Structural Properties and Phase Stability of Primary Y Phase (Ti2SC) in Ti-Stabilized Stainless Steel from Experiments and First Principles
Abstract
:1. Introduction
2. Experimental Procedure and Calculation Details
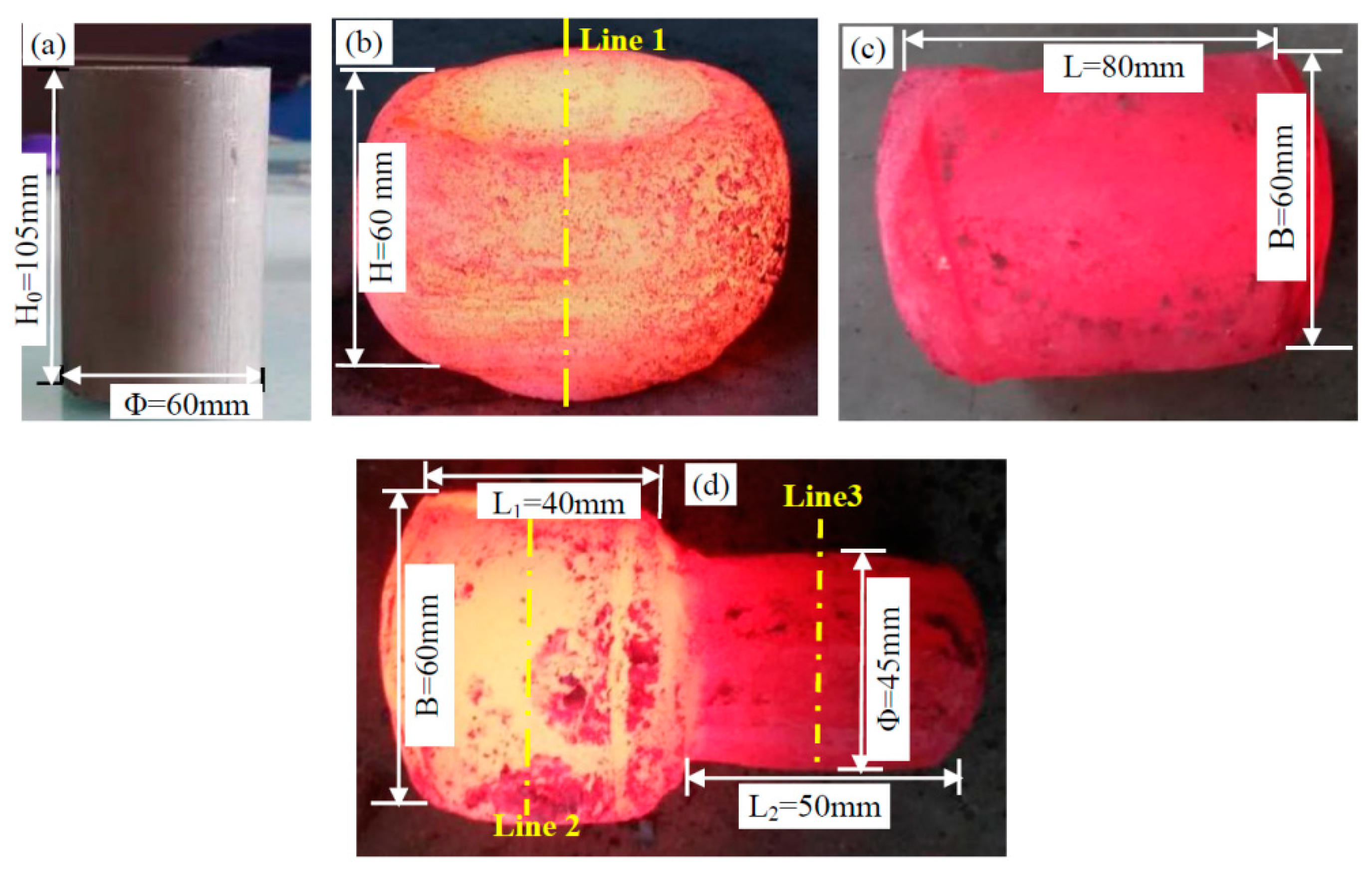
2.1. Experimental Method
2.2. Calculation Details
3. Results and Discussion
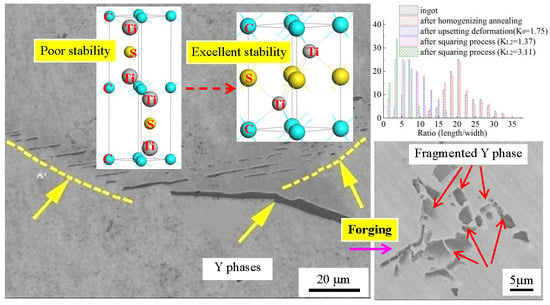
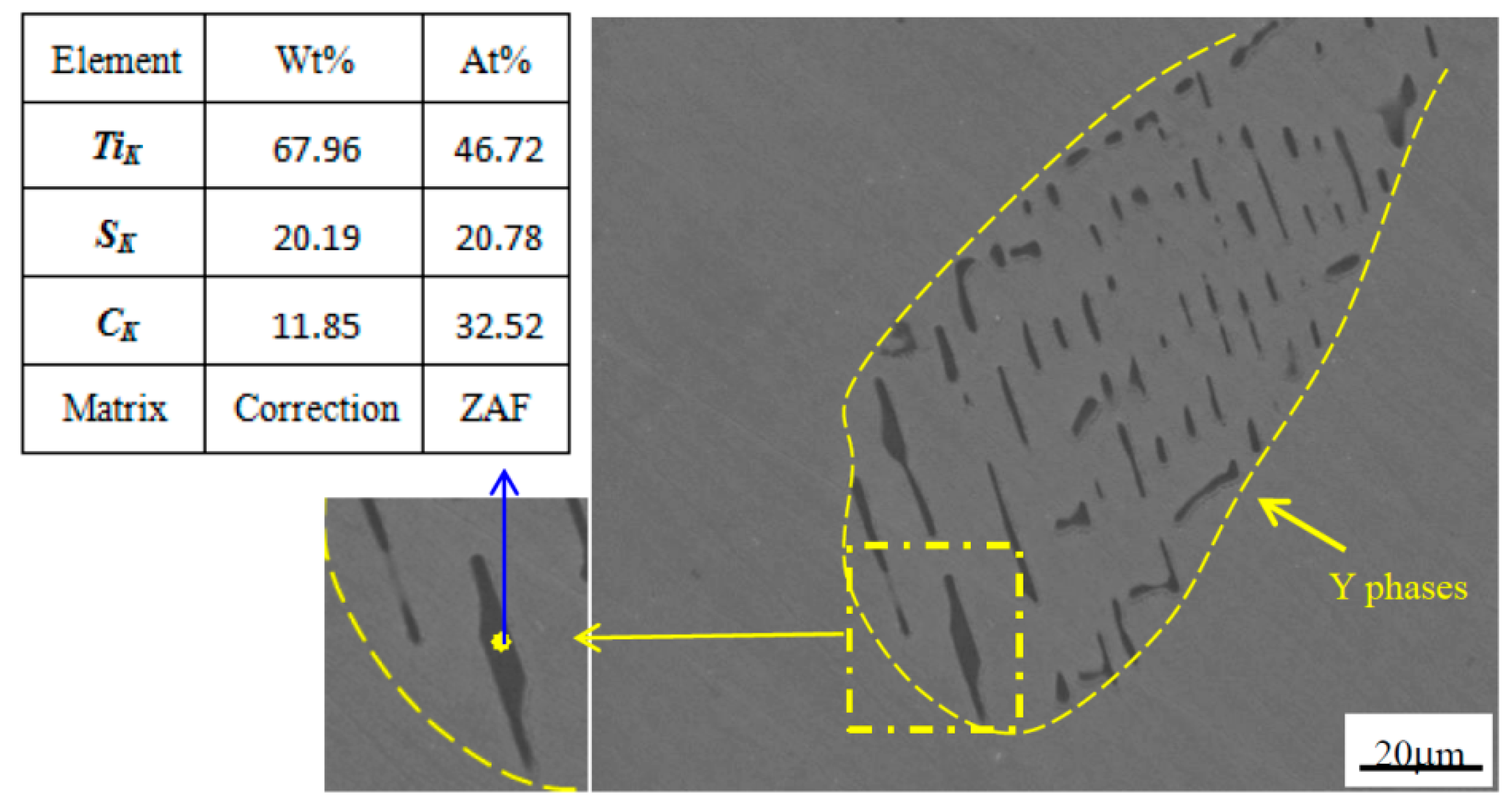
3.1. Morphology of Y Phase in Cast Ingot and Annealing Structure
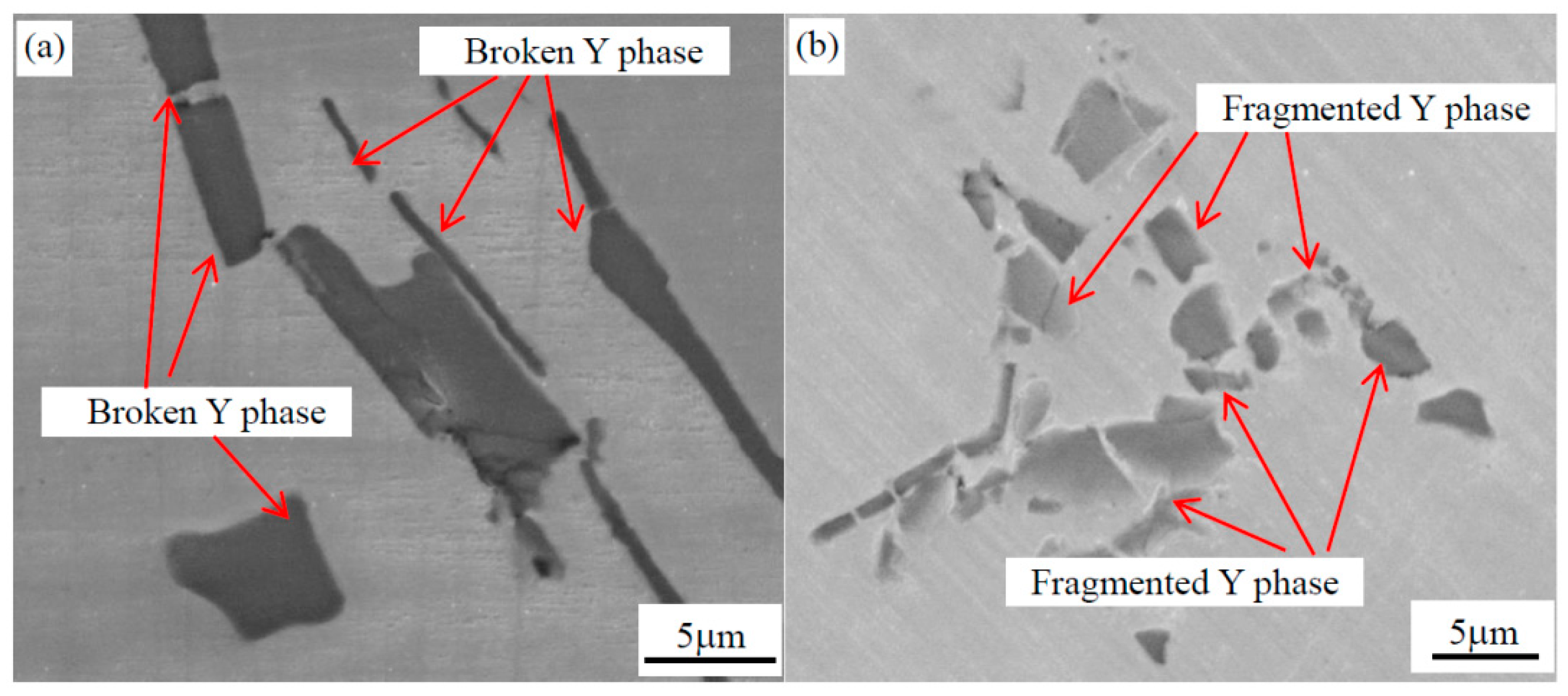
3.2. Morphology of Y Phase after Forging
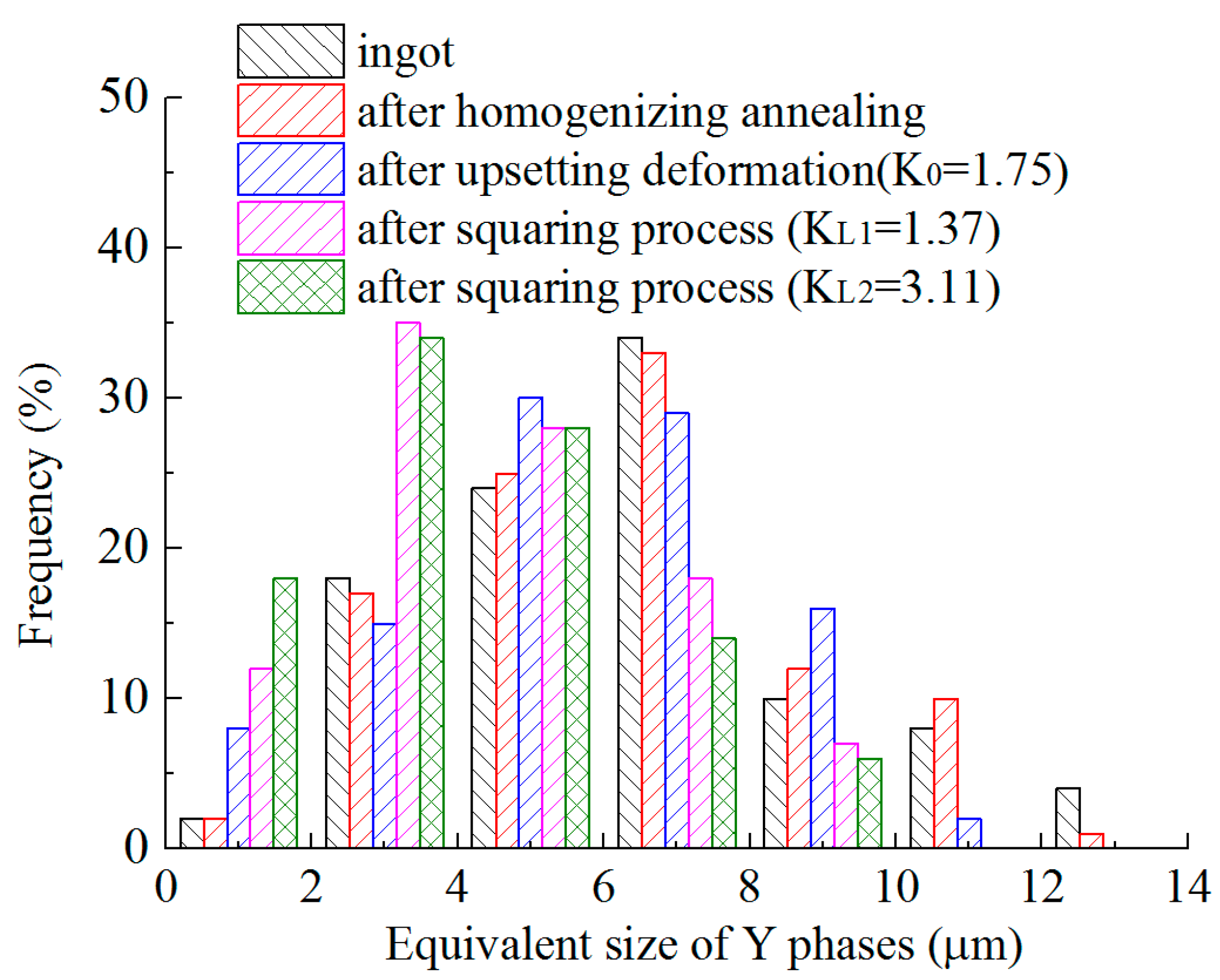
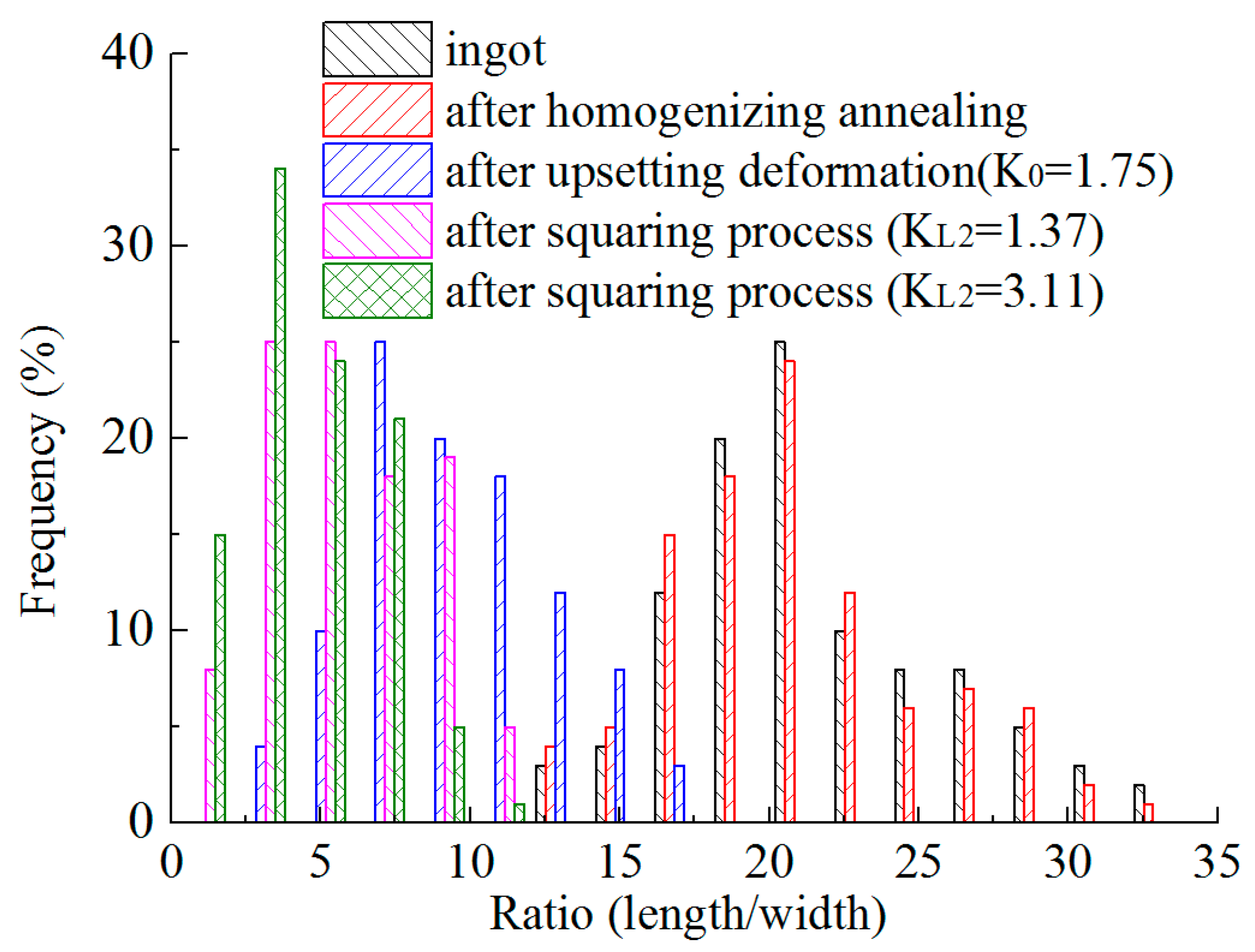
3.3. Size Distribution of Y Phases
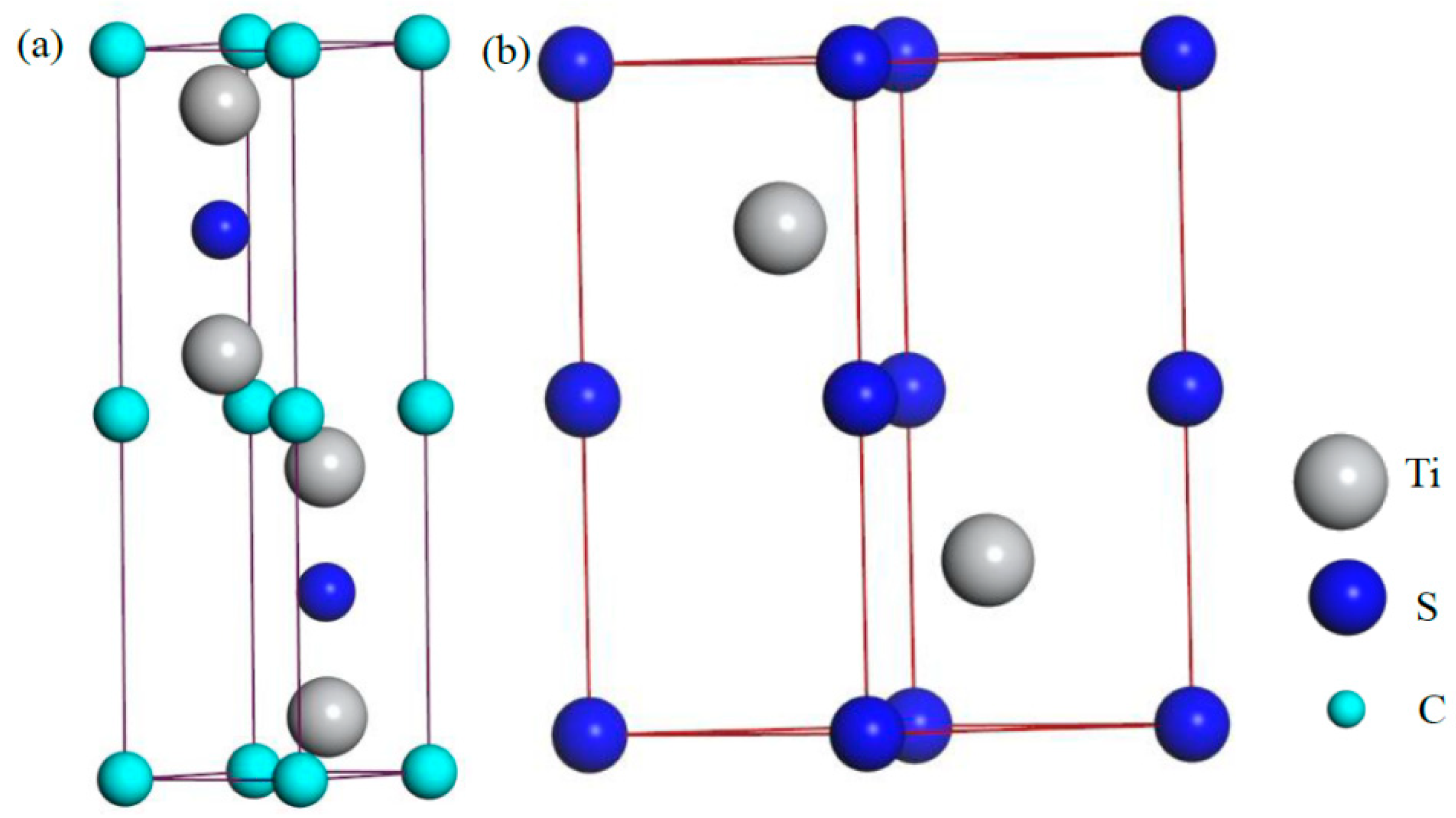
3.4. Crystal Structure and Phase Stability
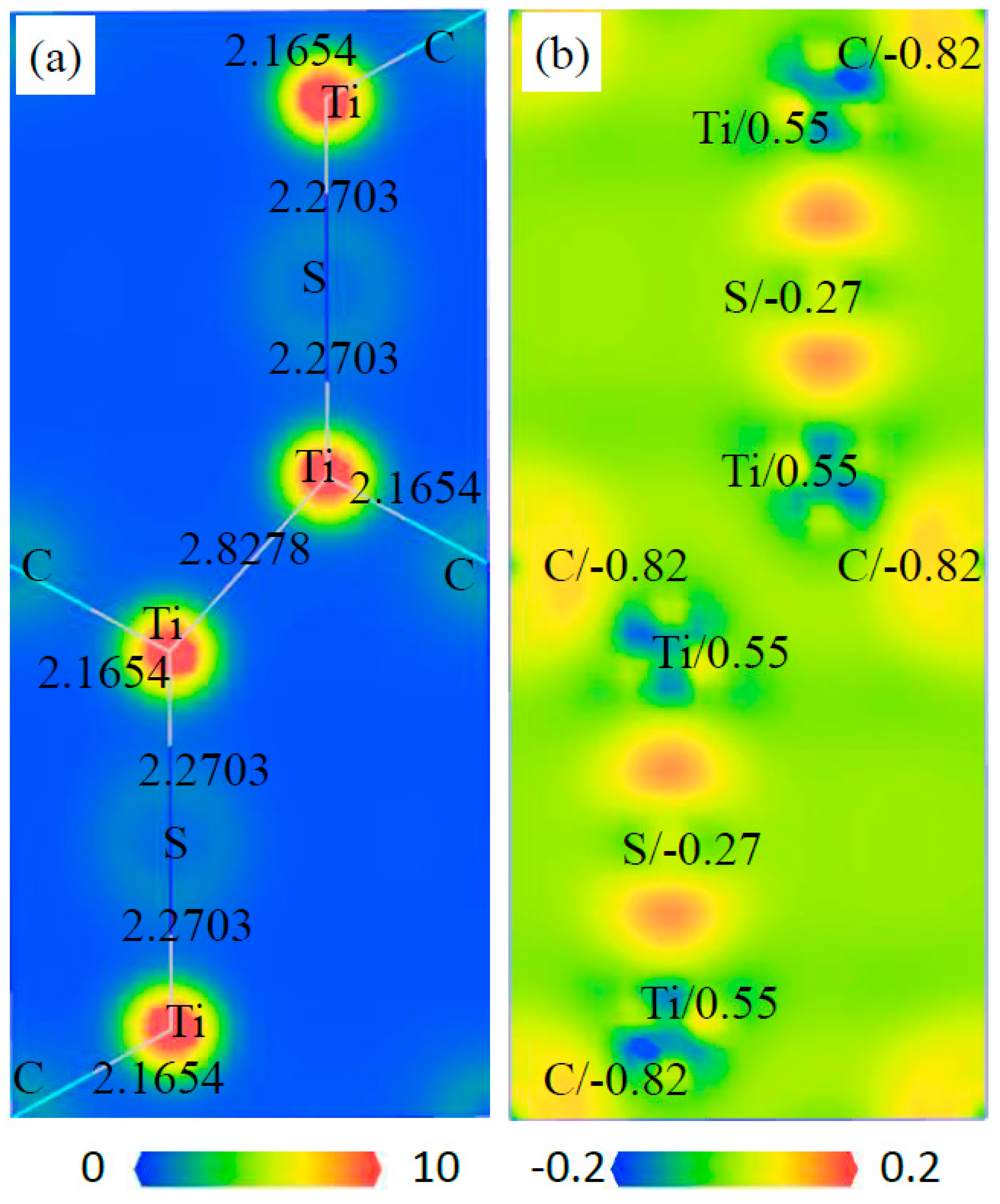
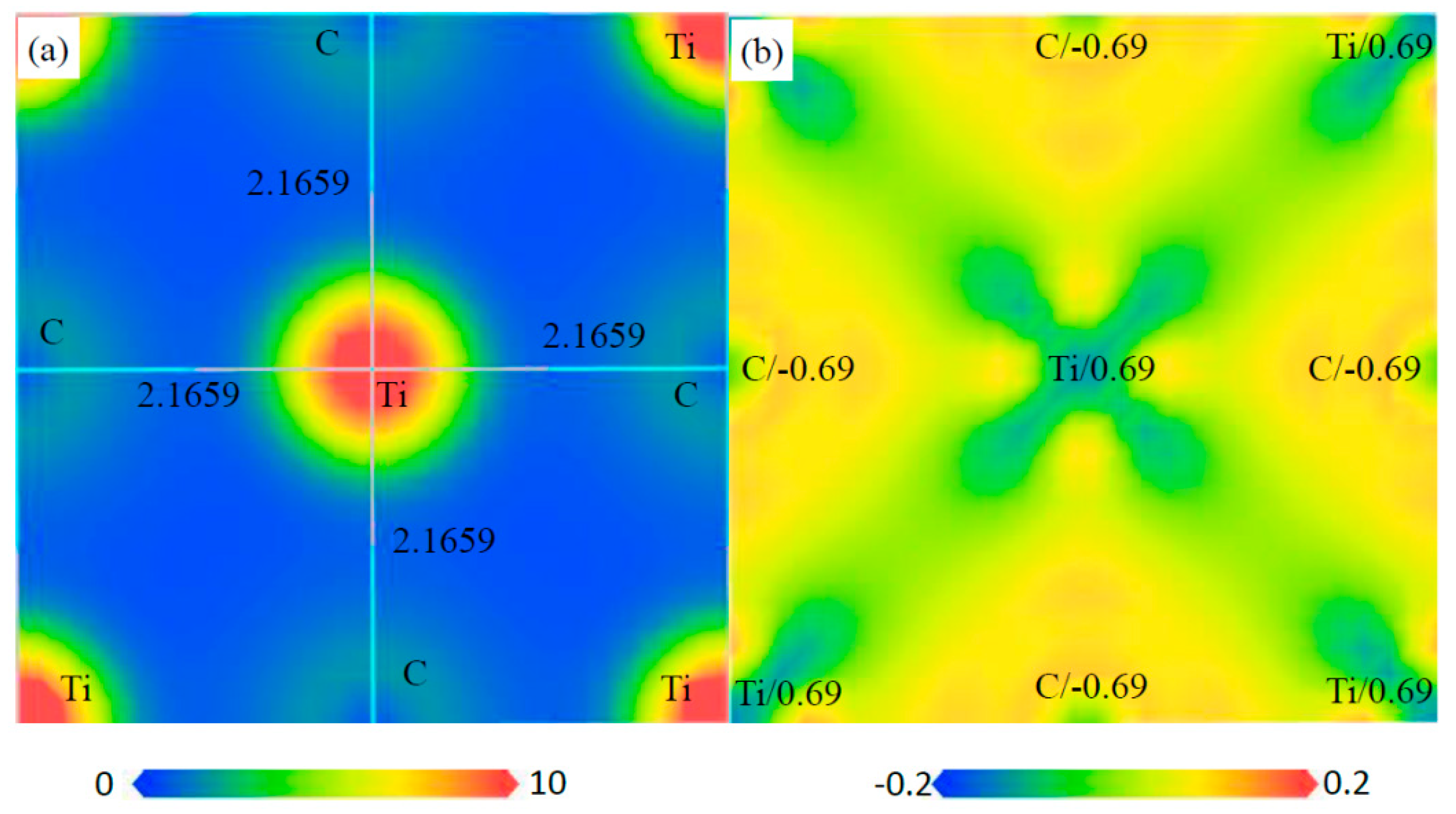
3.5. Electronic Structure of Phases
3.6. Elastic Properties of Phases
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pardo, A.; Merino, M.; Coy, A.; Viejo, F.; Carboneras, M.; Arrabal, R. Influence of Ti, C and N concentration on the intergranular corrosion behaviour of AISI 316Ti and 321 stainless steels. Acta Mater. 2007, 55, 2239–2251. [Google Scholar]
