Anodic Fabrication of Ti-Ni-Si-O Nanostructures on Ti10Ni5Si Alloy
Abstract
:1. Introduction
2. Materials and Methods
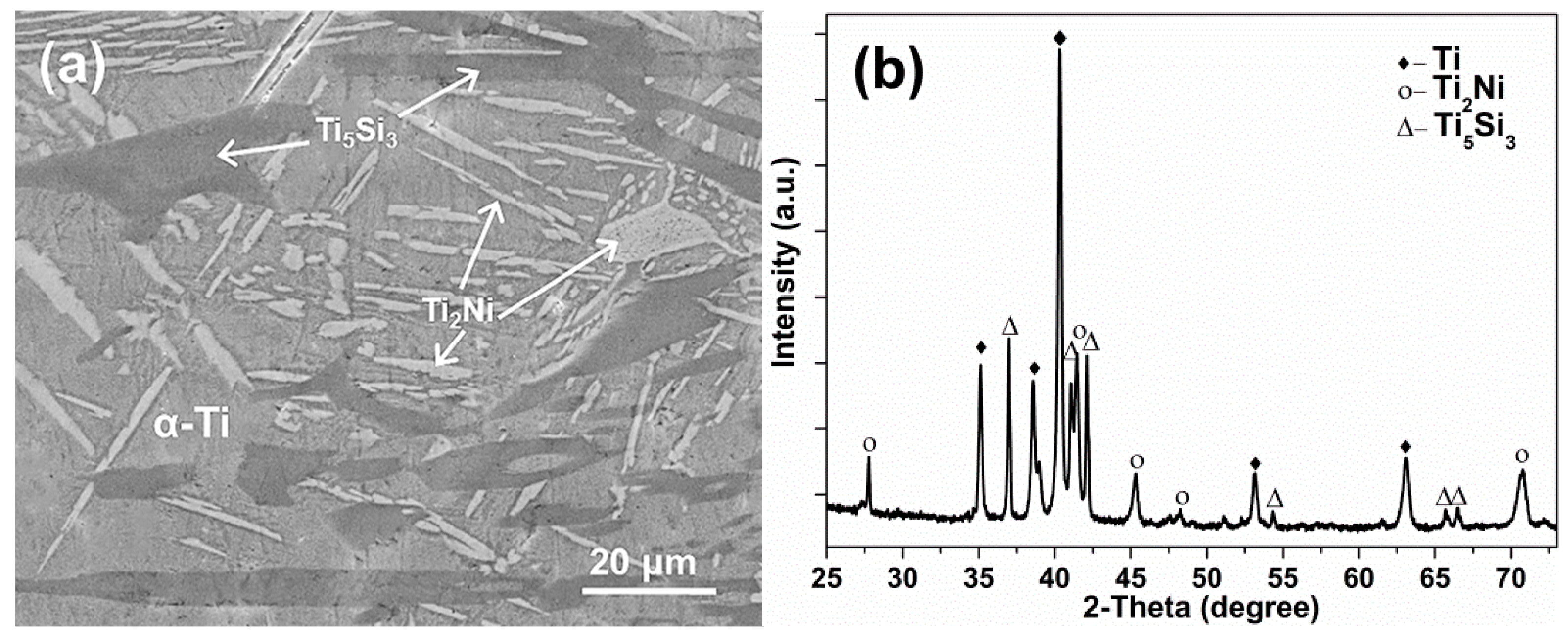
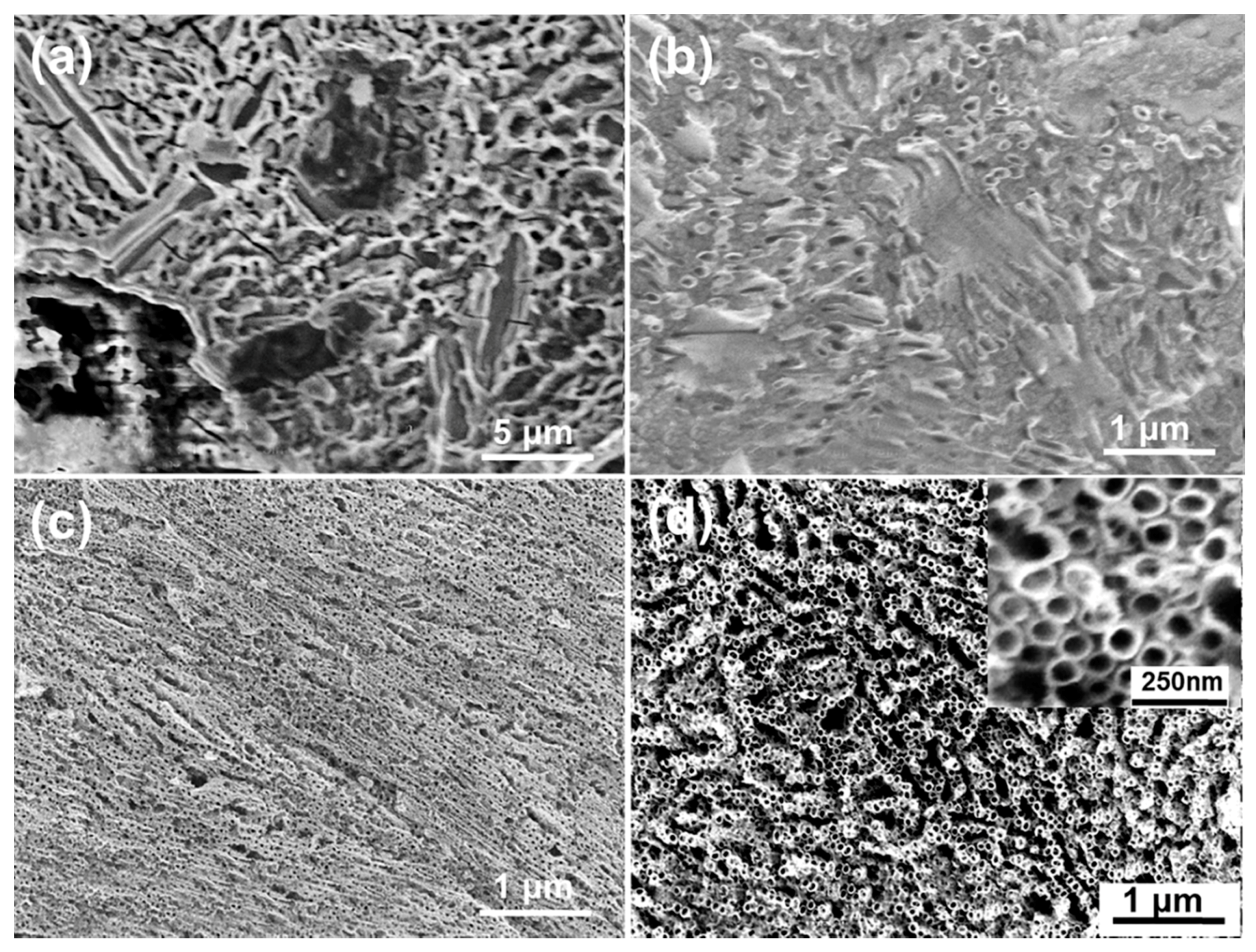
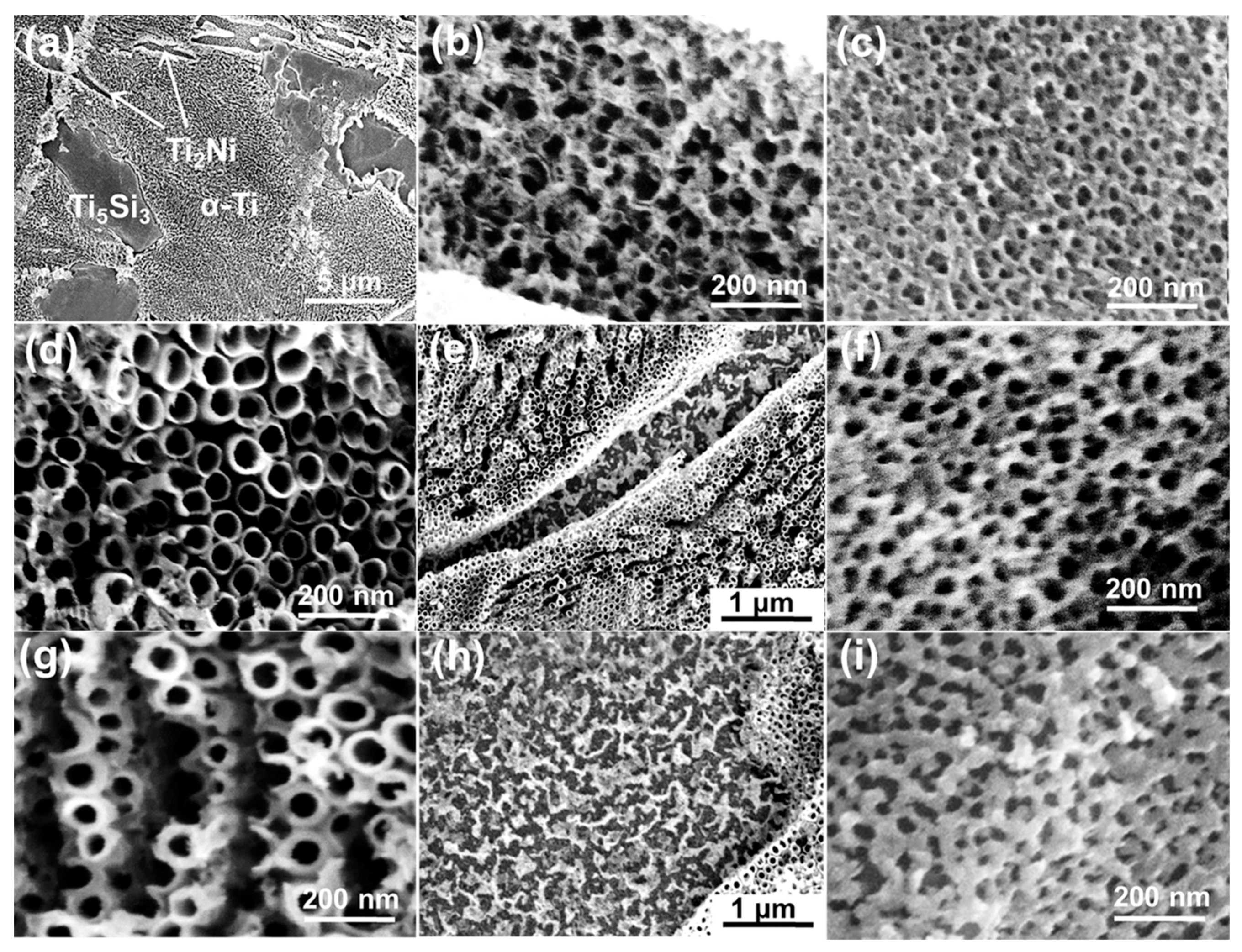
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ou, K.L.; Weng, C.C.; Lin, Y.H.; Huang, M.S. A promising of alloying modified beta-type titanium-niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Shi, X.; Jeong, H.; Oh, S.J.; Ma, M.; Zhang, K.; Kwon, J.; Choi, I.T.; Choi, I.Y.; Kim, H.K.; Kim, J.K.; et al. Unassisted photoelectrochemical water splitting exceeding 7% solar-to-hydrogen conversion efficiency using photon recycling. Nat. Commun. 2016, 7, 11943. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Hong, Y.; Zang, T.; Liu, Q.; Yu, L.; Dong, L. The application of heterostructured SrTiO3-TiO2 nanotube arrays in dye-sensitized solar cells. J. Electrochem. Soc. 2018, 165, 3069–3075. [Google Scholar] [CrossRef]
- Banerjee, S.; Mohapatra, S.K.; Das, P.P.; Misra, M. Synthesis of coupled semiconductor by filling 1D TiO2 nanotubes with CdS. Chem. Mater. 2008, 20, 6784–6791. [Google Scholar] [CrossRef]
- Zlamal, M.; Macak, J.M.; Schimuki, P.; Krysa, J. Electrochemically assisted photocatalysis on self-organized TiO2 nanotubes. Electrochem. Commun. 2007, 9, 2822–2826. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Navarrete, J.; Gomez, R. Synthesis, characterization and photocatalytic activity of TiO2 nanostructures: Nanotubes, nanofibers, nanowires and nanoparticles. Catal. Today 2016, 266, 90–101. [Google Scholar] [CrossRef]
