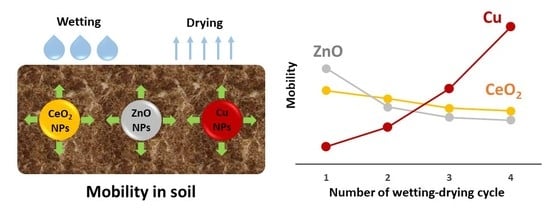
Mobility and Fate of Cerium Dioxide, Zinc Oxide, and Copper Nanoparticles in Agricultural Soil at Sequential Wetting-Drying Cycles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Characterization of NPs and Soil Sample
2.3. Spiking Soil with NPs
2.4. Performing Wetting-Drying Procedures
2.5. Leaching NPs from Soil Samples
2.6. Analysis of Leachates
2.7. Control Experiments
3. Results and Discussion
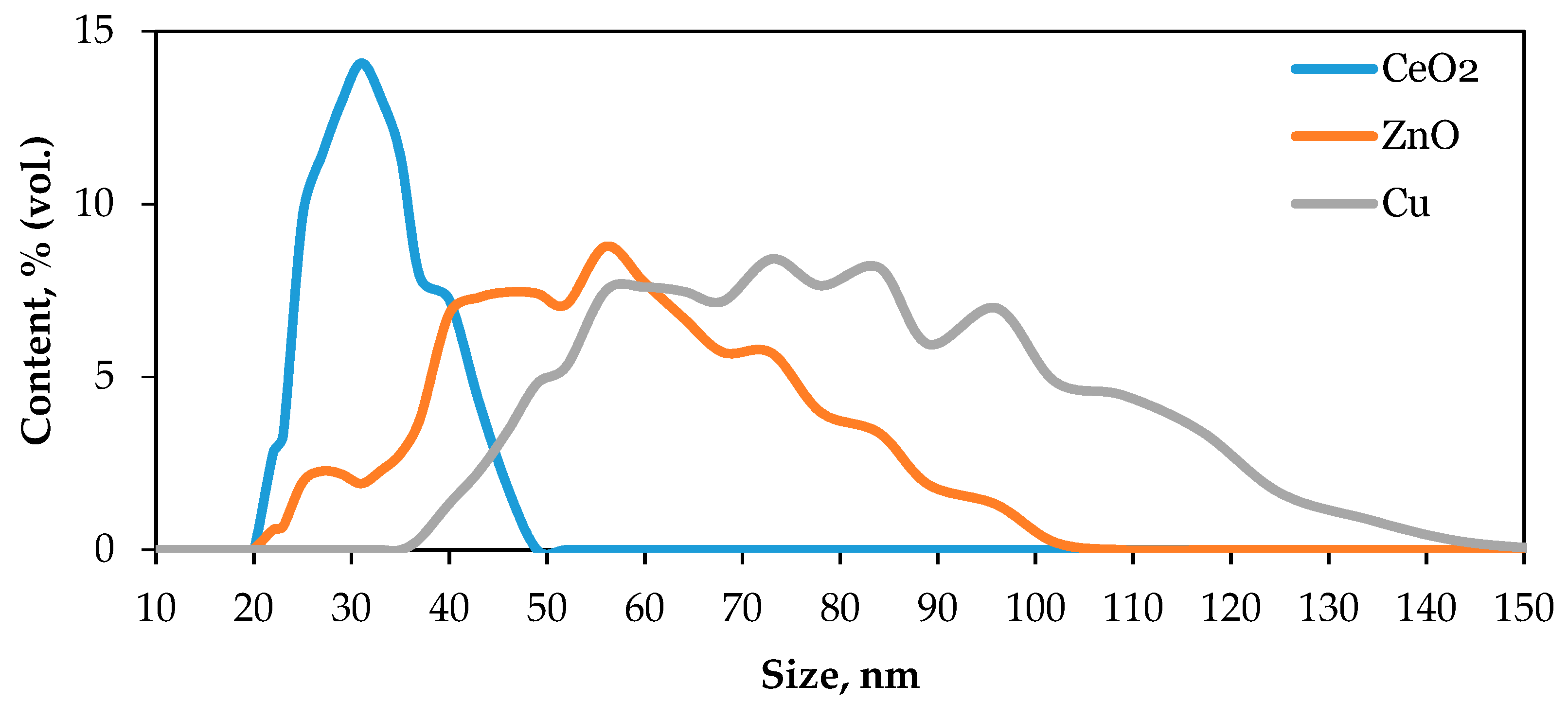
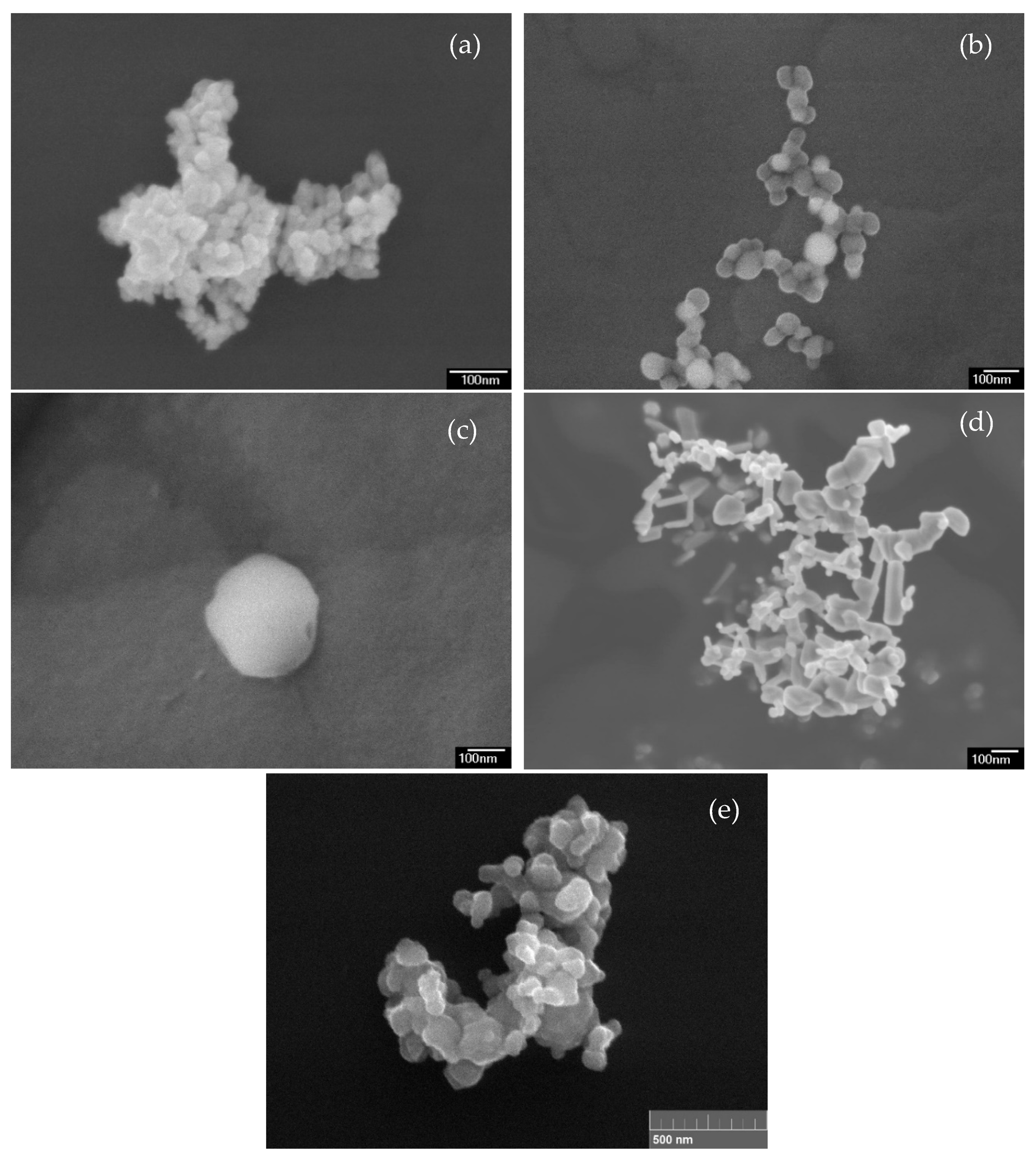
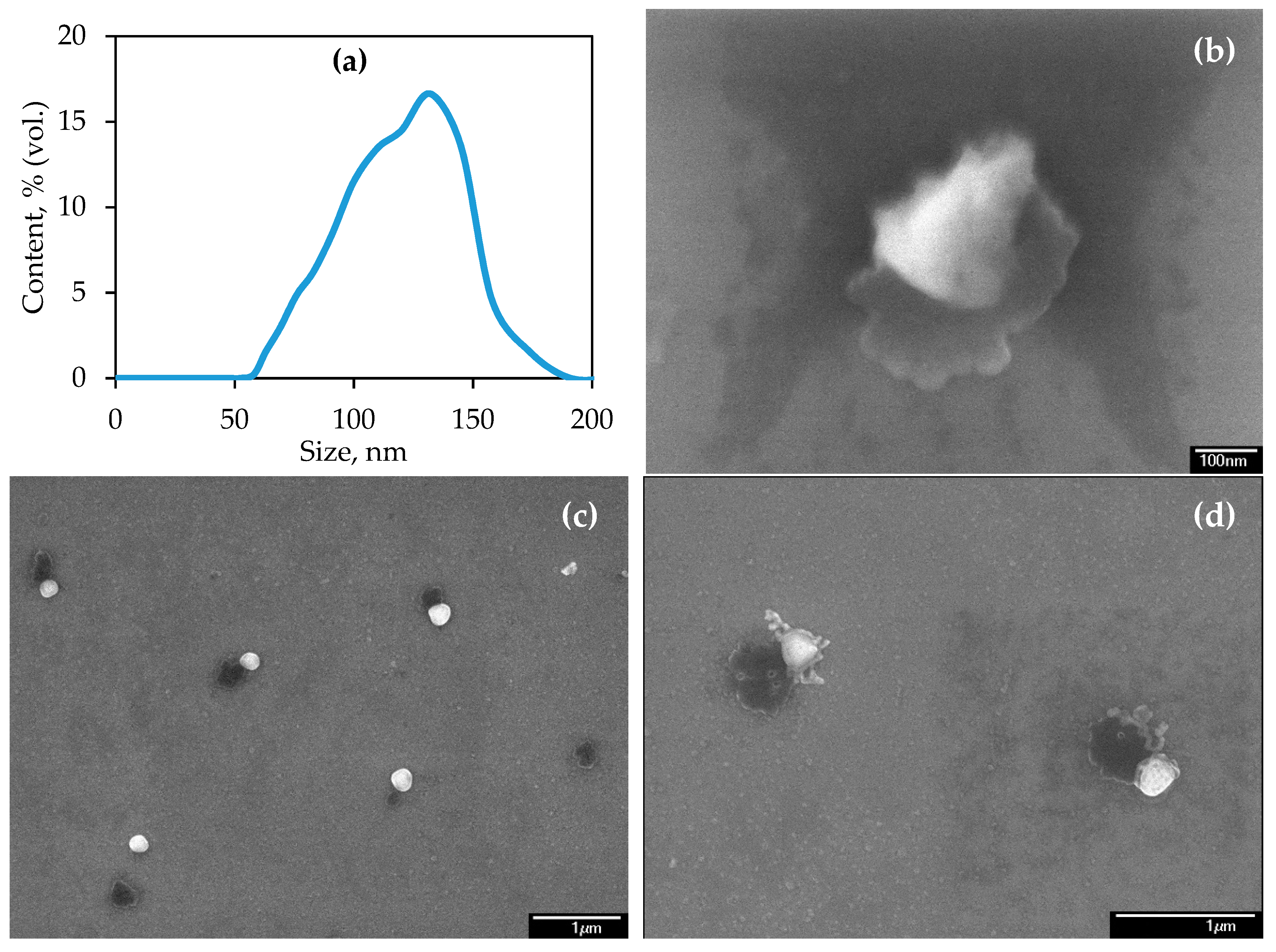
3.1. Characterization of NPs and Soil
3.2. The Mobility of CeO2, ZnO, and Cu NPs in Soil
3.3. Mass Balance of Ce, Zn, and Cu in Soil Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Bollag, J.M.; Myers, C.J.; Minard, R.D. Biological and chemical interactions of pesticides with soil organic matter. Sci. Total Environ. 1992, 123, 205–217. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Millan, G.; Agosto, F.; Vazquez, M. Use of clinoptilolite as a carrier for nitrogen fertilizers in soils of the Pampean regions of Argentina. Cienc. Investig. Agrar. 2008, 35, 293–302. [Google Scholar]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 6. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.; Niu, G.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO Nanoparticles Change the Nutritional Qualities of Cucumber (Cucumis sativus). J. Agric. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Y.; Hernandez-Viezcas, J.A.; Servin, A.D.; Hong, J.; Niu, G.; Peralta-Videa, J.R.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: A life cycle study. J. Agric. Food Chem. 2013, 61, 11945–11951. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef]