- Zhou, Y.; Li, Y.; Wang, W.; Qian, L.; Xiao, S.; Lv, Z. Effect of interstitial nitrogen in Fe18Cr6Mn8 austenitic alloys from density functional theory. J. Magn. Magn. Mater. 2018, 463, 57–63. [Google Scholar]
- Rezaeia, H.A.; Shaban Ghazanib, M.; Eghbalia, B. Effect of post deformation annealing on the microstructure and mechanical properties of cold rolled AISI 321 austenitic stainless steel. Mater. Sci. Eng. A 2018, 736, 364–374. [Google Scholar]
- Karthik, D.; Swaroop, S. Effect of laser peening on electrochemical properties of titanium stabilized 321 steel. Mater. Chem. Phys. 2017, 193, 147–155. [Google Scholar]
- Ramkumar, K.D.; Pavan, B.; Chandrasekar, V. Development of improved microstructural traits and mechanical integrity of stabilized stainless steel joints of AISI 321. J. Manuf. Process. 2018, 32, 582–594. [Google Scholar] [CrossRef]
- Mankari, K.; Acharyya, S.G. Failure analysis of AISI 321 stainless steel welded pipes in solar thermal power plants. Eng. Fail. Anal. 2018, 86, 33–43. [Google Scholar]
- Chen, Y.; Chou, L.; Shih, H. Effect of solution pH on the electrochemical polarization and stress corrosion cracking of Alloy 690 in 5M NaCl at room temperature. Mater. Sci. Eng. A 2005, 396, 129–137. [Google Scholar]
- Nkhoma, R.K.C.; Siyasiya, C.W.; Stumpf, W.E. Hot workability of AISI 321 and AISI 304 austenitic stainless steels. J. Alloys Compd. 2014, 595, 103–112. [Google Scholar] [Green Version]
