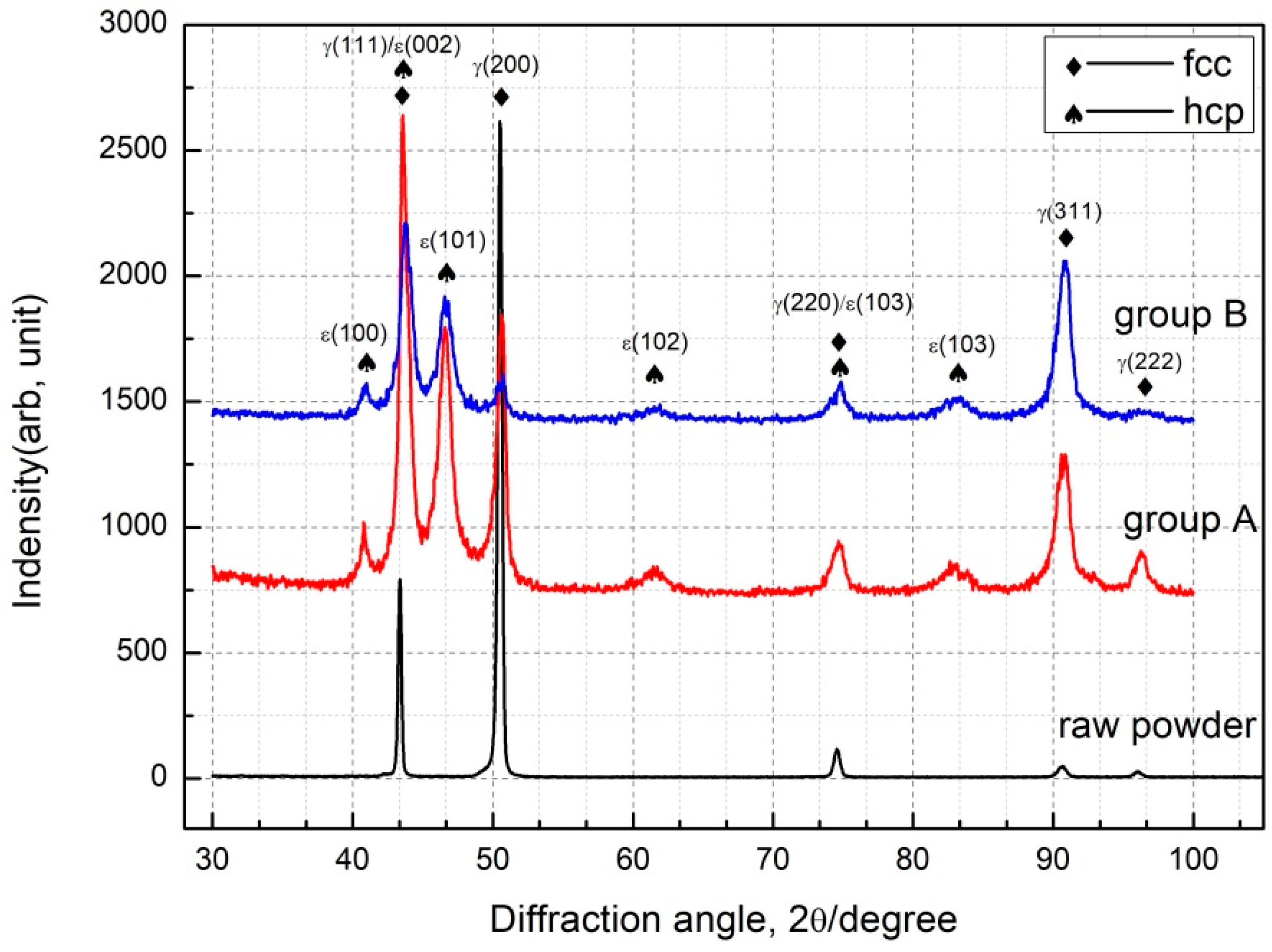
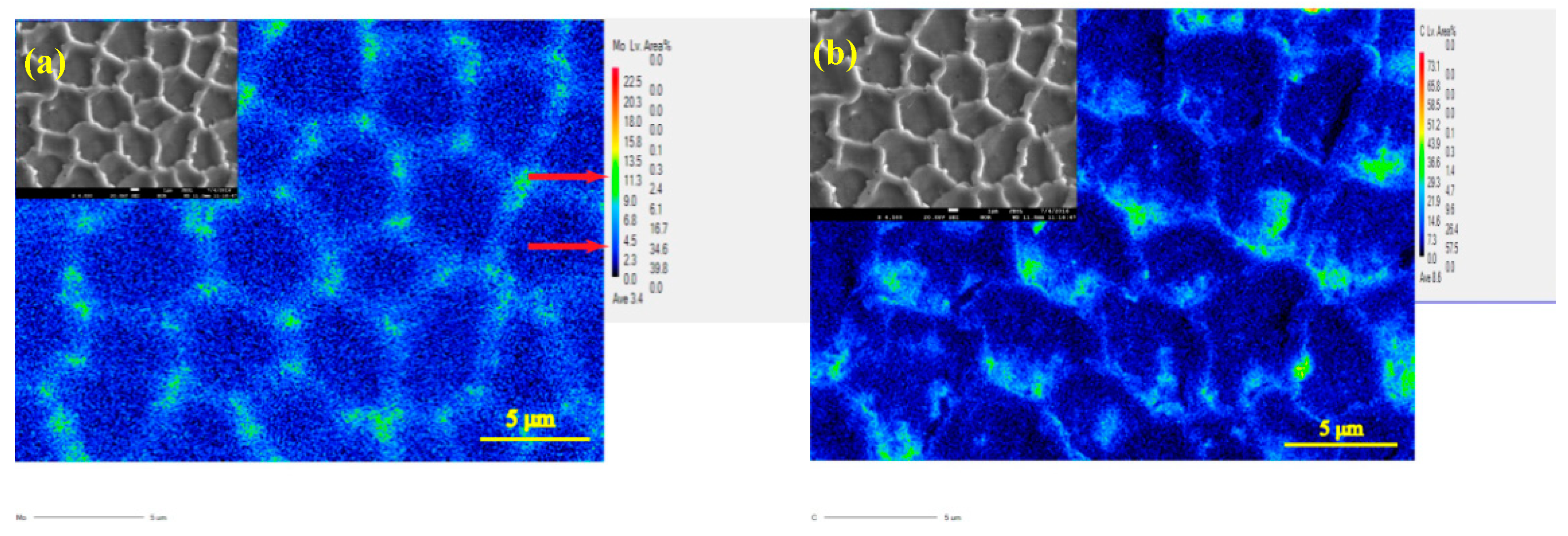
3.1. Morphology and Phase Analysis
3.2. Corrosion Resistance of Deposited Specimen
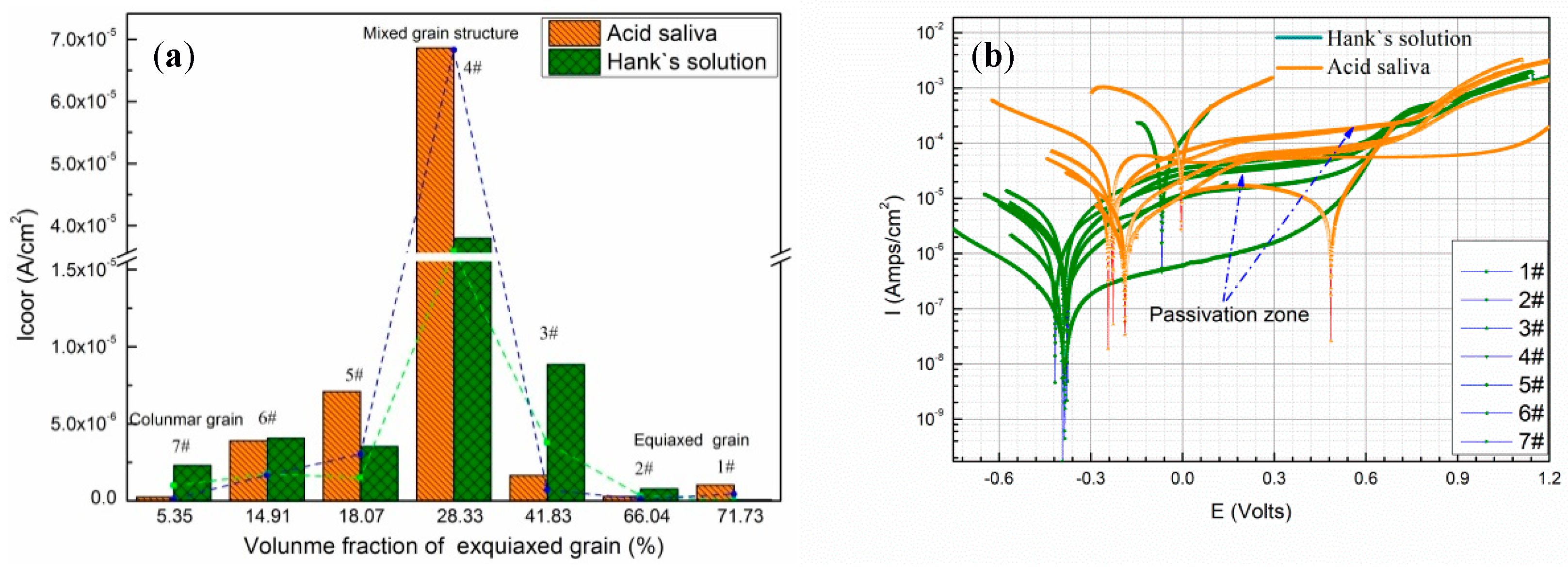
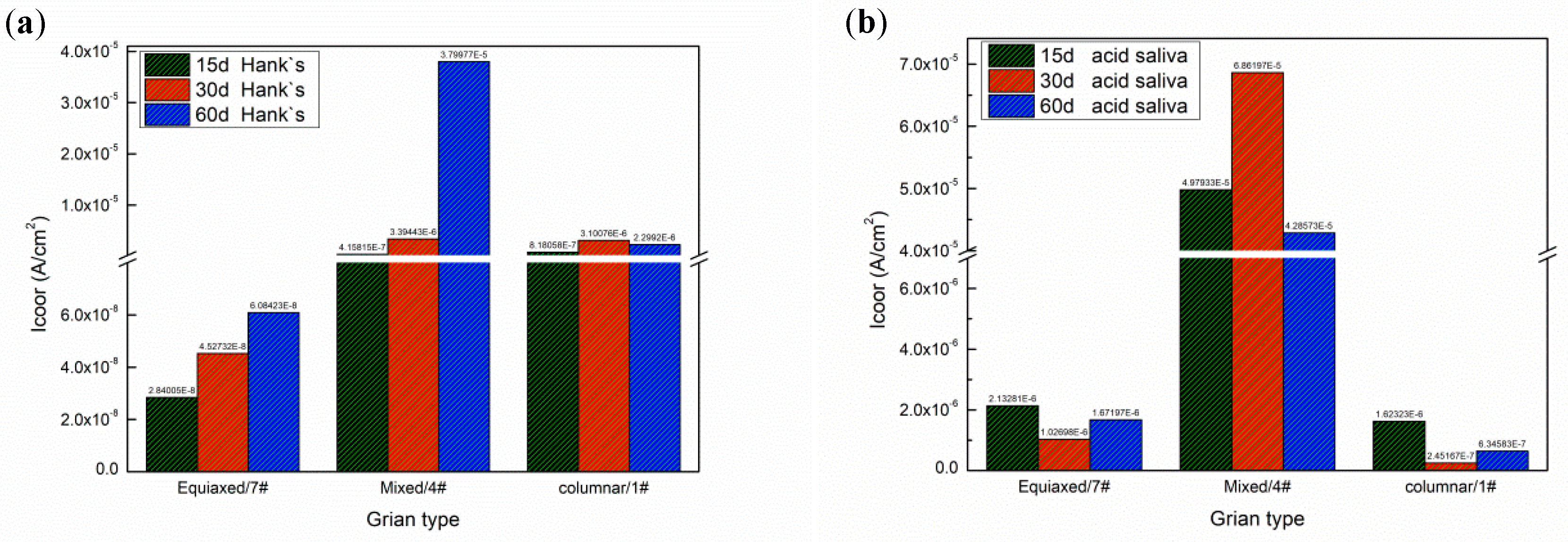
3.2.1. The Effect of Specific Energy Density on Corrosion Current
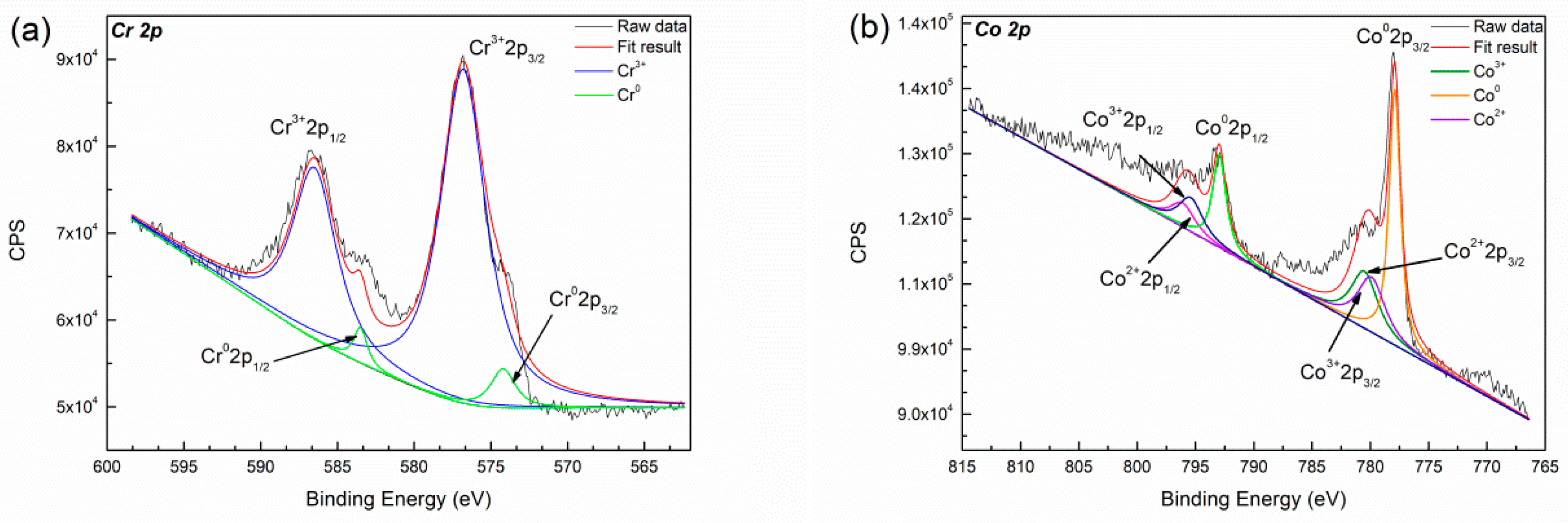
3.2.2. Passive Film Formation Analysis
4. Conclusions
- (1)
- The main phases observed in deposited specimens are γ-Co and partially transformed in ε-Co with the deposition processing. Equiaxed and columnar grain have a competitive growth mechanism in deposited specimens. The second dendrite arm spacing of columnar grain increases from bottom to top. Higher specific energy density promotes the formation of columnar grain and leads to coarse grain size.
- (2)
- The corrosion current of deposited specimens increases from 2.071 × 10−6 A/cm2 to 6.86 × 10−5 A/cm2 and rapidly drops to 9.88 × 10−7 A/cm2 with increase of specific energy density from 318.8 J/g to 2752.3 J/g. The columnar and equiaxed structure of deposited specimens have lower corrosion current than mixed structure due to finer grain and less Mo segregation.
- (3)
- The deposited specimens in acidic saliva forms a more stable, dense passive film than that in Hank’s solution. The passive film is composed of Cr2O3 and displays a wide range of passivation in acidic saliva, while it consists of CoO, Co3O4, CoOOH, Cr2O3 and minor MoO3 and shows a lower corrosion potential in Hank’s solution. The component fabricated by LMD has low level of released metal in both solutions.