- Barrios, A.C.; Rico, C.M.; Trujillo-Reyes, J.; Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of uncoated and citric acid coated cerium oxide nanoparticles, bulk cerium oxide, cerium acetate, and citric acid on tomato plants. Sci. Total Environ. 2016, 563–564, 956–964. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Vilchis-Nestor, A.R.; Majumdar, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Citric acid modifies surface properties of commercial CeO2 nanoparticles reducing their toxicity and cerium uptake in radish (Raphanus sativus) seedlings. J. Hazard. Mater. 2013, 263, 677–684. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011, 7. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Biswas, A.K.; Tarafdar, J.C.; Subba Rao, A. Characterization of zinc oxide nano particles and their effect on growth of maize (Zea mays L.) plant. J. Plant Nutr. 2015, 38, 1505–1515. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Adhikari, T.; Sarkar, D.; Mashayekhi, H.; Xing, B. Growth and enzymatic activity of maize (Zea mays L.) plant: Solution culture test for copper dioxide nano particles. J. Plant Nutrit. 2016, 39, 99–115. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Owolade, O.; Ogunleti, D. Effects of titanium dioxide on the diseases, development and yield of edible cowpea. J. Plant Protect. Res. 2008, 48, 329–336. [Google Scholar] [CrossRef]
- Parveen, A.; Mazhari, B.B.Z.; Rao, S. Impact of bio-nanogold on seed germination and seedling growth in Pennisetum glaucum. Enzym. Microb. Technol. 2016, 95, 107–111. [Google Scholar] [CrossRef]
- Rajesh, S.; Raja, D.P.; Rathi, J.M.; Sahayaraj, K. Biosynthesis of silver nanoparticles using Ulva fasciata (Delile) ethyl acetate extract and its activity against Xanthomonas campestris pv. Malvacearum. J. Biopestic. 2012, 5, 119–128. [Google Scholar]
- Bramhanwade, K.; Shende, S.; Bonde, S.; Gade, A.; Rai, M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016, 14, 229–235. [Google Scholar] [CrossRef]