- Zhang, J.S.; Chen, J.G. A titanium carbosulphide phase in alloy steels and superalloys. Acta Metall. Sin. 1980, 16, 159–164. [Google Scholar]
- Guo, K.X. Phases in high alloy steels and superalloys. Acta Metall. Sin. 1978, 14, 73–95. [Google Scholar]
- Liu, W.J.; Jonas, J.J. Calculation of the Ti(CyN1−y)-Ti4C2S2-MnS-austenite equilibrium in Ti-bearing steels. Metall. Trans. A 1989, 20, 1361–1374. [Google Scholar] [CrossRef]
- Casa, C.; Nileshwar, V.B. Precipitation in Unstabilized Austenitic Stainless Steel. J. Iron Steel Inst. 1969, 207, 1003. [Google Scholar]
- Wallace, W.; Holt, R.T.; Terada, T. Nature of the sulfo-carbides observed in nickel-base superalloys. Metallography 1973, 6, 511–526. [Google Scholar] [CrossRef]
- Maloney, J.L.; Garrison, W.M. The effect of sulfide type on the fracture behavior of HY180 steel. Acta Mater. 2005, 53, 533–551. [Google Scholar] [CrossRef]
- Wilson, P.; Chen, Z. TEM characterisation of iron titanium sulphide in titanium- and niobium-containing low manganese steel. Scr. Mater. 2007, 56, 753–756. [Google Scholar] [CrossRef]
- Bandi, W.R.; Krapf, G. Identification and determination of titanium sulphide and carbosulphide compounds in steel. Analyst 1979, 104, 812. [Google Scholar] [CrossRef]