Author Contributions
Funding
Conflicts of Interest
References
- Lindahl, C.; Xia, W.; Engqvist, H.; Snis, A.; Lausmaa, J.; Palmquist, A. Biomimetic calcium phosphate coating of additively manufactured porous CoCr implants. Appl. Surf. Sci. 2015, 353, 40–47. [Google Scholar] [CrossRef]
- Gatto, A.; Bortolini, S.; Iuliano, L. Characterization of Selective Laser Sintered Implant Alloys: Ti6Al4V And Co-Cr-Mo. In Global Product Development; Bernard, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 729–736. [Google Scholar]
- Hazlehurst, K.B.; Wang, C.J.; Stanford, M. The potential application of a Cobalt Chrome Molybdenum femoral stem with functionally graded orthotropic structures manufactured using Laser Melting technologies. Med. Hypotheses 2013, 81, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qiu, J. Combination of cloud manufacturing and 3D printing: Research progress and prospect. Int. J. Adv. Manuf. Technol. 2018, 96, 1929–1942. [Google Scholar] [CrossRef]
- Thompson, S.M.; Bian, L.; Shamsaei, N.; Yadollahi, A. An overview of Direct Laser Deposition for additive manufacturing; Part I: Transport phenomena, modeling and diagnostics. Addit. Manuf. 2015, 8, 36–62. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Chen, X.; Cui, W.; Liou, F. Evaluation of component repair using direct metal deposition from scanned data. Int. J. Adv. Manuf. Technol. 2018, 95, 3335–3348. [Google Scholar] [CrossRef]
- Matthews, M.J.; Guss, G.; Khairallah, S.A.; Rubenchik, A.M.; Depond, P.J.; King, W.E. Denudation of metal powder layers in laser powder bed fusion processes. Acta Mater. 2016, 114, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Ravi, G.A.; Attallah, M.M. Microstructural control during direct laser deposition of a β-titanium alloy. Mater. Des. 2015, 81, 21–30. [Google Scholar] [CrossRef]
- Mahamood, R.M.; Akinlabi, E.T. Scanning speed and powder flow rate influence on the properties of laser metal deposition of titanium alloy. Int. J. Adv. Manuf. Technol. 2017, 91, 2419–2426. [Google Scholar] [CrossRef]
- Takaichi, A.; Suyalatu, S.; Nakamoto, T.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A. Microstructures and mechanical properties of Co–29Cr–6Mo alloy fabricated by selective laser melting process for dental applications. J. Mech. Behav. Biomed. Mater. 2013, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Gradzka-Dahlke, M.; Dabrowski, J.R.; Dabrowski, B. Modification of mechanical properties of sintered implant materials on the base of Co–Cr–Mo alloy. J. Mater. Process. Technol. 2008, 204, 199–205. [Google Scholar] [CrossRef]
- Shim, D.-S.; Baek, G.-Y.; Seo, J.-S.; Shin, G.-Y.; Kim, K.-P.; Lee, K.-Y. Effect of layer thickness setting on deposition characteristics in direct energy deposition (DED) process. Opt. Laser Technol. 2016, 86, 69–78. [Google Scholar] [CrossRef]
- Zhong, C.; Gasser, A.; Schopphoven, T.; Poprawe, R. Experimental study of porosity reduction in high deposition-rate Laser Material Deposition. Opt. Laser Technol. 2015, 75, 87–92. [Google Scholar] [CrossRef]
- Campanelli, S.L.; Contuzzi, N.; Ludovico, A.D.; Caiazzo, F.; Cardaropoli, F.; Sergi, V. Manufacturing and Characterization of Ti6Al4V Lattice Components Manufactured by Selective Laser Melting. Materials 2014, 7, 4803–4822. [Google Scholar] [CrossRef] [PubMed]
- Del Val, J.; Comesaña, R.; Riveiro, A.; Lusquiños, F.; Quintero, F.; Boutinguiza, M.; Pou, J. Laser direct writing of Co-superalloy lines for micro-fabrication applications. Surf. Coat. Technol. 2018, 345, 76–88. [Google Scholar] [CrossRef]