- Mondal, K.K.; Mani, C. Investigation of the antibacterial properties of nanocopper against Xanthomonas axonopodis pv. punicae, the incitant of pomegranate bacterial blight. Ann. Microbiol. 2012, 62, 889–893. [Google Scholar] [CrossRef]
- Giannousi, K.; Avramidis, I.; Dendrinou-Samara, C. Synthesis, characterization and evaluation of copper-based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv. 2013, 3, 21743–21752. [Google Scholar] [CrossRef]
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; Van den Brink, N.; Nickel, C. Fate and Bioavailability of Engineered Nanoparticles in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- Peijnenburg, W.; Praetorius, A.; Scott-Fordsmand, J.; Cornelis, G. Fate assessment of engineered nanoparticles in solids dominated media—Current insights and the way forward. Environ. Pollut. 2016, 218, 1365–1369. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Darlington, T.K.; Neigh, A.M.; Spencer, M.T.; Nguyen, O.T.; Oldenburg, S.J. Nanoparticle characteristics affecting environmental fate and transport through soil. Environ. Toxicol. Chem. 2009, 28, 1191–1199. [Google Scholar] [CrossRef]
- Zhao, L.; Peralta-Videa, J.R.; Hernandez-Viezcas, J.A.; Hong, J.; Gardea-Torresdey, J.L. Transport and retention behavior of ZnO nanoparticles in two natural soils: Effect of surface coating and soil composition. J. Nano Res. 2012, 17, 229–242. [Google Scholar] [CrossRef]
- Quevedo, I.R.; Tufenkji, N. Mobility of functionalized quantum dots and a model polystyrene nanoparticle in saturated quartz sand and loamy sand. Environ. Sci. Technol. 2012, 46, 4449–4457. [Google Scholar] [CrossRef]
- Cornelis, G.; Doolette, C.; Thomas, M.; McLaughlin, M.J.; Kirby, J.K.; Beak, D.G.; Chittleborough, D. Retention and dissolution of engineered silver nanoparticles in natural soils. Soil Sci. Soc. Am. J. 2012, 76, 891–902. [Google Scholar] [CrossRef]