- Ball, C.J. X-ray powder diffraction patterns of titanium carbosulphide and τ titanium sulphide. Met. Sci. 1984, 18, 577–579. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J.; Sham, L. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, M. Inhomogeneous Electron Gas. Phys. Rev. B 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Liu, B.-L.; Lü, Z.-Q.; Feng, W.-W.; Ren, T.-Z.; Fu, W.-T. Precipitation and decomposition behaviors of carbides in AISI M2 high-speed steel with nitrogen and mischmetal. J. Cent. Univ. 2017, 24, 782–788. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, B.; Wang, Z.; Sun, S.; Fu, W. Effect of cyclic heat treatments on spheroidizing behavior of cementite in high carbon steel. Mater. Sci. Eng. A 2013, 574, 143–148. [Google Scholar] [CrossRef]
- Zheng, C.S.; Li, L.F. Mechanical behavior of ultrafine-grained eutectoid steel containing Nano-cementite particles. Mater. Sci. Eng. A 2018, 713, 35–42. [Google Scholar] [CrossRef]
- Prasad, C.; Bhuyan, P.; Kaithwas, C.; Saha, R.; Mandal, S. Microstructure engineering by dispersing nano-spheroid cementite in ultrafine-grained ferrite and its implications on strength-ductility relationship in high carbon steel. Mater. Des. 2018, 139, 324–335. [Google Scholar] [CrossRef]