- Fotovvati, B.; Wayne, S.F.; Lewis, G.; Asadi, E. A Review on Melt-Pool Characteristics in Laser Welding of Metals. Adv. Mater. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Yavari, S.A.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Additively Manufactured Open-Cell Porous Biomaterials Made from Six Different Space-Filling Unit Cells: The Mechanical and Morphological Properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef] [Green Version]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. Fatigue performance of selective laser melted Ti6Al4V components: State of the art. Mater. Res. Express 2019, 6, 012002. [Google Scholar] [CrossRef]
- Demir, A.G.; Previtali, B. Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater. Des. 2017, 119, 338–350. [Google Scholar] [CrossRef]
- Barucca, G.; Santecchia, E.; Majni, G. Structural characterization of biomedical Co–Cr–Mo components produced by direct metal laser sintering. Mater. Sci. Eng. C 2015, 48, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Liverani, E.; Fortunato, A.; Leardini, A. Fabrication of Co–Cr–Mo endoprosthetic ankle devices by means of Selective Laser Melting (SLM). Mater. Des. 2016, 106, 60–68. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, H. Evaluation of the mechanical properties and porcelain bond strength of cobalt-chromium dental alloy fabricated by selective laser melting. J. Prosthet. Dent. 2013, 111, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Xiang, N.; Wei, B. A comparison of corrosion resistance of cobalt-chromium-molybdenum metal ceramic alloy fabricated with selective laser melting and traditional processing. J. Prosthet. Dent. 2014, 112, 1217–1724. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.S.; Qian, B.; Shen, Z. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dent. Mater. 2014, 30, 525–534. [Google Scholar] [CrossRef]
- Xin, X.Z.; Chen, J.; Xiang, N.; Gong, Y.; Wei, B. Surface characteristics and corrosion properties of selective laser melted Co–Cr dental alloy after porcelain firing. Dent. Mater. 2014, 30, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L. Study on Mechanical Property and Corrosion Behavior of Co-Cr Alloy Fabricated by Laser Rapid Forming Technology. Master’s Thesis, Fourth Military Medical University, Xi’an, China, 2010. [Google Scholar]
- Wang, Y.-D.; Yang, Y.-Q.; Song, C.-H.; Liu, F.-M.; Mai, S.-Z. Process optimization and electrochemical behavior of CoCrMo alloy fabricated by selective laser melting based on response surface method. Chin. J. Nonferr. Met. 2014, 24, 2497–2505. [Google Scholar]
- International Organization for Standardization. Dentistry-Corrosion Test Methods for Metallic Materials, 2nd ed.; ISO 10271; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Biological Evaluation of Medical Devices-Part12: Sample Preparation and Reference Materials, English version: EN ISO 10993-12; European Committee for Standardization: Brussels, Belgium, 2012.
- Gan, Z.; Liu, H.; Li, S.; He, X.; Yu, G. Modeling of thermal behavior and mass transport in multi-layer laser additive manufacturing of Ni-based alloy on cast iron. Int. J. Heat Mass Transf. 2017, 111, 709–722. [Google Scholar] [CrossRef]
- Gan, Z.; Yu, G.; He, X.; Li, S. Numerical simulation of thermal behavior and multicomponent mass transfer in direct laser deposition of Co-base alloy on steel. Int. J. Heat Mass Transf. 2017, 104, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Milosˇev, I.; Strehblow, H.-H. The composition of the surface passive film formed on CoCrMo alloy in simulated physiological solution. Electrochim. Acta 2003, 48, 2767–2774. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Y.Y.; Zhang, S.Q.; Tang, H.B.; Wang, H.M. Grain morphology evolution behavior of titanium alloy components during laser melting deposition additive manufacturing. J. Alloy. Compd. 2015, 632, 505–513. [Google Scholar] [CrossRef]
- Huang, W.D.; Lin, X.; Chen, J.; Liu, Z.X.; Li, Y.M. Laser solid forming. In Formation Principle of Laser Solid Forming, 3rd ed.; Lei, J., Ed.; Northwestern Polytechnical University Press: Xi’an, China, 2007; pp. 107–108. [Google Scholar]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book, 8th ed.; Elsevier Butterworth-Heinemann: Burlington, NJ, USA, 2004. [Google Scholar]