- Fang, J.; Shan, X.Q.; Wen, B.; Lin, J.M.; Owens, G. Stability of titania nanoparticles in soil suspensions and transport in saturated homogeneous soil columns. Environ. Pollut. 2009, 157, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.G.; Li, Y.S.; Kim, H.; Walker, S.L.; Abriola, L.M.; Pennell, K.D. Transport and retention of fullerene nanoparticles in natural soils. J. Environ. Qual. 2010, 39, 1925–1933. [Google Scholar] [CrossRef]
- Cornelis, G.; Pang, L.; Doolette, C.; Kirby, J.K.; McLaughlin, M.J. Transport of silver nanoparticles in saturated columns of natural soils. Sci. Total Environ. 2013, 463–464, 120–130. [Google Scholar] [CrossRef]
- Jaisi, D.P.; Elimelech, M. Single-walled carbon nanotubes exhibit limited transport in soil columns. Environ. Sci. Technol. 2009, 43, 9161–9166. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.; Nelson, S. Gas displacement and aggregate stability of soil. Soil Sci. Soc. Am. J. 1985, 49, 25–28. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Bossuyt, H.; Frey, S.D.; Elliott, E.T.; Merckx, R.; Paustian, K. Influence of dry–wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem. 2001, 3, 1599–1611. [Google Scholar] [CrossRef]
- Peng, X.; Horn, R.; Smucker, A. Pore shrinkage dependency of inorganic and organic soils on wetting and drying cycles. Soil Sci. Soc. Am. J. 2007, 71, 1095–1104. [Google Scholar] [CrossRef]
- Shang, J.; Flury, M.; Chen, G.; Zhuang, J. Impact of flow rate, water content, and capillary forces on in situ colloid mobilization during infiltration in unsaturated sediments. Water Resour. Res. 2008, 44, W06411. [Google Scholar] [CrossRef]
- Gao, B.; Steenhuis, T.S.; Zevi, Y.; Morales, V.L.; Nieber, J.L.; Richards, B.K.; McCarthy, J.F.; Parlange, J.Y. Capillary retention of colloids in unsaturated porous media. Water Resour. Res. 2008, 44, W04504. [Google Scholar] [CrossRef]