- Pankratova, O.Y.; Novakovskii, V.A.; Vladimirova, V.A.; Zvinchuk, R.A. The synthesis and structure of titanium monosulphide. Zhurnal Neorganicheskoi Khimii (Russ. J. Org. Chem.) 1986, 31, 1615–1618. [Google Scholar]
- Zhou, Y.C.; Wang, X.H.; Sun, Z.M.; Chen, S.Q. Electronic and structural properties of the layered ternary carbide Ti3AlC2. J. Mater. Chem. 2001, 11, 2335–2339. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef]
- Shanker, J.; Kushwah, S.; Sharma, M. On the universality of phenomenological isothermal equations of state for solids. Phys. B Condens. 1999, 271, 158–164. [Google Scholar] [CrossRef]
- Fang, C.; Van Huis, M.; Sluiter, M. Formation, structure and magnetism of the γ-(Fe,M)23C6 (M = Cr, Ni) phases: A first-principles study. Acta Mater. 2016, 103, 273–279. [Google Scholar] [CrossRef]
- Lv, Z.; Xiao, S.; Xiao, Z.; Qian, L.; Wang, W.; Zhou, Y.; Fu, W. Structural properties and bonding characteristic of interfaces between VN and VC from density functional calculations. J. Alloy Compd. 2017, 718, 139–149. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.; Yu, C.; Chen, J. First-principles calculations of mechanical properties of TiC and TiN. J. Alloy Compd. 2009, 485, 542–547. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.H.; Heo, Y.U.; Suh, D.W. Stability of (Ti,M)C (M = Nb, V, Mo and W) carbide in steels using first-principles calculations. Acta Mater. 