- Acharyya, D.; Bhattacharyya, P. Highly efficient room-temperature gas sensor based on TiO2 nanotube-reduced graphene-oxide hybrid device. IEEE Electron. Device Lett. 2016, 37, 656–659. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Yin, Y.; Lin, C.; Xue, C. Anodic formation of ordered and bamboo-type TiO2 anotubes arrays with different electrolytes. J. Alloys Compd. 2010, 490, 247–252. [Google Scholar] [CrossRef]
- Zhang, M.; Bando, Y.; Wada, K. Sol-gel template preparation of TiO2 nanotubes and nanorods. J. Mater. Sci. Lett. 2001, 20, 167–170. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, W.M.; Li, H.L. Template synthesis of well-aligned titanium dioxide nanotubes. Appl. Phys. A 2005, 80, 317–320. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, G.; Yuan, X.; Xie, T.; Zhang, L. Fabrication and optical properties of TiO2 nanowire arrays made by sol gel electrophoresis deposition into anodic alumina membranes. J. Phys. Condens. Matter 2003, 15, 2917–2922. [Google Scholar] [CrossRef]
- Deen, K.M.; Farooq, A.; Raza, M.A.; Haider, W. Effect of electrolyte composition on TiO2 nanotubular structure formation and its electrochemical evaluation. Electrochim. Acta 2014, 117, 329–335. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Syrek, K.; Pawlik, A.; Jarosz, M.; Tynkevych, O.; Sulka, G.D. Effects of anodizing potential and temperature on the growth of anodic TiO2 and its photoelectrochemical properties. Appl. Surf. Sci. 2017, 396, 1119–1129. [Google Scholar] [CrossRef]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J. Mater. Res. 2011, 20, 230–236. [Google Scholar] [CrossRef]