- Baumann, T. Nanoparticles in Groundwater—Occurrence and Applications. In Nanoparticles in the Water Cycle; Frimmel, F.H., Niessner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; p. 27. [Google Scholar]
- Karandashev, V.K.; Turanov, A.N.; Orlova, T.A.; Lezhnev, A.E.; Nosenko, S.V.; Zolotareva, N.I.; Moskvina, I.R. Use of the inductively coupled plasma mass spectrometry for element analysis of environmental objects. Inorg. Mater. 2008, 44, 1491–1500. [Google Scholar] [CrossRef]
- Ermolin, M.S.; Fedyunina, N.N.; Karandashev, V.K.; Fedotov, P.S. Study on mobility of cerium oxide nanoparticles in soils using dynamic extraction in microcolumn and rotating coiled column. J. Anal. Chem. 2019, 74. in press. [Google Scholar]
- Utomo, W.H.; Dexter, A.R. Changes in soil aggregate water stability induced by wetting and drying cycles in non-saturated soil. Eur. J. Soil Sci. 1982, 33, 623–631. [Google Scholar] [CrossRef]
- Wagner, S.; Gondikas, A.; Neubauer, E.; Hofmann, T.; von der Kammer, F. Spot the difference: Engineered and natural nanoparticles in the environment—Release, behavior, and fate. Angew. Chem. Int. Ed. 2014, 53, 12398–12419. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Conway, J.R.; Adeleye, A.S.; Gardea-Torresdey, J.; Keller, A.A. Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ. Sci. Technol. 2015, 49, 2749–2756. [Google Scholar] [CrossRef]



| Element Oxide/ Element | Soil | Standard Sample | |||
|---|---|---|---|---|---|
| Andesite, AGV-2 | Gabbro, GSO 521-84P | ||||
| Determined | Certified Value | Determined | Certified Value | ||
| Concentration, mg g−1 | |||||
| Na2O | 8.2 ± 0.3 | 41 | 41.9 ± 1.3 | 27 | 27.2 ± 0.7 |
| MgO | 10.5 ± 0.1 | 18 | 17.9 ± 0.3 | 67 | 68.1 ± 0.9 |
| Al2O3 | 103 ± 2 | 168 | 169.1 ± 2.1 | 149 | 149.3 ± 1.6 |
| P2O5 | 1.69 ± 0.01 | 4.6 | 4.8 ± 0.2 | 11 | 10.3 ± 0.2 |
| K2O | 22.5 ± 0.7 | 28 | 28.8 ± 1.1 | 31 | 30.9 ± 1.1 |