- Girardin, E.; Barucca, G.; Mengucci, P.; Fiori, F.; Bassoli, E.; Gatto, A.; Iuliano, L.; Rutkowski, B. Biomedical Co-Cr-Mo components produced by Direct Metal Laser Sintering. Mater. Today Proceed. 2016, 3, 889–897. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Wang, L.; Lin, Y.; Zeng, Z.; Liu, W.; Xue, Q.; Hu, L.; Zhang, J. Electrochemical corrosion behavior of nanocrystal line Co coatings explained by higher grain boundary density. Electrochim. Acta 2007, 52, 4342–4350. [Google Scholar] [CrossRef]
- Liu, C. Microstructure and Antibacterial Property of CoCrMoCu Alloy. Master’s Thesis, Jiamusi University, Jiamusi, China, 2015. [Google Scholar]
- Lu, Y.; Wu, S.; Gan, Y.; Li, J.; Zhao, C.; Lin, J. Investigation on the microstructure, mechanical property and corrosion behavior of the selective laser melted CoCrW alloy for dental application. Mater. Sci. Eng. C 2015, 49, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Hagelin-Weaver, H.A.E.; Hoflund, G.B.; Minahan, D.M.; Salaita, G.N. Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar+ bombardment. Appl. Surf. Sci. 2004, 235, 420–448. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co-Cr-Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005–075005-13. [Google Scholar] [CrossRef]





| Co21 | Cr | Mo | C | Fe | W | Ni | Si | S | P | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 27.26 | 8.56 | 0.24 | 1.07 | 0.07 | 3.58 | 1.14 | 0.012 | 0.002 | Bal |
| 316L | C | Cr | Ni | Mo | Mn | S | P | Si | N | Fe |
| Content (wt.%) | 0.016 | 16.33 | 10.24 | 2.05 | 1.11 | 0.001 | 0.019 | 0.52 | 0.025 | Bal. |
| Order | 1# | 2# | 3# | 4# | 5# | 6# | 7# |
|---|---|---|---|---|---|---|---|
| Power (W) | 900 | 800 | 700 | 600 | 500 | 400 | 300 |
| Powder-Feed rate (g/s) | 0.327 | 0.408 | 0.529 | 0.685 | 0.73 | 0.851 | 0.941 |
| Scanning speed (mm/s) | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Line energy density (J/mm) | 128.6 | 114.3 | 100 | 85.7 | 71.4 | 57.1 | 42.9 |
| Powder-Feed rate per unit length (g/(mm×s)) | 0.047 | 0.058 | 0.076 | 0.098 | 0.104 | 0.122 | 0.134 |
| Specific energy * (J/g) | 2752.3 | 1960.8 | 1323.3 | 875.9 | 684.9 | 470.1 | 318.8 |
| Hank’s Solution | NaCl | KCl | CaCl2 | NaHCO3 | KH2PO4 | MgSO4·7H2O | MgCl2·6H20 | Glucose |
| Content (g/L) | 8 | 0.4 | 0.14 | 0.35 | 0.6 | 0.06 | 0.1 | 0.4 |
| Acidic Saliva | NaCl | KCl | CaCl2·2H2O | Carbamide | NaH2PO4·2H2O | Na2S·2 H2O | - | |
| Content (g/L) | 0.4 | 0.4 | 0.795 | 1.0 | 0.78 | 0.005 | - | |
| Acidic Saliva | Co(μg/L) | Cr(μg/L) | Fe(μg/L) | Mo(μg/L) |
| 318.8 J/g | 1.62 | 0.003 | 0.003 | 0.278 |
| 875.9 J/g | 0.214 | 0.002 | 0.002 | 0.047 |
| 2752.3 J/g | 0.441 | 0.002 | 0.003 | 0.067 |
| Hank’s Solution | Co(μg/L) | Cr(μg/L) | Fe(μg/L) | Mo(μg/L) |
| 318.8 J/g | 0.604 | 0.018 | 0.005 | 0.266 |
| 875.9 J/g | 0.337 | 0.005 | 0.011 | 0.064 |
| 2752.3 J/g | 0.288 | 0.007 | 0.012 | 0.038 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ren, H.; Liu, C.; Shang, S. The Effect of Specific Energy Density on Microstructure and Corrosion Resistance of CoCrMo Alloy Fabricated by Laser Metal Deposition. Materials 2019, 12, 1321. https://doi.org/10.3390/ma12081321
Li J, Ren H, Liu C, Shang S. The Effect of Specific Energy Density on Microstructure and Corrosion Resistance of CoCrMo Alloy Fabricated by Laser Metal Deposition. Materials. 2019; 12(8):1321. https://doi.org/10.3390/ma12081321
Chicago/Turabian StyleLi, Jinbao, Huijiao Ren, Changsheng Liu, and Shuo Shang. 2019. "The Effect of Specific Energy Density on Microstructure and Corrosion Resistance of CoCrMo Alloy Fabricated by Laser Metal Deposition" Materials 12, no. 8: 1321. https://doi.org/10.3390/ma12081321