- Fraoucene, H.; Sugiawati, V.A.; Hatem, D.; Belkaid, M.S.; Vacandio, F.; Eyraud, M.; Pasquinelli, M.; Djenizian, T. Optical and electrochemical properties of self-organized TiO2 nanotube arrays from anodized Ti−6Al−4V alloy. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Ocampo, R.A.; Echeverría, F.E. Effect of the anodization parameters on TiO2 nanotubes characteristics produced in aqueous electrolytes with CMC. Appl. Surf. Sci. 2019, 469, 994–1006. [Google Scholar] [CrossRef]
- Berger, S.; Tsuchiya, H.; Schmuki, P. Transition from nanopores to nanotubes: Self-ordered anodic oxide structures on titanium-aluminides. Chem. Mater. 2008, 20, 3245–3247. [Google Scholar] [CrossRef]
- Wei, W.; Jha, H.; Yang, G.; Hahn, R.; Paramasivam, I.; Berger, S.; Spiecker, E.; Schmuki, P. Formation of self-organized superlattice nanotube arrays–embedding heterojunctions into nanotube walls. Adv. Mater. 2010, 22, 4770–4774. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, D.; Ning, C.; Bai, S.; Huang, L.; Li, M.; Mao, D. Thermal stability and in vitro bioactivity of Ti-Al-V-O nanostructures fabricated on Ti6Al4V alloy. Nanotechnology 2009, 20, 065708. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Partida, E.; Moreno-Ulloa, A.; Valdez-Salas, B.; Velasquillo, C.; Carrillo, M.; Escamilla, A.; Valdez, E.; Villarreal, F. Improved osteoblast and chondrocyte adhesion and viability by surface-modified Ti6Al4V alloy with anodized TiO2 nanotubes using a super-oxidative solution. Materials 2015, 8, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.K.; Deyab, N.M.; Ghany, N.A. Ternary Ti-Mo-Ni mixed oxide nanotube arrays as photoanode materials for efficient solar hydrogen production. Phys. Chem. Chem. Phys. 2013, 15, 12274–12282. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Huang, J.; Zhang, J.; Wei, S.; Lin, G.; Liu, H. A study of Ti-Ni-Si coatings by reactive braze coating process. Mater. Lett. 2006, 60, 2240–2242. [Google Scholar] [CrossRef]