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, B.-J. Modified embedded-atom method interatomic potentials for the Ti-C and Ti-N binary systems. Acta Mater. 2008, 56, 3481–3489. [Google Scholar] [CrossRef]
- Ramalingam, B.; Vanek, J.; MacLaren, J.M.; McHenry, M.E.; Garrisonjr, W.M. Electronic structure and bonding in titanium carbosulphide. Philos. Mag. B 2000, 80, 379–394. [Google Scholar] [CrossRef]
- Lv, Z.; Shi, Z.; Gao, Y.; Wang, Z.; Sun, S.; Fu, W. Electronic and elastic properties of ε-phases Cr2−xVxN (x = 0, 1, 2) from density-functional calculations. J. Alloy Compd. 2014, 583, 79–84. [Google Scholar] [CrossRef]
- Zhang, W.H.; Lv, Z.Q.; Shi, Z.P.; Sun, S.H.; Wang, Z.H.; Fu, W.T. Electronic, magnetic and elastic properties of ε-phases Fe3X (X = B, C, N) from density-functional theory calculations. J. Magn. Magn. Mater. 2012, 324, 2271–2276. [Google Scholar] [CrossRef]
- Gilman, J.J.; Roberts, B.W. Elastic constants of TiC and TiB2. J. Appl. Phys. 1961, 32, 1405. [Google Scholar] [CrossRef]
- Lv, Z.; Gao, Y.; Sun, S.; Qv, M.; Wang, Z.; Shi, Z.; Fu, W. Electronic, magnetic and elastic properties of γ-Fe4X (X = B/C/N) from density functional theory calculations. J. Magn. Magn. Mater. 2013, 333, 39–45. [Google Scholar] [CrossRef]