| CaO | 16.4 ± 0.7 | 50 | 52.0 ± 1.3 | 108 | 106.8 ± 1.4 |
| TiO2 | 5.76 ± 0.02 | 10.6 | 10.5 ± 2.2 | 17 | 17.2 ± 0.5 |
| MnO | 0.84 ± 0.02 | 1.0 | 1.0 ± 0.03 | 1.8 | 1.7 ± 0.1 |
| Fe2O3 | 37.1 ± 0.3 | 66 | 66.9 ± 1.3 | 112 | 113 ± 1 |
| Concentration, µg g−1 | |||||
| Ce | 66 ± 1 | 51.5 | 53 ± 4 | 53.4 | 58 ± 5 |
| Zn | 60 ± 2 | 93.4 | 86 ± 8 | 120 | 120 ±15 |
| Cu | 19 ± 1 | 67.2 | 68 ± 3 | 180 | 163 ± 20 |
| Element | Number of WDC | Concentration, µg g−1 | ||
|---|---|---|---|---|
| NPs-Derived Fraction | Naturally Occurring Fraction | Residual Fraction | ||
| Ce | 1 | 0.073 ± 0.004 | 0.007 ± 0.001 | 123 ± 3 |
| 2 | 0.066 ± 0.008 | 0.009 ± 0.001 | ||
| 3 | 0.053 ± 0.007 | 0.011 ± 0.001 | ||
| 4 | 0.049 ±0.009 | 0.011 ± 0.003 | ||
| Zn | 1 | 0.091 ± 0.006 | 0.088 ± 0.006 | 108 ± 4 |
| 2 | 0.046 ± 0.008 | 0.084 ± 0.007 | ||
| 3 | 0.034 ± 0.010 | 0.087 ± 0.010 | ||
| 4 | 0.033 ± 0.009 | 0.075 ± 0.009 | ||
| Cu | 1 | - | 0.133 ± 0.005 | 35 ± 3 |
| 2 | 0.028 ± 0.007 | 0.136 ± 0.007 | ||
| 3 | 0.084 ± 0.015 | 0.147 ± 0.015 | ||
| 4 | 0.173 ± 0.005 | 0.139 ± 0.005 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolin, M.; Fedyunina, N.; Katasonova, O. Mobility and Fate of Cerium Dioxide, Zinc Oxide, and Copper Nanoparticles in Agricultural Soil at Sequential Wetting-Drying Cycles. Materials 2019, 12, 1270. https://doi.org/10.3390/ma12081270
Ermolin M, Fedyunina N, Katasonova O. Mobility and Fate of Cerium Dioxide, Zinc Oxide, and Copper Nanoparticles in Agricultural Soil at Sequential Wetting-Drying Cycles. Materials. 2019; 12(8):1270. https://doi.org/10.3390/ma12081270
Chicago/Turabian StyleErmolin, Mikhail, Natalia Fedyunina, and Olesya Katasonova. 2019. "Mobility and Fate of Cerium Dioxide, Zinc Oxide, and Copper Nanoparticles in Agricultural Soil at Sequential Wetting-Drying Cycles" Materials 12, no. 8: 1270. https://doi.org/10.3390/ma12081270