- Lu, B.C.; Wang, Y.L.; Xu, J. Revisiting the glass-forming ability of Ti-Ni-Si ternary alloys. J. Alloys Compd. 2009, 475, 157–164. [Google Scholar] [CrossRef]
- Yang, Y.F.; Mu, D. Effect of Ni addition on the formation mechanism of Ti5Si3 during self-propagation high-temperature synthesis and mechanical property. J. Eur. Ceram. Soc. 2014, 34, 2177–2185. [Google Scholar] [CrossRef]
- Yang, Y.F.; Luo, S.D.; Bettles, C.J.; Schaffer, G.B.; Qian, M. The effect of Si additions on the sintering and sintered microstructure and mechanical properties of Ti-3Ni alloy. Mater. Sci. Eng. A 2011, 528, 7381–7387. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, Y.; Xiao, J.; Xie, G. Micro-solid phase equilibrium extraction with highly ordered TiO2 nanotube arrays: A new approach for the enrichment and measurement of organochlorine pesticides at trace level in environmental water samples. Anal. Bioanal.Chem. 2011, 400, 205–212. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, S. Self-organized regular arrays of anodic TiO2 nanotubes. Nano Lett. 2008, 8, 3171–3173. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Peng, W.; Ning, C.; Hu, Q.; Dong, H. Nanostructure transition on anodic titanium: Structure control via a competition strategy between electrochemical oxidation and chemical etching. J. Phys. Chem. C 2012, 116, 22359–22364. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, H.; Xia, Q.; Liu, Q.; Mou, L. Density, dynamic viscosity and electrical conductivity of two hydrophobic phosphonium ionic liquids. Acta Phys. Chim. Sin. 2017, 33, 736–744. [Google Scholar]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res. A 2005, 75, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Yang, M.; Schmuki, P. Self-ordered nanoporous nickel oxide/fluoride composite film with strong electrochromic contrast. Electrochem. Solid-State Lett. 2010, 13, C21. [Google Scholar] [CrossRef]
- Yang, M.; Huo, L.; Pei, L.; Pan, K.; Gan, Y. Enhanced photoelectrochemical performance and charge transfer properties in self-organized NiOx-doped TiO2 nanotubes. Electrochim. Acta 2014, 125, 288–293. [Google Scholar] [CrossRef]


| Composition | EL-1 | EL-2 | EL-3 | EL-4 |
|---|---|---|---|---|
| NH4F | 0.3 M | 0.3 M | 0.3 M | 0.3 M |
| Ethylene glycol | 100 vol.% | - | - | 7 vol.% |
| Glycerol | - | 100 vol.% | 95 vol.% | 90 vol.% |
| Water | - | - | 5 vol.% | 3 vol.% |
| Phase Region | Ti (wt.%/at.%) | Ni (wt.%/at.%) | Si (wt.%/at.%) |
|---|---|---|---|
| α-Ti | 89.2/90.4 | 10.1/8.3 | 0.7/1.3 |
| Ti2Ni | 69.9/73.1 | 28.7/24.5 | 1.4/2.4 |
| Ti5Si3 | 73.1/64.5 | 6.4/4.6 | 20.5/30.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ding, D.; Li, N. Anodic Fabrication of Ti-Ni-Si-O Nanostructures on Ti10Ni5Si Alloy. Materials 2019, 12, 1315. https://doi.org/10.3390/ma12081315
Li T, Ding D, Li N. Anodic Fabrication of Ti-Ni-Si-O Nanostructures on Ti10Ni5Si Alloy. Materials. 2019; 12(8):1315. https://doi.org/10.3390/ma12081315
Chicago/Turabian StyleLi, Ting, Dongyan Ding, and Nan Li. 2019. "Anodic Fabrication of Ti-Ni-Si-O Nanostructures on Ti10Ni5Si Alloy" Materials 12, no. 8: 1315. https://doi.org/10.3390/ma12081315