- Mehl, M.J.; Barry, B.M.; Papaconstantopoulos, D.A. Intermetallic compounds: Principle and practice. In Principles; Westbrook, J.H., Fleischeir, R.L., Eds.; Wiley: London, UK, 1995; Volume 1, p. 195. [Google Scholar]
- Ranganathan, S.I.; Starzewshi, M.O. Universal Elastic Anisotropy Index. Phys. Rev. B 2008, 101, 055504. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Fan, J.; Guan, K.; Wu, Z.; Zhao, D.; Fu, W. Effects of interstitial atoms (N/O) in bcc Fe from first-principle calculations. Eng. Des. 2018, 137, 22–29. [Google Scholar] [CrossRef]
- Wu, S.; Hou, Z.; Zhu, Z.; Hou, Z. Elastic properties and electronic structures of CdCNi3: A comparative study with MgCNi3. Solid State Sci. 2009, 11, 251–258. [Google Scholar] [CrossRef]





| Phase | a (Å) | b (Å) | c (Å) | c/a | Vcell (Å3) | ∆Ef-cell | ∆Ef-f.u. | ∆Ef |
|---|---|---|---|---|---|---|---|---|
| Ti4S2C2(Z = 2) | 3.2802 | 3.2802 | 13.2810 | 4.05 | 123.752 | −8.46 | −2.12 | −1.06 |
| Ti2S2(Z = 2) | 3.3342 | 3.3342 | 6.3494 | 1.90 | 61.13 | −7.61 | −3.81 | −1.90 |
| Ti2SC | 3.1702 | 3.1702 | 5.8550 | 1.85 | 50.96 | −6.70 | −6.70 | −1.46 |
| Ti4C4(Z = 4) | 4.3317 | 4.3317 | 4.3317 | - | 81.28 | −7.54 | −1.88 | −0.94 |
| Phase | C11 | C12 | C13 | C33 | C44 |
|---|---|---|---|---|---|
| Ti4S2C2 (Z = 2) | 154.4 | 16.4 | 71.0 | 419.1 | −23.2 |
| Ti2S2 (Z = 2) | 195.7 | 82.5 | 99.5 | 194.3 | 70.4 |
| Ti2SC | 328.3 | 79.6 | 82.4 | 348.2 | 86.1 |
| Ti4C4(Z = 4) | 516.6 | 115.1 | - | - | 182.8 |
| Phase | E | B | G | ν | AU | θD |
|---|---|---|---|---|---|---|
| Ti4S2C2 (Z = 2) | −111.4 | 100.4 | −33.1 | - | - | - |
| Ti2S2 (Z = 2) | 153.2 | 127.5 | 58.9 | 0.300 | 0.146 | 492 |
| Ti2SC | 265.5 | 165.9 | 107.6 | 0.233 | 0.224 | 684 |
| Ti4C4 (Z = 4) | 454.9 | 248.9 | 189.8 | 0.196 | 0.011 | 944 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Zhou, Y.; Fan, J.; Liu, T.; Nie, Y.; Fu, W.; Lv, Z. Structural Properties and Phase Stability of Primary Y Phase (Ti2SC) in Ti-Stabilized Stainless Steel from Experiments and First Principles. Materials 2019, 12, 1118. https://doi.org/10.3390/ma12071118
Zhao D, Zhou Y, Fan J, Liu T, Nie Y, Fu W, Lv Z. Structural Properties and Phase Stability of Primary Y Phase (Ti2SC) in Ti-Stabilized Stainless Steel from Experiments and First Principles. Materials. 2019; 12(7):1118. https://doi.org/10.3390/ma12071118
Chicago/Turabian StyleZhao, Deli, Yu Zhou, Jiangyu Fan, Tianyu Liu, Yihong Nie, Wantang Fu, and Zhiqing Lv. 2019. "Structural Properties and Phase Stability of Primary Y Phase (Ti2SC) in Ti-Stabilized Stainless Steel from Experiments and First Principles" Materials 12, no. 7: 1118. https://doi.org/10.3390/ma12071118